Performance-Based Measures of Everyday Function in Mild Cognitive Impairment
Abstract
Objective
The view that everyday function is preserved in mild cognitive impairment may be problematic. The objectives of this study were to determine the magnitude of impairment in everyday function in patients with mild cognitive impairment and Alzheimer's disease using a novel sensitive performance-based measure (the UCSD Performance-Based Skills Assessment; UPSA), contrast it with use of an informant-based measure (the Alzheimer's Disease Cooperative Study–Activities of Daily Living Inventory; ADCS-ADL), and model the relationship between cognitive measures and the performance-based measure.
Method
Fifty cognitively normal elders, 26 patients who met criteria for amnestic mild cognitive impairment, and 22 patients who suffered from mild to moderate Alzheimer's disease were assessed on the UPSA, the ADCS-ADL, and a battery of neurocognitive tests.
Results
Patients with mild cognitive impairment had significant impairments on the UPSA but not on the ADCS-ADL. The magnitude of the effect size between the cognitively healthy and the mild cognitive impairment group for the UPSA was large (d=0.86). A strong and significant relationship was observed between cognitive performances in speed (R2=0.37), episodic memory (R2=0.10), and semantic processing (R2=0.03) and UPSA score using multiple regression models. The psychometric properties of the UPSA were acceptable, as were its sensitivity and specificity in contrasts between cognitively normal elders and patients with mild cognitive impairment and between the latter group and patients with Alzheimer's disease.
Conclusions
These findings indicate that performance-based measures of function may be a sensitive tool in studies of Alzheimer's disease and mild cognitive impairment and suggest the need for a reconceptualization of the relationship between cognition and function in mild cognitive impairment so that they can be usefully aligned.
Mild cognitive impairment is a less-than-benign diagnosis because it is associated with an elevated risk of incident Alzheimer's disease and more rapid cognitive decline (1, 2). The rate of conversion from mild cognitive impairment to Alzheimer's disease may be 10%–12% per year (3). Neuropathologically, mild cognitive impairment (in many but not all cases) appears to be a transitional state of evolving Alzheimer's disease (4, 5). Recent in vivo quantified imaging using the amyloid binding ligand carbon-11-PIB has suggested that the amyloid burden in mild cognitive impairment is intermediate between healthy comparison subjects and patients with Alzheimer's disease (6, 7). By recommended diagnostic criteria, individuals with mild cognitive impairment have an impairment of 1.5 standard deviations in episodic memory but essentially preserved everyday function (8, 9).
Several lines of evidence suggest that the diagnostic criterion relating to function may be problematic. First, neuropsychiatric conditions with associated cognitive impairments are also reliably associated with sequelae in everyday function. These include such disorders as Alzheimer's disease itself, various amnestic syndromes, traumatic brain injury, focal epilepsy, stroke, multiple sclerosis, and HIV infection (10–16). Second, if the cognitive impairment and underlying neuropathology of amnestic mild cognitive impairment and Alzheimer's disease are on a continuum, it would be unlikely that functional impairment could be dichotomized as preserved (in mild cognitive impairment) or impaired (in Alzheimer's disease). Thus, when pathology and cognitive impairment are dimensional, they are also likely to exact a graduated cost on function in mild cognitive impairment.
Another factor that might artifactually maintain views of intact or preserved function in mild cognitive impairment is related to test measurement issues. If more sensitive measures of everyday function were used, they might indeed be found to be compromised in populations with mild cognitive impairment. There has been little empirical study of the exact nature of activities of daily living or instrumental activities of daily living in individuals with mild cognitive impairment (see the Discussion section for more on previous work), and several investigators have suggested that new instruments capable of objectively measuring functional impairments and their decline in patients with mild cognitive impairment would advance the field (17–20). Basic activities such as bathing, toileting, dressing, and eating remain preserved in the face of mild cognitive deterioration, but more complex abilities, such as managing schedules, managing finances, planning trips, shopping, and using public transportation, which usually involve interactions with technology, use of higher-level cognitive operations, or knowledge of cultural expectations (i.e., instrumental activities of daily living), are sometimes thought to be impaired, albeit subtly (21). Because instrumental activities of daily living are more complex and demanding and rely less on routines and habits, they may be more sensitive to the early effects of cognitive deterioration. Another measurement issue that can affect test sensitivity is the use of informant-based measures, which can suffer from the inherent problems associated with halo effects, memory lapses, or lacunae in knowledge, generally resulting in an overestimation of the patient's abilities (22). Thus, one way to increase sensitivity in these functional measures might be to use performance-based measures. In these functional outcome assessments, individuals are tested and objectively scored on their ability to perform real-world task analogues.
In this study, we carefully assayed functional disability using a performance-based measure of everyday function that included many ecologically relevant items in healthy comparison subjects, patients with mild cognitive impairment, and patients with Alzheimer's disease and then assessed its relationship to performances on a variety of cognitive tests, as well as its psychometric properties. We were also able to compare it to a widely used informant-based measure of function.
Method
Staging Instruments
The Mini-Mental State Examination (MMSE) (23) was used for screening of cognitive level. The Clinical Dementia Rating scale (24) was used to assess dementia; this instrument consists of items relating to memory, orientation, problem solving, personal care, function at home and in hobbies, and function in community affairs. Domain scores were integrated in an algorithm in order to yield the global value.
Participants
All participants were between the ages of 55 and 85 years. There were no restrictions based on gender or ethnicity. Patients were recruited because they had subjective complaints. All potential participants underwent examination by a neurologist or a geriatric psychiatrist to determine study eligibility.
Patients with evidence of clinically significant and active pulmonary, gastrointestinal, renal, hepatic, endocrine, or cardiovascular system disease were excluded, as were individuals with clinically significant folate or vitamin B12 deficiency and those undergoing cancer treatment. Individuals with Hachinski Ischemic Scores >4 were excluded (25). Patients with evidence of other neurological disorders, including but not limited to stroke, Parkinson's disease, seizure disorder, hydrocephalus, and head injury with loss of consciousness greater than 30 minutes within the past 5 years, were excluded. Patients with a current DSM-IV axis I disorder other than Alzheimer's disease (including substance use diagnoses within the previous year) were excluded. Controlled diabetes, hypertension, and hypothyroidism were not among the exclusion criteria.
Alzheimer's disease. Twenty-two individuals met National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders Association criteria for probable Alzheimer's disease. Diagnostic criteria include memory impairment (defined below for mild cognitive impairment) and at least one other area of impaired cognition, including speed of processing, executive ability, and/or semantic processing/language; report of decline in memory and other areas of cognition; and impairments in activities of daily living. Patients in the Alzheimer's disease group had MMSE scores <24 and >12 (i.e., in the mild to moderate range) and Clinical Dementia Ratings of 1.0 or greater on the global scale.
Mild cognitive impairment
The diagnosis of mild cognitive impairment was made according to the criteria of Petersen et al. (8) for "amnestic" mild cognitive impairment in 26 individuals. These individuals had memory impairment of greater than 1.5 standard deviations on either selective reminding or logical memory and had ostensibly preserved activities of daily living (i.e., were "functioning well"). Individuals who had additional impairments in other nonmnemonic domains of cognition were also included, as long as activities of daily living were ostensibly preserved (i.e., "amnestic plus"). This approach is similar to that used in the study of Tabert et al. (26). All participants with mild cognitive impairment had MMSE scores ≥24 (i.e., they did not have dementia) and Clinical Dementia Ratings of 0.5 (as specified by the National Institute of Aging Working Group on Study Design) (27).
Cognitively healthy elders
Fifty elders had MMSE scores ≥24 and did not meet psychometric criteria for mild cognitive impairment or Alzheimer's disease. All formal neurocognitive test scores for these participants were within 1.5 standard deviations of normative data in published studies or manuals. Cognitively healthy elders were usually the spouses of study participants.
Medications
Of the cognitively healthy participants, 34 were not receiving any medication; nine were receiving a selective serotonin reuptake inhibitor (SSRI) and seven were receiving levothyroxine. Of the patients with mild cognitive impairment, 11 were not receiving any medication, while eight were receiving cholinesterase inhibitors, four were receiving memantine, eight were receiving an SSRI, and two were receiving a second-generation antipsychotic. Of the patients with Alzheimer's disease, four were not receiving any medication, seven were receiving cholinesterase inhibitors, eight were receiving memantine, seven were receiving an SSRI, and two were receiving a second-generation antipsychotic.
Cognitive Measures
For verbal list learning, we used a version of the Buschke Selective Reminding Test (28) that consisted of six trials of a 12-word list, followed after 20 minutes by free recall and recognition; total recall (trials 1–6) and delayed free recall were the dependent measures. To test memory for stories, we used the Wechsler Memory Scale–Revised logical memory subscale (29); immediate memory and memory after a delay were the dependent measures. To test verbal working memory, we used the WAIS-R version of the digit span test; the raw score was the dependent measure.
To assess speed of processing that involves visual scanning and psychomotor speed, we used the Trail Making Test, Part A (30); time in seconds was the dependent measure. We used the WAIS-R version of the digit symbol test to measure speed of processing; the raw score was the dependent measure. We used the clock drawing test (31) to measure semantic memory-related time, number, and/or linguistic facility and visual motor processing; we used a 10-point scoring system. Finally, we used the semantic fluency test to measure word production and speed; we measured number of words generated in 60 seconds.
Functional Measures
The Alzheimer's Disease Cooperative Study–Activities of Daily Living Inventory (ADCS-ADL) (32) has a range of items assessing both complex abilities (shopping, hobbies, personal appliances) and more basic activities of daily living (including walking and eating). Some of these latter items might dilute the instrument's sensitivity at preclinical stages of the disease, as they are rarely impaired. This 23-item questionnaire was completed by the cognitively healthy group and by caregivers for the participants in the mild cognitive impairment and Alzheimer's disease groups. The total score (possible scores range from 0 to 78) was the dependent variable.
The University of California, San Diego (UCSD) Performance-Based Skills Assessment (UPSA) is a performance-based measure of functional abilities that has ecological validity; it includes analogue measures of real-world activities—for example, planning an activity, such as a trip to the beach or the zoo, determining a route, dialing a phone number, and writing a check. It was validated in middle-aged and older healthy individuals and in an outpatient sample of older patients with schizophrenia (33, 34). Interrater reliability was high, correlation with another measure of function (informant based) was high, and test-retest reliability was 0.92 (33). A second study demonstrated its high correlations with both neurocognitive measures derived from the Clinical Dementia Rating Scale and level of independence in community living in older stable psychotic outpatients (35). Bowie et al. (36) demonstrated in a path analysis that the UPSA appeared to occupy a key node as a mediating variable in the path from cognition to functional disability. The UPSA includes domains involving comprehension and planning; transportation; communication; and financial procedures. Accommodations to the New York metropolitan area (e.g., local maps) were made for all relevant items after consultation with the test's author. We used the composite score derived as the mean of percent correct for each UPSA subtest as our primary dependent measure. We did not administer the household management section of the UPSA because of time constraints and concerns about its lack of contribution to the total score.
Procedure
To establish eligibility, a medical and psychiatric history and a neurological examination were conducted, and the MMSE, the Clinical Dementia Rating scale (using a trained rater), the Geriatric Depression Scale (37), and the Hachinski Ischemia Scale were administered. The cognitive tests listed above, followed by the UPSA, were then administered to each participant. Cognitively healthy participants completed the ADCS-ADL. Informants for participants in the mild cognitive impairment and Alzheimer's disease groups completed the ADCS-ADL.
Statistical Analyses
Statistical analyses were conducted using SAS, version 9.1.3 (SAS Institute, Cary, N.C.). All parametric analyses that contrasted groups on key measures of function and cognition were analyses of covariance (ANCOVAs) (using Proc GLM) in which age and education served as covariates. Multiple regression models (using Proc Stepwise) were used to assess the relation between function and cognition; age and education were forced to enter prior to other independent variables. Receiver-operating-characteristic curve analyses were conducted using Proc Logistic; such analyses have the advantage of providing a single estimate of diagnostic accuracy.
Results
Demographic Characteristics and Cognition
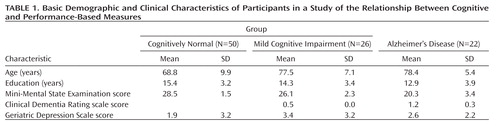
The basic demographic and clinical characteristics of the study participants are summarized in Table 1. The groups differed significantly in age and education by analysis of variance; they did not differ in sex ratio (cognitively normal men, N=19; men with mild cognitive impairment, N=8; men with Alzheimer's disease, N=10).
 |
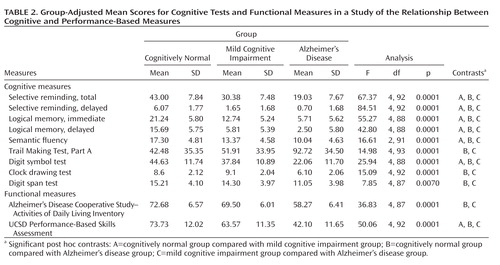
The performance of the three groups on cognitive tests and functional measures is summarized in Table 2. On all cognitive tests, significant between-group differences were observed by ANCOVA. Post hoc contrasts generally indicated that cognitively healthy participants outperformed those in the mild cognitive impairment group, who in turn outperformed those in the Alzheimer's disease group.
 |
Functional Measures
We subjected UPSA scores to ANCOVA parametric analyses. (Age and education served as covariates, given group differences.) Differences among the groups were highly significant (Table 2). Notably, post hoc Bonferroni-corrected contrasts indicated that the mild cognitive impairment group performed significantly worse than the cognitively healthy group (p=0.002). The effect size (Cohen's d) of this difference was large at 0.86.
We examined the ADCS-ADL similarly. Differences among the groups were highly significant by ANCOVA (Table 2). However, post hoc contrasts indicated that the cognitively healthy participants did not significantly differ from those with mild cognitive impairment. The effect size of this latter difference was moderate at 0.56.
Refinement of Analyses in Restricted Samples
Next, we performed a more rigorous analysis in which we excluded any patient with mild cognitive impairment whose ADCS-ADL score was more than 1.5 standard deviations below the mean of the cognitively healthy group. By so doing, we created a "purified" sample that directly and objectively met criteria for preserved function as reflected in the responses of spouses or other family members. Critically for our hypothesis, when we compared the resulting purified mild cognitive impairment group to the cognitively healthy group on UPSA performance by ANCOVA, the groups continued to differ at a highly significant level in Bonferroni-corrected post hoc contrasts (p=0.008).
Because of the difference in age and education between the cognitively healthy group and the mild cognitive impairment and Alzheimer's disease groups, we also conducted an analysis in which we compared a subset of 34 older, slightly less educated cognitively healthy participants (mean age=74 years, mean education=14 years) now matched to the mild cognitive impairment and Alzheimer's disease groups on these demographic variables. Differences among the groups on the UPSA remained highly significant (F=64.52, df=2, 80, p=0.0001). Notably, post hoc contrasts indicated that the mild cognitive impairment group performed significantly worse than the cognitively healthy group (p<0.001). The effect size of this difference was large at 1.43. Similarly, the difference between the mild cognitive impairment and Alzheimer's disease groups was significant (p<0.001), with a large effect size at 1.71.
Psychometric Properties of the UPSA
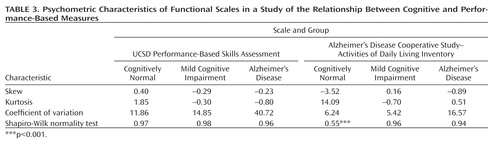
The UPSA was not generally prone to ceiling effects in the cognitively healthy group, nor to floor effects in the Alzheimer's disease group. Thus, only one participant in the cognitively healthy group obtained a score above 90% and only one in the Alzheimer's disease group obtained a score under 10%. For the ADCS-ADL, 14 participants were at ceiling.
As shown in Table 3, the UPSA was generally less skewed than the ADCS-ADL. It also generally demonstrated less extreme kurtotic distributions than did the ADCS-ADL. The ADCS-ADL distribution violated assumptions of normality in the cognitively healthy group at least partly because of ceiling effects. The coefficient of variation was consistently greater in the UPSA than in the ADCS-ADL.
 |
Receiver-operating-characteristic curves for the UPSA are shown in Figure 1. The area under the curve was 0.84 for the comparison between the cognitively healthy and mild cognitive impairment groups (odds ratio=1.16, 95% confidence interval [CI]=1.07–1.25, p<0.0001)—that is, it indicated that for any randomly drawn pair of participants from these two groups, the probability that the participant with mild cognitive impairment would have a lower UPSA score was 0.84. At a cutoff of p=0.50, sensitivity for identification of cognitively healthy participants was 0.88 and specificity was 0.58. At p=0.44, correct classification was maximal (0.816). For the mild cognitive impairment and Alzheimer's disease contrast (odds ratio=1.16, 95% CI=1.06–1.26, p=0.0009), the area under the curve was 0.88—that is, it indicated that for any randomly drawn pair of participants from these two groups, the probability that the patient with Alzheimer's disease would have a lower UPSA score was 0.88. At p=0.50, sensitivity for identification of participants with mild cognitive impairment was 0.85 and specificity was 0.73. At p=0.44, correct classification was maximal (0.813). Thus, sensitivity and specificity were acceptable for both contrasts, and group separation was robust.
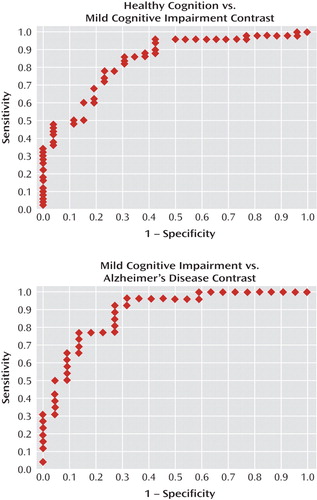
aArea under the curve, a general marker of sensitivity and specificity, was acceptable in both contrasts (>0.80). Note that the "elbow" for both contrasts is in the upper left corner of the panels, consistent with this interpretation.
Relationship Between the Functional and Cognitive Measures
Using a variant of stepwise regression in which age and education were forced to enter, we determined that a three-variable solution comprising the Trail Making Test, Part A (a test of processing speed, complex visual attention, and scanning); logical memory, immediate (a measure of verbal episodic memory); and semantic fluency (speeded semantic access and executive control), predicted 51% of the remaining variance in the UPSA. Beta weights, partial R2 values, and significance values are listed in Table 4.
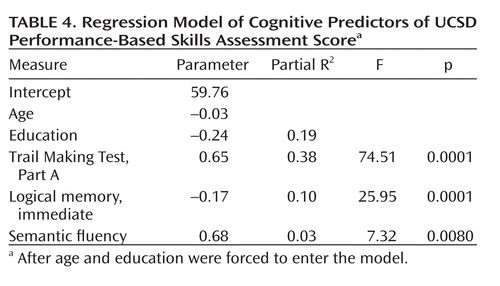 |
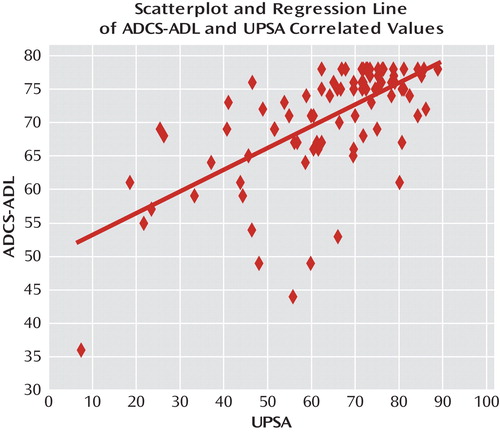
The UPSA and the ADCS-ADL were also significantly correlated using Spearman's rank order method (r=0.63, p=0.0001). The scatterplot is shown in Figure 2. However, the magnitude of the correlation suggested that the amount of nonshared variance was also substantial (>0.60).
Discussion
Our study yielded four important findings. First, we demonstrated that patients with mild cognitive impairment showed significant compromises on the UPSA, a performance-based measure of everyday function that comprises ecologically relevant tasks. The effect size between the cognitively healthy participants and those with mild cognitive impairment was large (>0.85). Second, when we restricted our analysis to only participants whose everyday function was deemed within normal limits on an informant-based measure, we nevertheless continued to observe significant impairments on the UPSA. Third, we found that cognitive scores in speed of processing, episodic memory, and semantic processing and fluency accounted for a significant share of the variance on the UPSA (about 0.50), thus suggesting a principled relationship between the two types of measures. Fourth, the psychometric properties of the UPSA were good.
Our results indicate that patients with mild cognitive impairment have functional impairments, as indicated on a performance-based measure of everyday functional ability. Moreover, in our stringent and critical analysis of a purified sample, we were able to demonstrate that patients with mild cognitive impairment had impairments even when there was otherwise "objective" informant-based information suggesting preserved function. These results argue against the notion of mild cognitive impairment exceptionalism—that is, the idea that patients with mild cognitive impairment, unlike those with nearly all other neuropsychiatric disorders, and despite their marked impairment in an important domain of cognition, have preserved everyday function.
Early conceptions of mild cognitive impairment suggested that little change would occur in daily function in the face of ongoing cognitive decline (38). As this view evolved, subtle impairments in instrumental activities were included in the consensus criteria for amnestic mild cognitive impairment (21). However, the specific instrumental performances of patients with mild cognitive impairment have not been well characterized (38). Thus, the diagnosis of mild cognitive impairment did not necessarily imply that there were no functional consequences, but rather it indicated that patients did not exhibit gross functional impairment in the course of normal daily activities as observed and reported by a knowledgeable observer but could have subtle, undetected impairment. (See the Patient Perspective box for some of the clinical implications.)
The method we used in this study allowed us to align cognitive and functional impairments. Thus, the majority of the variance in the UPSA could be predicted by key cognitive measures, including verbal episodic memory, semantic processing, executive ability, and speed/attention. This approach also provided convergent validity for findings of compromised function in the mild cognitive impairment group. Moreover, such a principled relationship has often been difficult to discern in informant-based measures of function (39).
A pragmatic advantage of performance-based measures is that they are free of the possible informant biases or lacunae in knowledge that may distort informant-based reports. From a psychometric standpoint, the UPSA was also sensitive, was not prone to ceiling or floor effects, and demonstrated acceptable receiver-operating-characteristic curves. As the field moves to earlier diagnostics and interventional strategies, the psychometric strengths of the UPSA also make it attractive. It might also be an appropriate coprimary endpoint in clinical trials of Alzheimer's medications designed to improve cognition and function on instrumental activities. Certainly the need for more sensitive and objective measures of everyday function has been widely discussed in the literature (20, 21, 36, 39–41).
It could be argued that we have created a "straw man" in this article. While several reports have identified functional impairments in cohorts with psychometrically defined mild cognitive impairment (40, 42, 43), including in one study using a narrowly based performance-type measure of function (44), no study has directly contrasted performance-based and informant-based measures, as we did here, and provided empirical support for the predictions that the magnitude of these compromises are large and can be robustly and systematically related to cognitive failures in mild cognitive impairment and Alzheimer's disease. In this manner, it becomes possible to place our results within a broader context of neuropsychiatric disorders so as to better understand the implications of a variety of cognitive impairments for function. Moreover, we note the rather large number of studies of mild cognitive impairment in which preserved function remains a diagnostic criterion, along with its implicit assumptions.
It should be noted that the ADCS-ADL was designed for a very different purpose than that used here, namely, to stage decline in Alzheimer's disease. We deployed it to bring into sharper relief points about conceptualizing mild cognitive impairment, performance-based measures of function, and the pragmatics of assessing function at earlier points in the Alzheimer's disease process. Even so, the effect size of 0.56 that we observed on this measure was moderate and would have been statistically significant in a larger sample. Newer versions of the test specifically tailored to emphasize the more complex activities that are likely to be impaired in mild cognitive impairment show much promise (45). These issues can be thought of as involving detection and sensitivity, which may in turn be dependent on what is being asked and who is being asked. Of course, both types of scales (informant-based and performance-based) need to address cultural variability and sensory and motor disabilities.
Several criticisms have been raised about performance-based measures. These include the idea that they are neuropsychological tests by another name. Our view is more nuanced, in that we believe that the analogue tasks in performance-based measures engage fundamental cognitive operations. We believe that the UPSA is ecologically valid because it assesses the performance of tasks that must be frequently and efficiently managed by individuals living in the United States and similar cultures. The tasks tested by the UPSA are important in and of themselves and may be surrogates for a wider range of activities (e.g., remembering key documents to bring to a doctor's office in the UPSA assessment may also have relevance to remembering documents or items to bring to other types of appointments). Our multiple regression results empirically support this argument. Another argument has to do with the possibility that these measures may be too sensitive—that patients appear to be doing well in their home environment but score poorly on tests. In our view, patients' adaptation to their environment may be dependent on procedural learning and relatively automatic routines that do not accurately reflect the ability to perform more novel, yet ecologically critical, tasks. Such tasks might require the integration of a variety of attentional/speed, semantic, and episodic memory demands that are indirectly captured by the UPSA.
In summary, we found that patients with mild cognitive impairment had significant impairments on a performance-based measure of everyday function and that the magnitude of the contrast between cognitively healthy participants and those with mild cognitive impairment (in effect size units) was greater for the performance-based measure than for an informant-based measure. Furthermore, as predicted, we found a strong relationship between cognitive performance and the performance-based measure of function using multiple regression models. Our work also suggests the need for a reconceptualization of the relationship between cognition and function in mild cognitive impairment so that they can more effectively be aligned.

1 : Mild cognitive impairment: risk of Alzheimer disease and rate of cognitive decline. Neurology 2006; 67:441–445 Crossref, Medline, Google Scholar
2 : The course of cognitive impairment in preclinical Alzheimer disease: three- and 6-year follow-up of a population-based sample. Arch Neurol 2000; 57:839–844 Crossref, Medline, Google Scholar
3 : Mild cognitive impairment: prevalence, prognosis, aetiology, and treatment. Lancet Neurol 2003; 2:15–21 Crossref, Medline, Google Scholar
4 : Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol 2006; 63:665–672 Crossref, Medline, Google Scholar
5 : Neuropathologic outcome of mild cognitive impairment following progression to clinical dementia. Arch Neurol 2006; 63:674–681 Crossref, Medline, Google Scholar
6 : 11C PiB and structural MRI provide complementary information in imaging of Alzheimer's disease and amnestic mild cognitive impairment. Brain 2008; 131:665–680 Crossref, Medline, Google Scholar
7 : PET amyloid ligand [11C]PIB uptake is increased in mild cognitive impairment. Neurology 2007; 68:1603–1606 Crossref, Medline, Google Scholar
8 : Current concepts in mild cognitive impairment. Arch Neurol 2001; 58:1985–1992 Crossref, Medline, Google Scholar
9 : Mild cognitive impairment as a clinical entity and treatment target. Arch Neurol 2005; 62:1160–1163 Crossref, Medline, Google Scholar
10 : Using neuropsychological and personality tests to assess the likelihood of patient employment. J Nerv Ment Dis 1978; 166:408–416 Crossref, Medline, Google Scholar
11 : Neuropsychological impairment in human immunodeficiency virus infection: implications for employment. Psychosom Med 1994; 56:8–17 Crossref, Medline, Google Scholar
12 : Long-term memory impairment in subjects with focal epilepsy. Epilepsia 2007; 48(suppl 9):26–29 Crossref, Medline, Google Scholar
13 : "Oops!": performance correlates of everyday attentional failures in traumatic brain injured and normal subjects. Neuropsychologia 1997; 35:747–758 Crossref, Medline, Google Scholar
14 : Neuropsychological assessment of monozygotic twins discordant for schizophrenia. Arch Gen Psychiatry 1990; 47:1066–1072 Crossref, Medline, Google Scholar
15 : Cognitive impairment after stroke: frequency, patterns, and relationship to functional abilities. J Neurol Neurosurg Psychiatry 1994; 57:202–207 Crossref, Medline, Google Scholar
16 : Training use of compensatory memory books: a three stage behavioral approach. J Clin Exp Neuropsychol 1989; 11:871–891 Crossref, Medline, Google Scholar
17 : Mild cognitive impairment: directions for future research. Neurology 2003; 61:438–444 Crossref, Medline, Google Scholar
18 : Identifying ADL changes in the early stages of cognitive impairment. Gerontologist 1998; 38:114–115 Google Scholar
19 : Activities of daily living instruments: optimizing scales for neurologic assessments. Neurology 2003; 60:738–742 Crossref, Medline, Google Scholar
20 : Complex activities of daily living in mild cognitive impairment: conceptual and diagnostic issues. Age Ageing 2006; 35:240–245 Crossref, Medline, Google Scholar
21 : Mild cognitive impairment: beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Int Med 2004; 256:240–246 Crossref, Medline, Google Scholar
22 : Caregivers' judgments of the functional abilities of the Alzheimer's disease patient: a comparison of proxy reports and objective measures. J Gerontol B Psychol Sci Soc Sci 2001; 56:78–84 Crossref, Google Scholar
23 : Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA 1993; 269:2386–2391 Crossref, Medline, Google Scholar
24 : The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993; 43:2412–2414 Crossref, Medline, Google Scholar
25 : Cerebral blood flow in dementia. Arch Neurol 1975; 32:632–637 Crossref, Medline, Google Scholar
26 : Neuropsychological prediction of conversion to Alzheimer disease in patients with mild cognitive impairment. Arch Gen Psychiatry 2006; 63:916–924 Crossref, Medline, Google Scholar
27 US National Institutes of Health, National Institute on Aging Working Group on Study Design: Inclusion/exclusion criteria table. http://www.nia.nih.gov/ResearchInformation/ExtramuralPrograms/NeuroscienceOfAging/Working+Group+on+Study+Design+-+Inclusion_Exclusion+Criteria+Table.htm Google Scholar
28 : Predicting development of dementia in the elderly with the selective reminding test. J Clin Exp Neuropsychol 1990; 12:529–538 Crossref, Medline, Google Scholar
29 : The Wechsler Memory Scale–Revised. San Antonio, Tex, Psychological Corp, 1987 Google Scholar
30 : Neuropsychological Assessment, 3rd ed. New York, Oxford University Press, 1995 Google Scholar
31 : The clock drawing test is a poor screen for very mild dementia. Neurology 2002; 59:898–903 Crossref, Medline, Google Scholar
32 : An inventory to assess activities of daily living for clinical trials in Alzheimer's disease (the Alzheimer's Disease Cooperative Study). Alzheimer Dis Assoc Disord 1997; 11(suppl 2):S33–S39 Crossref, Medline, Google Scholar
33 : Development of a brief scale of everyday functioning in persons with serious mental illness. Schizophr Bull 2007; 33:364–372 Google Scholar
34 : UCSD Performance-Based Skills Assessment: development of a new measure of everyday functioning for severely mentally ill adults. Schizophr Bull 2001; 27:235–245 Crossref, Medline, Google Scholar
35 : Generalized cognitive impairments, ability to perform everyday tasks, and level of independence in community living situations of older patients with psychosis. Am J Psychiatry 2002; 159:2013–2020 Link, Google Scholar
36 : Determinants of real-world functional performance in schizophrenia subjects: correlations with cognition, functional capacity, and symptoms. Am J Psychiatry 2006; 163:418–425 Link, Google Scholar
37 : Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1982–1983; 17:37–49 Crossref, Medline, Google Scholar
38 : Scales as outcome measures for Alzheimer's disease. Alzheimers Dement 2009; 5:324–339 Crossref, Medline, Google Scholar
39 (Committee on Research of the American Neuropsychiatric Association): The cognitive correlates of functional status: a review from the Committee on Research of the American Neuropsychiatric Association. J Neuropsychiatry Clin Neurosci 2007; 19:249–265 Link, Google Scholar
40 : Restriction in complex activities of daily living in MCI: impact on outcome. Neurology 2006; 67:461–466 Crossref, Medline, Google Scholar
41 : The Measurement of Everyday Cognition (ECog): scale development and psychometric properties. Neuropsychology 2008; 22:531–544 Crossref, Medline, Google Scholar
42 : Impairment of activities of daily living requiring memory or complex reasoning as part of the mild cognitive impairment syndrome. Int J Geriatric Psychiatry 2006; 21:158–162 Crossref, Medline, Google Scholar
43 : Functional significance of mild cognitive impairment in elderly subjects without a dementia diagnosis. Am J Geriatric Psychiatry 1999; 7:213–220 Crossref, Medline, Google Scholar
44 : Impaired financial abilities in mild cognitive impairment: a direct assessment approach. Neurology 2003; 60:449–457 Crossref, Medline, Google Scholar
45 (Alzheimer's Disease Cooperative Study): Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Arch Neurol 2004; 61:59–66 Crossref, Medline, Google Scholar



