Antidepressants May Mitigate the Effects of Prenatal Maternal Anxiety on Infant Auditory Sensory Gating
Abstract
Objective:
Prenatal maternal anxiety has detrimental effects on the offspring's neurocognitive development, including impaired attentional function. Antidepressants are commonly used during pregnancy, yet their impact on offspring attention and their interaction with maternal anxiety has not been assessed. The authors used P50 auditory sensory gating, a putative marker of early attentional processes measurable in young infants, to assess the impact of maternal anxiety and antidepressant use.
Method:
A total of 242 mother-infant dyads were classified relative to maternal history of anxiety and maternal prenatal antidepressant use. Infant P50 auditory sensory gating was recorded during active sleep at a mean age of 76 days (SD=38).
Results:
In the absence of prenatal antidepressant exposure, infants whose mothers had a history of anxiety diagnoses had diminished P50 sensory gating. Prenatal antidepressant exposure mitigated the effect of anxiety. The effect of maternal anxiety was limited to amplitude of response to the second stimulus, while antidepressant exposure had an impact on the amplitude of response to both the first and second stimulus.
Conclusions:
Maternal anxiety disorders are associated with less inhibition during infant sensory gating, a performance deficit mitigated by prenatal antidepressant exposure. This effect may be important in considering the risks and benefits of antidepressant use during pregnancy. Cholinergic mechanisms are hypothesized for both anxiety and antidepressant effects, although the cholinergic receptors involved are likely different for anxiety and antidepressant effects.
At least 8% of all pregnancies in the United States include treatment with antidepressants (1), primarily for anxiety and mood disorders. Yet, the use of antidepressants remains controversial. While there have been no randomized controlled trials of antidepressants in pregnant women, cessation of ongoing treatment is associated with psychiatric illness relapse (2), and risks and benefits for the pregnant woman are considered to be similar to those found in nonpregnant populations (3). Concerns arise because of a lack of knowledge about the potential effect of antidepressant exposure on the developing fetus.
In utero exposure to elevated maternal stress is generally accepted as having lifelong ramifications. Children with a history of in utero exposure to maternal anxiety, depression, or other forms of prenatal stress have an elevated risk for a variety of neuropsychiatric conditions, including general psychopathology such as internalizing and externalizing disorders (4), specific psychopathological symptoms such as those found in attention deficit hyperactivity disorder, anxiety, and depression (5–9), and generally lower neurocognitive performance, particularly in areas of attention and memory (10–12). The impact of maternal stress on offspring outcome has been demonstrated, even when controlling for confounding factors such as tobacco exposure, socioeconomic status, and later maternal psychopathology. Epidemiological studies, which often focus on a specific diagnosis, have found that in utero exposure to elevated levels of maternal stress is associated with an elevated risk for autism (13) and for schizophrenia (14). It has been estimated that as much as 15% of childhood emotional problems may be attributable to prenatal exposure to maternal psychopathology (15). At least some of the negative impact of in utero exposure remains even with postnatal treatment of the mother (16); whether effective prenatal treatment of the mother lowers the risk for her offspring remains unknown.
Antidepressant treatment during pregnancy may be associated with a small elevation in risk of negative birth outcomes, including spontaneous abortion (17), prematurity (18), small size for gestational age at birth (19), persistent pulmonary hypertension of the newborn (20), and congenital heart defects (20–22). Antidepressants cross both the placenta and the blood-brain barrier, yet few studies have examined brain or behavioral outcomes of prenatal maternal antidepressant use. In general, other than a slight delay in motor development (23), no significant effects of in utero antidepressant exposure on brain and behavior measures have been identified. However, sample sizes for such studies have been small, outcome measures have been nonspecific (e.g., general intelligence scores), and only a limited number of studies have controlled for maternal psychopathology (18, 23) in order to distinguish the neuropsychiatric effects of antidepressant exposure from the underlying maternal pathology leading to antidepressant use (20). Attentional deficits are among the most commonly identified deficits associated with in utero exposure to maternal stress; however, many measures of attention, such as cognitive tasks, can be assessed only later in childhood, when the child's rearing by an ill parent may have had its own effects (24). Hence, in this study, we assessed the development of an evoked potential infant correlate of attentional function, P50 sensory gating, soon after birth in offspring of women with and women without anxiety diagnoses, some of whom were treated with antidepressants.
Repeated stimuli, such as auditory clicks delivered in pairs, engage both excitatory and inhibitory cerebral mechanisms. Diminished response to the second stimulus of the pair occurs because of inhibitory mechanisms activated during the response to the first stimulus. In adults, P50 sensory gating is impaired in a number of psychiatric disorders characterized by attentional dysfunction, including schizophrenia (25), bipolar disorder (26), attention deficit hyperactivity disorder (27), posttraumatic stress disorder (28–31), and Parkinson's disease (32). In families with high rates of P50 sensory gating dysfunction, such as families of individuals with schizophrenia, P50 sensory gating deficits and attentional dysfunction cosegregate (33). P50 sensory gating is also correlated with attentional functioning within individuals, whether attention is assessed via neurocognitive testing (34) or based on self-reported ability to selectively attend (35).
The P50 auditory evoked potential is not fully developed in infants, occurring about 70 msec after the stimulus in infants, rather than the 50 msec generally seen in adult populations. The infant response is also somewhat broader than the adult P50. However, its inhibition to the second of paired stimuli occurs, and, to keep terminology consistent with that used in adult studies, this inhibition is referred to as P50 sensory gating (36). The infants are recorded in active sleep, a REM-like state in which they spend a majority of their sleeping time. Adults recorded in REM sleep show sensory gating inhibition and deficits similar to those seen in the awake state (37, 38). P50 sensory gating of 14 children assessed at age 14 weeks was correlated with P50 sensory gating at 47 months (r=0.75, p=0.002), suggesting that sensory gating is stable across early childhood (39). Elevated ratios (impaired sensory gating) in infancy are also associated with factors that suggest increased vulnerability to attentional deficits, such as increased genetic vulnerability from having a parent with psychosis and in utero exposure to nicotine, an environmental risk factor (40).
The most common maternal psychiatric illnesses involve anxiety and depression, which are often comorbid. There is some indication that maternal anxiety may be the more significant risk factor for the fetus (41–43). The object of this study was to assess the effects of antidepressant exposure on the development of inhibitory brain function in the fetus while accounting for the independent effects of maternal anxiety.
Method
Participants
Initial recruitment screening included the exclusionary criteria of known birth defects, chromosomal abnormality, and infant major neurological disorder. For a total of 313 mother-infant dyads, an infant physiological recording was completed and a maternal structured diagnostic psychiatric instrument was administered. Prenatal exposure to nicotine, nonnicotine substance use, and parental psychotic illness are associated with impaired infant sensory gating (40, 44); 71 dyads were excluded for presence of at least one of these exposures (56 for tobacco use, 16 for nonnicotine substance use disorder, and 16 for a parental psychotic diagnosis). Of the remaining 242 dyads, 133 (55%) were recruited via a state birth registry, 122 (42%) were recruited from local obstetrics clinics, four (2%) self-referred themselves to the research program, and three (1%) were referred from a local infant care treatment program. Written informed consent was obtained from mothers and participating fathers as monitored by the Colorado Multiple Institutional Review Board. Data on a subset of the mother-infant pairs have previously been reported (40).
Maternal Diagnosis
Mothers' psychiatric histories were elicited via the Structured Clinical Interview for DSM-IV Axis I Disorders (45). All interviews were conducted by an experienced psychiatric clinician (a physician or a master's-level social worker), with translation services when necessary. Mothers were considered to have an anxiety disorder if they had any history of chronic or sustained anxiety diagnoses, including agoraphobia, generalized anxiety disorder, obsessive-compulsive disorder, panic disorder, and posttraumatic stress disorder. Mild or intermittent anxiety disorders that could generally be avoided by behavioral changes, such as specific phobia and social phobia, were excluded. Current psychiatric symptoms were defined as a mother having sufficient symptoms to meet criteria for an anxiety or depression diagnosis during her pregnancy or an illness with onset prior to pregnancy with continued symptoms causing impairment during pregnancy. Mothers with previous or chronic illness who were not impaired during pregnancy, regardless of treatment, were considered not to have current anxiety symptoms.
Auditory Sensory Gating
Details of the recording procedures have been reported previously (36). EEG at site Cz, bipolar electro-oculogram, submental electromyogram, and respiration were continuously recorded while infants slept. Paired clicks were presented through two speakers positioned at either side of the infant's head, approximately 0.5 m from each ear. Auditory clicks were 85 dB (SPL) at the ear. Recording continued for as long as the infant remained asleep.
Sleep state was identified offline, and average waveforms were computed from the first 15 minutes of usable active sleep data. The amplitude and latency of the largest positive peak (P1) between 50 and 100 msec following a click and preceded by a negative trough was determined by an investigator who was blind to maternal diagnosis and antidepressant treatment. Sensory gating was measured by dividing the average amplitude of P1 evoked by the second click by the average amplitude of the first click, yielding a P50 sensory gating ratio (Figure 1). The test/retest reliability of this measure is 0.86 in infants (46).
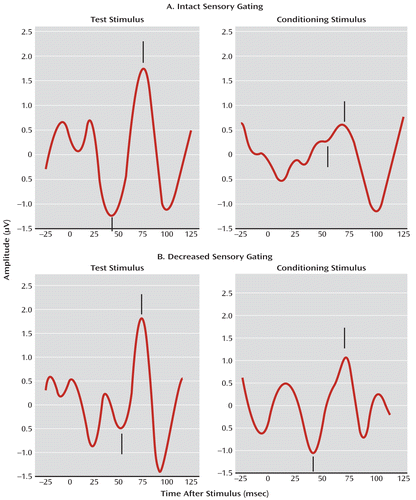
FIGURE 1. Individual Examples of P50 Sensory Gating Responses During Active Sleepa
a Clicks are presented 500 msec apart; the P50 response is noted by hash marks. The positive P50 peak (hash mark above the line) was measured relative to the preceding negative trough (hash mark below). Panel A shows an example of intact sensory gating in an infant at 44 weeks after the mother's last menstrual period (approximately 4 weeks of age). Note that the response test stimulus is suppressed in comparison with the conditioning stimulus for a P50 sensory gating ratio of 0.11. Panel B shows an example of an infant of about the same age with decreased sensory gating. This infant's P50 response to the test stimulus is similar to that for the conditioning stimulus, demonstrating lack of response suppression, with a sensory gating ratio of 0.94.
Statistical Analysis
Because recruitment was not stratified and assignment to antidepressants not randomized, the two-factor maternal anxiety and maternal antidepressant use analysis had sample sizes ranging from 13 to 169 subjects per cell. The small number of subjects in some cells resulted in relatively low power to test interactions; for an analysis of variance (ANOVA) with an alpha of 0.05, 80% power was achievable only for large interaction effect sizes of 1.18 standard deviations or greater. Insufficient power increases the risk of an interaction effect type II error, which decreases confidence in main effect statistical results. To avoid problems associated with interpretation of main effects in the presence of a potential interaction that cannot be detected because of low power, ANOVA was used to compare the four group cell means, as is common practice when it is known that interaction is present. Each dependent measure—P50 gating ratio and amplitudes of responses to the first and second stimuli—was assessed with separate ANOVAs comparing post hoc differences of least squares group means. The Tukey-Kramer method of multiple comparisons was used where appropriate.
Results
Demographic and Clinical Characteristics
Sixty of the 242 mothers (25%) had a history of anxiety disorder. Mothers with a history of anxiety disorder were more likely than those without to meet diagnostic criteria for current or residual anxiety (63% compared with 0%; χ2=136.7, p<0.001) and depressive disorders (23% compared with 4%; χ2=16.7, p<0.001) and to use antidepressants (24% compared with 7%; χ2=11.9, p=0.001) during pregnancy. On average, mothers with a history of anxiety disorder were also younger, less educated, and of lower socioeconomic status; they were also more likely to have a female infant, but this difference fell short of statistical significance. Infants exposed to maternal anxiety disorder did not significantly differ from those not exposed on race/ethnicity, frequency of living with both biological parents, gestational age at birth, or chronological age at time of physiological recording. Demographic and clinical information for the 242 dyads is summarized in Table 1.
| Characteristic | Total (N=242) | No History of Maternal Anxiety Disorder (N=182) | History of Maternal Anxiety Disorder (N=60) | Analysis | ||||
|---|---|---|---|---|---|---|---|---|
| Maternal characteristics | ||||||||
| Mean | SD | Mean | SD | Mean | SD | t | p | |
| Age at delivery (years) | 30 | 5 | 30 | 5 | 27 | 6 | 3.9 | <0.001 |
| Education (years) | 15 | 3 | 15 | 3 | 14 | 3 | 3.3 | 0.002 |
| Socioeconomic Index of Occupations scorea | 46 | 23 | 48 | 24 | 39 | 19 | 2.5 | 0.014 |
| N | % | N | % | N | % | χ2 | p | |
| Distribution of Socioeconomic Index of Occupations scores | ||||||||
| ≤34 | 109 | 45 | 78 | 43 | 31 | 52 | ||
| 35–60 | 54 | 22 | 37 | 20 | 17 | 28 | ||
| ≥61 | 76 | 31 | 65 | 36 | 11 | 18 | ||
| Unknown | 3 | 1 | 2 | 1 | 1 | 2 | ||
| Psychiatric illness during pregnancyb | 38 | 16 | 0 | 0 | 38 | 63 | 136.7 | <0.001 |
| Anxiety or depression | 49 | 20 | 8 | 4 | 41 | 68 | 114.2 | <0.001 |
| Depression | 22 | 9 | 8 | 4 | 14 | 23 | 19.6 | <0.001 |
| Antidepressant use during pregnancy | 27 | 11 | 13 | 7 | 14 | 24 | 11.9 | 0.001 |
| Sertraline | 11 | 5 | ||||||
| Fluoxetine | 4 | 2 | ||||||
| Citalopram/escitalopram | 4 | 2 | ||||||
| Bupropion | 4 | 2 | ||||||
| Other | 4 | 2 | ||||||
| Infant characteristics | ||||||||
| Female | 127 | 53 | 89 | 49 | 38 | 63 | 3.8 | 0.052 |
| Race/ethnicity | 0.6 | 0.75 | ||||||
| Caucasian non-Hispanic | 125 | 52 | 96 | 53 | 29 | 49 | ||
| Caucasian Hispanic | 72 | 30 | 54 | 30 | 18 | 30 | ||
| Other | 45 | 19 | 32 | 18 | 13 | 22 | ||
| Living with both biological parents | 198 | 82 | 153 | 84 | 45 | 75 | 2.5 | 0.11 |
| Mean | SD | Mean | SD | Mean | SD | t | p | |
| Gestational age at birth (days) | 273 | 12 | 273 | 11 | 271 | 15 | 1.1 | 0.29 |
| Chronological age at P50 recording (days) | 76 | 38 | 76 | 40 | 73 | 33 | 0.5 | 0.63 |
TABLE 1. Characteristics of Mothers and Infants in a Study of Prenatal Maternal Anxiety and Antidepressant Use and Infant Auditory Sensory Gating
Twenty-seven (11%) of the women used an antidepressant during pregnancy (see Table 1). Antidepressant treatment was confined to the first trimester in four women (15% of those utilizing antidepressants), the second trimester in one woman (4%), and the third trimester in six women (22%), and occurred during more than one trimester in 16 women (59%). Nineteen mothers (70% of those exposed during pregnancy) continued taking antidepressants after delivery. Of these, 16 (59% of those exposed prenatally) breast-fed their infants, exposing them to antidepressants postnatally.
Twenty-six of the 27 women (96%) who used antidepressants during pregnancy had a lifetime history of either a mood or an anxiety disorder; however, only 26 of 118 women with a lifetime history of a mood or anxiety disorder (22%) used antidepressants during pregnancy. Of those with a lifetime history of a mood or anxiety disorder, antidepressant use was associated with a nonsignificant elevation in age at delivery (30.9 years compared with 28.6 years; t=1.85, df=116, p=0.067); there were no significant associations with education, socioeconomic status, minority racial or ethnic status, marital status, duration of gestation, or gender of the fetus.
Effects on Infant Sensory Gating
Age at delivery, years of education, and socioeconomic status differed significantly between women with a history of anxiety disorder and those without. All analyses were conducted both with and without these covariates, with no notable difference in results. Analyses without the covariates are reported here.
Table 2 summarizes the P50 sensory gating results. For infant P50 sensory gating ratios, there was an overall significant ANOVA among the four groups formed by the presence or absence of a history of maternal anxiety disorder and by the presence or absence of prenatal antidepressant exposure (differences between the four means, F=5.60, df=3, 238, p=0.001). In the absence of antidepressants, maternal anxiety disorder was associated with elevated P50 sensory gating (Tukey-Kramer p<0.001) (Figure 2). Mean P50 sensory gating ratios were lower in infants of mothers treated with antidepressants, either with or without maternal anxiety disorder, than the mean for infants of untreated mothers with anxiety disorder (Tukey-Kramer p=0.007 for those without maternal anxiety disorder, p=0.041 [uncorrected] for those with maternal anxiety disorder).
| No Prenatal Antidepressant Use | Prenatal Antidepressant Use | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P50 Amplitude | P50 Amplitude | |||||||||||||
| First Stimulus | Second Stimulus | P50 Sensory Gating Ratio | First Stimulus | Second Stimulus | P50 Sensory Gating Ratio | |||||||||
| Anxiety Disorder Status | N | Mean | SD | Mean | SD | Mean | SD | N | Mean | SD | Mean | SD | Mean | SD |
| No anxiety disorder | 169 | 2.44 | 1.32 | 1.05 | 0.96 | 0.425 | 0.284 | 13 | 1.90 | 0.78 | 0.74 | 0.64 | 0.366 | 0.254 |
| Anxiety disorder present | 46 | 2.58 | 1.15 | 1.46 | 0.71 | 0.607 | 0.289 | 14 | 2.00 | 0.75 | 0.86 | 0.55 | 0.431 | 0.207 |
TABLE 2. Amplitude (μV) of the Evoked P50 Response to the First and Second Stimuli and the P50 Sensory Gating Ratio in Infants, by Presence of Maternal Anxiety Disorders and Maternal Use of Antidepressants During Pregnancy
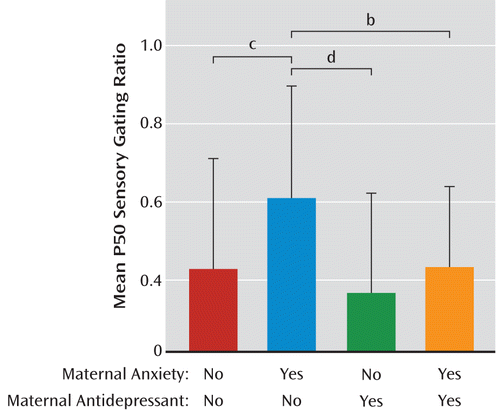
FIGURE 2. P50 Ratio, by Group, Among Infants of Mothers With and Without Anxiety Disorders and Depressant Usea
a There was a significant effect of group (F=5.60, df=3, 238, p=0.001). In infants with a maternal history of an anxiety disorder but without prenatal exposure to antidepressants, P50 ratios were significantly elevated compared with each of the other groups. The other groups did not significantly differ from each other. Error bars indicate standard deviations.
b p=0.041.
c p<0.001. Difference remained significant after Tukey-Kramer adjustment for multiple comparisons.
d p<0.007. Difference remained significant after Tukey-Kramer adjustment for multiple comparisons.
The limited sample sizes in the antidepressant-exposed groups restrict additional subgroup analyses. Exploratory analyses did not identify P50 sensory gating differences between infants with pre- and postnatal antidepressant exposure compared to infants with only prenatal exposure or between infants exposed to selective serotonin reuptake inhibitors compared to infants exposed to other antidepressants.
Because P50 sensory gating is calculated as a ratio, it can vary with the numerator (amplitude of response to the second stimulus), the denominator (amplitude of response to the first stimulus), or both. For all four groups of subjects, the amplitude of response to the second stimulus was significantly lower than the amplitude of response to the first stimulus (all p values <0.001), demonstrating the presence of sensory gating in all groups. To further explore the impact of group membership on P50 amplitude, two separate ANOVAs were conducted, one to explore the impact of group on amplitude of response to the first stimulus and the other to explore the impact of group on response to the second stimulus. There was no significant effect of group on P50 amplitude in response to the first stimulus; however, when groups were collapsed across anxiety status, there was a significant effect of antidepressant exposure (t=2.06, df=240, p=0.040) (Figure 3). An overall significant effect of group was identified for amplitude of response to the second stimulus (F=3.83, df=3, 238, p=0.010) (Figure 3). Anxiety disorder in the absence of antidepressant use significantly increased the amplitude of infant P50 response to the second stimulus (Tukey-Kramer p=0.005). Antidepressant use in the presence of an anxiety disorder reduced amplitude to levels similar to those found in infants who had no prenatal exposure to either maternal anxiety or antidepressants. Compared to infants of untreated mothers with anxiety, antidepressant use in mothers without anxiety disorder decreased the P1 amplitude in infant response to the second stimulus (Tukey-Kramer p<0.001), and there was indication of the same effect in infants of mothers with anxiety disorder (p=0.026 uncorrected).
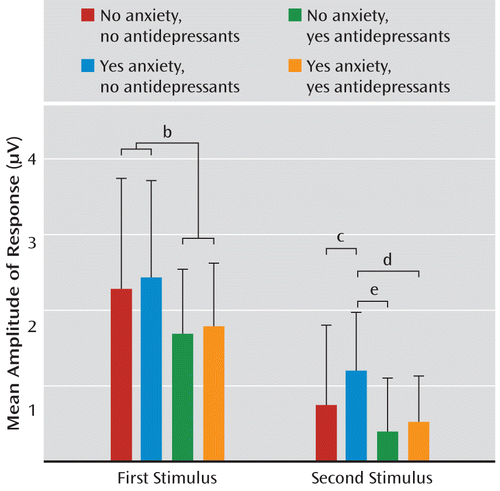
FIGURE 3. Infant P50 Amplitude in Response to Stimuli Presented 500 msec Apart in Infants of Mothers With and Without Anxiety Disorders and Depressant Usea
a For amplitude of P50 response to the first stimulus, the effect of group was not significant; however, when groups were collapsed across anxiety disorder status, there was a significant effect of antidepressant exposure (t=2.06, df=240, p=0.040). For amplitude of P50 response to the second stimulus, there was a significant effect of group (F=3.83, df=3, 238, p=0.010). For infants without prenatal antidepressant exposure, having a mother with a history of an anxiety disorder was associated with an elevated P50 amplitude in response to the second stimulus, an effect that was at least partially mitigated by prenatal exposure to antidepressants. Error bars indicate standard deviations.
b p=0.040.
c p=0.005.
d p=0.026
e p<0.001. Difference remained significant after adjustment for multiple comparisons.
Discussion
In the absence of prenatal exposure to antidepressants, infants born to a mother with an anxiety disorder had more impaired P50 sensory gating than infants born to a mother with no identified anxiety disorders. For infants whose mothers had an anxiety disorder, prenatal antidepressant treatment improved sensory gating. Because the effect's significance in mothers with anxiety disorders (p=0.041) was not verified by rigorous correction for multiple testing, the effect of maternal anxiety disorder should be considered mitigated but not fully reversed by antidepressant treatment. Two-thirds of the mothers with an anxiety disorder had continuing symptoms despite treatment, which may explain why treatment was only partially effective. Although tests of equivalence were not conducted, the P50 sensory gating ratios of infants whose mothers took antidepressants (whatever their diagnostic status) were within the range for P50 ratios in infants of untreated mothers with no history of anxiety disorders. Given the limited sample size, we cannot draw any firm conclusions about the relative effect sizes of maternal anxiety versus maternal antidepressant use; however, the effects are in opposite directions and suggest that antidepressant use at least partially mitigates the effect of anxiety. To our knowledge, this is the first report of antidepressant use protecting against adverse effects of a maternal history of anxiety on fetal brain development.
The relationship between prenatal exposure to maternal anxiety disorder and impaired P50 sensory gating is consistent with previous reports that attentional dysfunction is elevated in children prenatally exposed to maternal anxiety (48, 49). The mechanism by which maternal stress affects fetal brain development is unclear, but there are several possibilities. In older children and adults, anxiety and attentional dysfunction are often comorbid (50–52), and shared genetic risk between maternal anxiety and offspring attentional dysfunction is a possibility. Maternal trait anxiety may be associated with alterations in maternal cortisol secretion (53), and both state and trait anxiety may increase the percentage of maternal cortisol that crosses the placenta (54). A deleterious direct effect of cortisol on the developing brain is thus a second possibility. Other possibilities include effects of estrogen and other hormones, which affect other measures of sensory gating (55).
The increased impairment in sensory gating seen in infant offspring of mothers with maternal anxiety disorders is primarily due to an increase in response to the second stimulus, with little or no change in response to the first stimulus. The normal inhibition seen in response to the second stimulus is mediated by a local inhibitory neurocircuit involving GABA-ergic interneurons (56). Stimulation during early development of α7 nicotinic cholinergic receptors on the interneurons appears critical for normal development of the inhibitory neurocircuit, and elements that interfere with adequate early development, including both genetically mediated decreases in α7 nicotinic receptor density (57, 58) and decreases in the prenatal ligand choline (59), permanently impair inhibition to the second stimulus, increasing the resulting P50 sensory gating ratio. Stress leads to decreased serum choline levels (60–63), primarily as a result of corticosteroid-mediated increased sequestration of choline in the liver (64). Maternal anxiety disorders may result in a cortisol-mediated drop in maternal and fetal serum choline levels, resulting in decreased fetal brain α7 nicotinic receptor stimulation, diminished development of inhibitory neurocircuits, and a resultant impairment in the ability to gate the response to repetitive auditory stimuli.
Although our analysis did not survive rigorous adjustment for multiple testing, our results suggest that antidepressant exposure may be associated with improved P50 sensory gating. In adults, P50 sensory gating is impaired in those with a history of mood disorder but is not correlated with current mood state (65, 66). Antidepressant use in adults has been associated, in some but not all studies, with normalization of sensory gating even when mood symptoms were not fully treated (66, 67). One possibility is that improvement in infant P50 sensory gating related to antidepressant exposure is an acute effect of current antidepressant exposure; however, infants with pre- and postnatal antidepressant exposure did not differ in P50 sensory gating ratios from those with prenatal exposure only. Thus, postnatal exposure effects seem unlikely.
The mechanisms by which antidepressants could exert an effect on the fetal brain are unknown, but they could include a reduction in the duration or severity of the mother's symptoms or direct protective effects on the fetal brain. Antidepressant exposure was associated with reductions in evoked P50 response amplitude in response to both the first and second stimuli. As discussed above, response to the second stimulus is associated with α7 nicotinic receptor function; response to the first stimulus appears to be more closely related to α4β2 nicotinic receptor activity (68), at least in adult animal models. A pharmacological feature common to most antidepressants, including selective serotonin reuptake inhibitors, tricyclics, bupropion, and venlafaxine, is noncompetitive inhibition of a broad range of nicotinic receptors, including α7 and α4β2 (69–76). The fact that both α7 and α4β2 nicotinic receptors play a role in local inhibitory neurocircuit formation and function, and that antidepressants are noncompetitive inhibitors of both receptor types, suggests one avenue for additional research.
Our study had several limitations. Only 27 participants (11% of the sample) took antidepressants during pregnancy. When combined with an anxiety history classification, some subgroups were as small as 13 participants, limiting statistical power. In addition, while antidepressant use was almost completely limited to women with a history of either a mood or an anxiety disorder, only a minority of women with such a history used antidepressants, and antidepressant use was not randomized. Among women who had a history of mood and anxiety disorders, there was no difference on several demographic variables between those who did and those who did not take antidepressants; however, there are likely other factors that influence the choices individuals make on pharmacological treatment that were not identified or controlled for in this study. Additional efforts to replicate and extend the findings of this study should account for other potential confounding factors to establish the risk and benefit to the fetus of maternal antidepressant treatment.
1.
2. : Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA 2006; 295:499–507Crossref, Medline, Google Scholar
3. : The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry 2009; 31:403–413Crossref, Medline, Google Scholar
4. : Maternal antenatal anxiety and children's behavioral/emotional problems at 4 years: report from the Avon Longitudinal Study of Parents and Children. Br J Psychiatry 2002; 180:502–508Crossref, Medline, Google Scholar
5. : Chernobyl exposure as a stressor during pregnancy and behaviour in adolescent offspring. Acta Psychiatr Scand 2007; 116:438–446Crossref, Medline, Google Scholar
6. : Antenatal maternal anxiety is related to HPA-axis dysregulation and self-reported depressive symptoms in adolescence: a prospective study on the fetal origins of depressed mood. Neuropsychopharmacology 2008; 33:536–545Crossref, Medline, Google Scholar
7. : High antenatal maternal anxiety is related to ADHD symptoms, externalizing problems, and anxiety in 8- and 9-year-olds. Child Dev 2004; 75:4085–4097Crossref, Google Scholar
8. : Are maternal smoking and stress during pregnancy related to ADHD symptoms in children? J Child Psychol Psychiatry 2005; 46:246–254Crossref, Medline, Google Scholar
9. : Maternal depression and psychiatric outcomes in adolescent offspring: a 13-year longitudinal study. J Affect Disord 2007; 97:145–154Crossref, Medline, Google Scholar
10. : Does maternal prenatal stress adversely affect the child's learning and memory at age six? J Abnorm Child Psychol 2006; 34:789–798Crossref, Medline, Google Scholar
11. : Stress during pregnancy affects general intellectual and language functioning in human toddlers. Pediatr Res 2004; 56:400–410Crossref, Medline, Google Scholar
12. : High antenatal maternal anxiety is related to impulsivity during performance on cognitive tasks in 14- and 15-year-olds. Neurosci Biobehav Rev 2005; 29:259–269Crossref, Medline, Google Scholar
13. : Autism prevalence following prenatal exposure to hurricanes and tropical storms in Louisiana. J Autism Dev Disord 2008; 38:481–488Crossref, Medline, Google Scholar
14. : Higher risk of offspring schizophrenia following antenatal maternal exposure to severe adverse life events. Arch Gen Psychiatry 2008; 65:146–152Crossref, Medline, Google Scholar
15. : Sustained neurobehavioral effects of exposure to SSRI antidepressants during development: molecular to clinical evidence. Clin Pharmacol Ther 2009; 86:672–677Crossref, Medline, Google Scholar
16. : Change in child psychopathology with improvement in parental depression: a systematic review. J Am Acad Child Adolesc Psychiatry 2008; 47:379–389Crossref, Medline, Google Scholar
17. : Use of antidepressants during pregnancy and the risk of spontaneous abortion. CMAJ 2010; 182:1031–1037Crossref, Medline, Google Scholar
18. : Major depression and antidepressant treatment: impact on pregnancy and neonatal outcomes. Am J Psychiatry 2009; 166:557–566Link, Google Scholar
19. : Antidepressant use during pregnancy and the risk of preterm delivery and fetal growth restriction. J Clin Psychopharmacol 2009; 29:555–560Crossref, Medline, Google Scholar
20. : Delivery outcome after maternal use of antidepressant drugs in pregnancy: an update using Swedish data. Psychol Med 2010; 40:1723–1733Crossref, Medline, Google Scholar
21. : Paroxetine and fluoxetine in pregnancy: a prospective, multicentre, controlled, observational study. Br J Clin Pharmacol 2008; 66:695–705Medline, Google Scholar
22. : First-trimester use of paroxetine and congenital heart defects: a population-based case-control study. Birth Defects Res A Clin Mol Teratol 2010; 88:94–100Medline, Google Scholar
23. : Follow-up of children of depressed mothers exposed or not exposed to antidepressant drugs during pregnancy. J Pediatr 2003; 142:402–408Crossref, Medline, Google Scholar
24. : The effects of maternal postnatal depression and child sex on academic performance at age 16 years: a developmental approach. J Child Psychol Psychiatry 2010; 51:1150–1159Crossref, Medline, Google Scholar
25. : Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biol Psychiatry 1982; 17:639–654Medline, Google Scholar
26. : Physiology of schizophrenia, bipolar disorder, and schizoaffective disorder. Am J Psychiatry 2007; 164:1900–1906Link, Google Scholar
27. : The P50 auditory event-evoked potential in adult attention-deficit disorder: comparison with schizophrenia. Biol Psychiatry 2000; 47:969–977Crossref, Medline, Google Scholar
28. : Sensory filtering phenomenology in PTSD. Depress Anxiety 2008; 25:38–45Crossref, Medline, Google Scholar
29. : Impaired P50 sensory gating in post-traumatic stress disorder secondary to urban violence. Int J Psychophysiol 2004; 51:209–214Crossref, Medline, Google Scholar
30. : Sensory gating in chronic posttraumatic stress disorder: reduced auditory P50 suppression in combat veterans. Biol Psychiatry 1999; 46:1656–1664Crossref, Medline, Google Scholar
31. : Combat veterans with posttraumatic stress disorder exhibit decreased habituation of the P1 midlatency auditory evoked potential. Life Sci 1997; 61:1421–1434Crossref, Medline, Google Scholar
32. : Decreased habituation of midlatency auditory evoked responses in Parkinson's disease. Mov Disord 1997; 12:655–664Crossref, Medline, Google Scholar
33. : Neuropsychological dysfunction in parents of schizophrenics. Schizophr Res 1996; 20:253–260Crossref, Medline, Google Scholar
34. : Neurophysiological and neuropsychological evidence for attentional dysfunction in schizophrenia. Schizophr Res 1993; 10:131–141Crossref, Medline, Google Scholar
35. : Comparison of sensory gating to mismatch negativity and self-reported perceptual phenomena in healthy adults. Psychophysiology 2004; 41:604–612Crossref, Medline, Google Scholar
36. : Early postnatal development of sensory gating. Neuroreport 2003; 14:693–697Crossref, Medline, Google Scholar
37. : The effect of state on sensory gating: comparison of waking, REM, and non-REM sleep. Clin Neurophysiology 2001; 112:1154–1165Crossref, Medline, Google Scholar
38. : Sensory gating impairment associated with schizophrenia persists into REM sleep. Psychophysiology 2003; 40:29–38Crossref, Medline, Google Scholar
39. : Stability of P50 sensory gating in preschoolers (abstract). J Investig Med 2010; 58:154–155Google Scholar
40. : Diminished cerebral inhibition in neonates associated with risk factors for schizophrenia: parental psychosis, maternal depression, and nicotine use. Schizophr Bull 2011; 37:1200–1208Crossref, Medline, Google Scholar
41.
42. : Psychological factors in pregnancy and mixed-handedness in the offspring. Dev Med Child Neurol 2003; 45:557–561Crossref, Medline, Google Scholar
43. : Annual research review: prenatal stress and the origins of psychopathology: an evolutionary perspective. J Child Psychol Psychiatry 2011; 52:356–367Crossref, Medline, Google Scholar
44. : How does auditory gating in newborns with prenatal drug and/or nicotine exposure differ from newborns with normal pattern exposures? (abstract). J Investig Med 2009; 57:98Google Scholar
45. : Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition. New York, New York State Psychiatric Institute, 1997Google Scholar
46. : Reliability of P50 auditory sensory gating measures in infants during active sleep. Neuroreport 2008; 19:79–82Crossref, Medline, Google Scholar
47. : The 1989 Socioeconomic Index of Occupations: construction from the 1989 occupational prestige scores. General Social Survey Methodological Report No 74. Chicago, University of Chicago, National Opinion Research Center, May 1992Google Scholar
48. : Maternal anxiety and attention problems in children at 5 and 14 years. J Atten Disord 2010; 13:658–667Crossref, Medline, Google Scholar
49. : High antenatal maternal anxiety is related to ADHD symptoms, externalizing problems, and anxiety in 8- and 9-year-olds. Child Dev 2004; 75:1085–1097Crossref, Medline, Google Scholar
50. : The identification of attention complaints in the general population and their effect on quality of life. J Atten Disord 2011; 15:46–55Crossref, Medline, Google Scholar
51. : Higher-order genetic and environmental structure of prevalent forms of child and adolescent psychopathology. Arch Gen Psychiatry 2011; 68:181–189Crossref, Medline, Google Scholar
52. : Patterns of comorbidity, functioning, and service use for US children with ADHD, 2007. Pediatrics 2011; 127:462–470Crossref, Medline, Google Scholar
53. : Maternal trait anxiety, emotional distress, and salivary cortisol in pregnancy. Biol Psychology 2010; 83:169–175Crossref, Medline, Google Scholar
54. : Association between maternal and amniotic fluid cortisol is moderated by maternal anxiety. Psychoneuroendocrinology 2009; 34:430–435Crossref, Medline, Google Scholar
55. : Changes in sensorimotor inhibition across the menstrual cycle: implications for neuropsychiatric disorders. Biol Psychiatry 1997; 41:452–460Crossref, Medline, Google Scholar
56. : Cholinergic mechanisms, early brain development, and risk for schizophrenia. J Child Psychol Psychiatry 2010; 51:535–549Crossref, Medline, Google Scholar
57. : Genetic correlation of inhibitory gating of hippocampal auditory evoked response and alpha-bungarotoxin-binding nicotinic cholinergic receptors in inbred mouse strains. Neuropsychopharmacology 1996; 15:152–162Crossref, Medline, Google Scholar
58. : Altered hippocampal circuit function in C3H alpha7 null mutant heterozygous mice. Brain Res 2008; 1194:138–145Crossref, Medline, Google Scholar
59. : Perinatal choline deficiency produces abnormal sensory inhibition in Sprague-Dawley rats. Brain Res 2008; 1237:84–90Crossref, Medline, Google Scholar
60. : Decreased serum choline concentrations in humans after surgery, childbirth, and traumatic head injury. Neurochem Res 1998; 23:727–732Crossref, Medline, Google Scholar
61. : Declines in serum free and bound choline concentrations in humans after three different types of major surgery. Clin Chem Lab Med 2004; 42:1390–1395Crossref, Medline, Google Scholar
62. : Free and phospholipid-bound choline concentrations in serum during pregnancy, after delivery, and in newborns. Arch Physiol Biochem 2002; 110:393–399Crossref, Medline, Google Scholar
63. : The decline in serum choline concentration in humans during and after surgery is associated with the elevation of cortisol, adrenocorticotropic hormone, prolactin, and beta-endorphin concentrations. Neruosci Lett 2002; 324:41–44Crossref, Medline, Google Scholar
64. : Serum free and phospholipid-bound choline decrease and surgery and methylprednisolone administration in dogs. Neurosci Lett 2003; 339:195–198Crossref, Medline, Google Scholar
65. : Diminished suppression of the P50 auditory evoked potential in bipolar disorder subjects with a history of psychosis. Am J Psychiatry 2005; 162:43–49Link, Google Scholar
66. : A follow-up study on featuers of sensory gating P50 in treatment-resistant depression patients. Chin Med J (Engl) 2009; 122:2956–2960Medline, Google Scholar
67. : The P50 midlatency auditory evoked potential in patients with chronic low back pain (CLBP). Clin Neurophysiol 2005; 116:681–689Crossref, Medline, Google Scholar
68. : Stimulation of the [alpha]4[beta]2 nicotinic receptor by 5-I A-85380 improves auditory gating in DBA/2 mice. Brain Res 2008; 1224:29–36Crossref, Medline, Google Scholar
69. : Interaction of selective serotonin reuptake inhibitors with neuronal nicotinic acetylcholine receptors. Biochemistry 2010; 49:5734–5742Crossref, Medline, Google Scholar
70. : Tricyclic antidepressants and mecamylamine bind to different sites in the human [alpha]4[beta]2 nicotinic receptor ion channel. Int J Biochem Cell Biol 2010; 42:1007–1018Crossref, Medline, Google Scholar
71. : Inhibitory effect of antidepressants on the NMDA-evoked [3H]noradrenaline release from rat hippocampal slices. Neurochem Int 2009; 55:383–388Crossref, Medline, Google Scholar
72. : Is the inhibition of nicotinic acetylcholine receptors by bupropion involved in its clinical actions? Int J Biochem Cell Biol 2009; 41:2098–2108Crossref, Medline, Google Scholar
73. : Bupropion inhibits the cellular effects of nicotine in the ventral tegmental area. Biochem Pharmacol 2007; 74:1283–1291Crossref, Medline, Google Scholar
74. : Review of the pharmacology and clinical profile of bupropion, an antidepressant and tobacco use cessation agent. CNS Drug Rev 2006; 12:178–207Crossref, Medline, Google Scholar
75. : Effects of clomipramine on neuronal nicotinic acetylcholine receptors. Eur J Pharmacol 2002; 444:13–19Crossref, Medline, Google Scholar
76. : Bupropion is a nicotinic antagonist. J Pharmacol Exp Ther 2000; 295:321–327Medline, Google Scholar



