Physiology of Schizophrenia, Bipolar Disorder, and Schizoaffective Disorder
Abstract
Objective: Endophenotypes have been proposed to identify the genetic and biological substrates of complex disorders. Three physiological inhibitory endophenotypes of large effect size in schizophrenia include suppression of P50 auditory evoked responses, inhibition of leading (small anticipatory) saccades during smooth pursuit eye movements, and cancellation of reflexive saccades in the antisaccade eye movement task. The aim of this study was to determine if the pattern of endophenotype abnormalities within individuals with schizophrenia differed from that within individuals with bipolar disorder. A second aim was to determine whether subjects with schizoaffective disorder, bipolar type, were neurophysiologically more similar to subjects with schizophrenia or subjects with bipolar disorder. Method: Endophenotypes were recorded for subjects diagnosed with schizophrenia (N=29), bipolar disorder (DSM-IV-TR) (N=40), and schizoaffective disorder, bipolar type (N=18). Data from normal comparison subjects were used to establish normal performance. Results: Logistic regression determined that P50 ratio and frequency of leading saccades identified subjects with schizophrenia and bipolar disorder with a sensitivity of 95% and a specificity of 83%. The schizoaffective disorder group was split, with six subjects physiologically classified as schizophrenia-like and 12 subjects as bipolar-like. Those classified as schizophrenia-like were significantly younger at illness onset and had higher symptom ratings. Conclusion: A composite endophenotype of P50 ratio and frequency of leading saccades is consistent with the current clinical nosology of schizophrenia and bipolar disorder and parses patients with schizoaffective disorder, bipolar type, into two subgroups.
Debates about the conceptualization of schizophrenia, schizoaffective disorder, and bipolar mood disorder as forming a pathological spectrum arise from the clinical, therapeutic, and genetic similarities between these illnesses. While classic presentations of euphoric mania and schizophrenia are easily distinguished by clinicians, mania is frequently marked by bizarre behavior and psychosis that can prompt a diagnosis of schizophrenia (1) . Equally problematic for diagnosis are those patients with schizophrenia who present with multiple affective episodes, whether they are diagnosed with schizoaffective disorder or schizophrenia. A better description of the underlying neurobiology of these illnesses might help determine to what extent schizophrenia with affective features and bipolar disorder with psychotic features are separate disorders (2) .
The clinical observation of failure to engage inhibitory filtering in schizophrenia (3) and the prominence of distractibility in bipolar disorder indicate impairment of inhibition of sensory response in both illnesses. Three biological markers proposed as inhibitory endophenotypes for schizophrenia are suppression of the P50 auditory evoked response (4) , inhibition of intrusive saccade generation during smooth pursuit eye movements (5) , and ability to cancel reflexive saccade generation in the antisaccade eye movement task (6) . Although other inhibitory endophenotypes, notably prepulse inhibition of startle, could also have been included in this study, the three measures chosen range from entirely passive and automatic inhibition (suppression of the P50 auditory evoked response) to more top-down or voluntary inhibition (prosaccade cancellation), with leading saccades as an intermediate example of failure to inhibit an automatic reflex intruding upon a relatively low-level voluntary task. Furthermore, the selected endophenotypes have effect sizes for differences between individuals with psychiatric illness within the large range (2) .
Normal suppression of the P50 auditory evoked response involves inhibitory gating of the response to repeated stimuli. Patients with schizophrenia, bipolar disorder with a history of psychosis, or schizoaffective disorder, bipolar type, exhibit poor P50 suppression (4 , 7 – 9) . The smooth pursuit system is used to maintain foveal position on a moving object. One specific abnormality of smooth pursuit performance in patients with schizophrenia is the loss of the ability to inhibit the intrusion of small anticipatory, or leading, saccades (10) . The frequency of leading saccades during smooth pursuit eye movement has not been studied in persons with bipolar disorder. Finally, the antisaccade task measures the ability to inhibit the prepotent reflex to look quickly toward a peripheral target. Antisaccade inhibition is impaired in patients with schizophrenia (6) . In patients with bipolar disorder, no impairment (11) , nonsignificant impairment (12) , and significant impairment (13 – 16) have been reported.
We hypothesized that inhibitory deficits would be present in both subjects with schizophrenia and subjects with bipolar disorder, but differences in the pattern of neurophysiological abnormalities between the two groups might separate the two, in accordance with current clinical nosology. Furthermore, we hypothesized that subjects with schizoaffective disorder, bipolar type, would have varied neurophysiological performance, with some performing more similarly to schizophrenia subjects and some performing more similarly to bipolar disorder subjects, consistent with their mixed clinical presentation.
Method
Subjects
Twenty-nine subjects with schizophrenia (18 men and 11 women; Caucasian: N=22 [76%]) 18 to 55 years of age (mean=38.1 years, SD=10) were recruited from the Colorado Psychiatric Hospital outpatient program and the Denver VA Medical Center. Forty subjects with bipolar disorder (11 men and 29 women; Caucasian: N=32 [80%]) 19 to 61 years of age (mean=43.6 years, SD=11) and 18 subjects with schizoaffective disorder, bipolar type (10 men and eight women; Caucasian: N=12 [67%]), 22 to 59 years of age (mean=41.0, SD=11) were recruited from the Colorado Psychiatric Hospital outpatient program and depressive/manic depressive support groups in the Denver metropolitan area as part of a larger study (8) . The Structured Clinical Interview for DSM-IV or the Diagnostic Interview for Genetic Studies was used to determine the diagnosis. The Beck Depression Inventory (BDI), the Young Mania Rating Scale, and a modified Positive and Negative Syndrome Scale (PANSS) were used to assess the level of severity of illness in subjects with bipolar or schizoaffective disorder. After a complete description of the study to the subjects, monitored by a local Institutional Review Board, written informed consent was obtained.
To establish normal levels of performance for each of the individual tasks, data from three separate comparison groups were used ( Table 1 ). Comparison subjects were used for the P50 paradigm (N=42, mean=41.8 years, SD=8, 50% male, 88% Caucasian) (17) , the smooth pursuit paradigm (N=42, mean=40.2 years, SD=13, 45% male, 86% Caucasian) (18) , and the antisaccade paradigm (N=40, mean=34.8 years, SD=8, 48% male, 88% Caucasian) (19) .
Suppression of the P50 Auditory Evoked Response
Details of the recording procedure have been reported previously (8) . A conditioning-testing paradigm presented auditory stimuli with an intrapair interval of 0.5 seconds and an interstimulus interval of 10 seconds. At least five recordings were collected for each subject, with each recording containing at least 16 trials. The recordings were then added together to create an average which was used for analysis. For the conditioning response, a computer algorithm selected the most prominent peak between 40 and 75 msec poststimulus. The P50 ratio was calculated as the testing P50 wave amplitude divided by the conditioning P50 wave amplitude. Results from these subjects have previously been reported as part of a larger group (8) .
Leading Saccades During Smooth Pursuit Eye Movements
Details of the eye movement recording procedure and analysis are reported in detail elsewhere (10) . An infrared photoelectric limbus eye-tracking device recorded horizontal eye movements (Eye Trac Model 210, Applied Science Laboratories, Waltham, Mass.). During the smooth pursuit task, the target (a small lighted square subtending a visual angle of less than 0.4°) moved back and forth horizontally at a rate of 16.7°/sec over 30° of visual angle, with a 1.4-second fixation period at the end of each ramp. Each trial consisted of 60 seconds of recording time and each subject completed two trials. Leading saccades were defined as saccades that 1) occurred in the direction of the moving target, 2) either began and ended ahead of the target location or increased position error by 100%, 3) were followed by a 50-msec interval of eye velocity less than 50% of target velocity, and 4) had an amplitude of less than 4° (10 , 20) .
Inhibition of Saccades During the Antisaccade Task
Details of the eye movement recording procedure and analysis are reported in Ross et al. (21) . During the antisaccade task the subject initially fixated on a central target, with onset of a peripheral target after 2000 msec. In an attempt to induce express saccades, the fixation target extinguished 75 msec prior to onset of the peripheral target (a “gap” paradigm) (22) . The peripheral target appeared either to the left or right at a distance of 5° to 15° from the central fixation point for a period of 100 msec. After 100 msec, the peripheral target disappeared for a period of 700 msec, and the fixation target reappeared at the correct point. The task was then repeated. Subjects completed four trials, each consisting of 14 stimuli, after completing a practice trial to ensure they understood the directions. Antisaccades were defined as correct eye movements to the side of the screen opposite from the peripheral target. Prosaccades were defined as incorrect eye movements toward the same side of the screen as the peripheral target. The percentage of prosaccades generated during the task was used as the measure of disinhibition. Eye tracking measurements were first converted to z scores, based on age-matched comparison subjects (19 , 23) .
Statistical Analysis
For each physiological task there were three subject groups: subjects with schizophrenia, subjects with bipolar disorder, and comparison subjects for that particular task. Differences in demographic and physiological characteristics and medication status between the subject groups were assessed using either one-way analysis of variance with Tukey post hoc analysis for continuous variables or chi-square tests for categorical variables (Fisher’s exact test was used if cells contained fewer than five individuals). Pearson correlations were used to investigate the relationship between the clinical and physiological variables as well as the relationships among the individual physiological variables.
Logistic regression analysis (24 , 25) was performed using age, gender, and physiological variables to determine which factors would differentiate the subjects with schizophrenia from the subjects with bipolar disorder. A receiver operating characteristic curve analysis was then used to separate the subjects, using the physiological variables identified in the logistic regression. Post hoc analysis of the clinical characteristics of the subjects with schizophrenia or bipolar disorder was performed using Student’s t test for continuous variables and Fisher’s exact test for categorical variables. The logistic regression model was then applied to subjects with schizoaffective disorder to classify them physiologically as schizophrenia-like or bipolar-like. The clinical characteristics of these two groups were then compared using Student’s t tests. The logistic regression was performed using SAS procedure LOGISTIC (24) .
Results
Neurophysiology of Schizophrenia and Bipolar Disorder
Subjects with schizophrenia differed from comparison subjects on all three measures. Subjects with bipolar disorder differed from comparison subjects on only two measures, P50 ratio and frequency of prosaccade generation. Eighty-three percent of subjects with schizophrenia and 43% of subjects with bipolar disorder had P50 ratios two or more standard deviations above the mean for the comparison group. There were significant group differences in the mean P50 ratio ( Table 1 ). In post hoc analyses derived from the analysis of variance, subjects with schizophrenia had a significantly greater mean P50 ratio than both subjects with bipolar disorder (p<0.001) and comparison subjects (p<0.001); subjects with bipolar disorder had a significantly greater mean P50 ratio than comparison subjects (p=0.041).
Thirty-four percent of subjects with schizophrenia and 18% of subjects with bipolar disorder had frequencies of leading saccades two or more standard deviations above the mean for the comparison group. The mean frequency of leading saccades during smooth pursuit eye movement was significantly different between the groups ( Table 1 ). Subjects with schizophrenia had a significantly greater mean frequency of leading saccades than subjects with bipolar disorder (p=0.004) and comparison subjects (p<0.001) in the post hoc analysis. Subjects with bipolar disorder were no different from comparison subjects.
For prosaccade generation during the antisaccade task, 62% of the subjects with schizophrenia and 35% of the subjects with bipolar disorder had levels of impairment two or more standard deviations above the mean for the comparison group. There were significant group differences in the mean frequency of prosaccade generation during the antisaccade task ( Table 1 ). Post hoc analysis showed that on average, subjects with schizophrenia made significantly more prosaccade errors than comparison subjects (p<0.001), but not subjects with bipolar disorder. On average, subjects with bipolar disorder made significantly more prosaccade errors than comparison subjects (p<0.001).
Classification of Schizophrenia and Bipolar Disorder
Table 2 reports the details of the logistic regression model used for classifying schizophrenia and bipolar disorder subjects. The P50 ratio and frequency of leading saccades endophenotypes significantly contributed to the discrimination between subjects with schizophrenia and subjects with bipolar disorder, but not the frequency of prosaccade generation during the antisaccade task ( Table 2 ). Details of the logistic regression model used to discriminate between these illnesses are presented in Table 3 . The resulting model estimated the probability that a patient would be classified in the bipolar disorder group as

and the probability that a patient would be classified in the schizophrenia group as
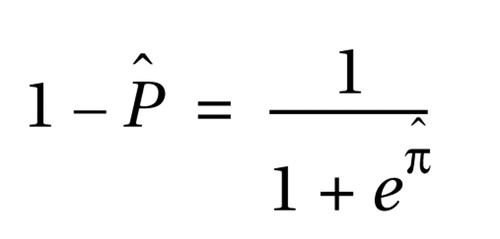
where πˆ = –2.2440+0.0869(age)+2.4680(gender)–0.0282(P50 ratio)–0.7945(frequency of leading saccades). The receiver operating characteristic curve created from this model had an estimated area under the curve of 0.92 ( Figure 1 ). At the point where the probability of classifying a subject as bipolar was >0.50 and the probability of classifying a subject as schizophrenic was 0.50, the model produced a sensitivity of 95% for correctly classifying a patient who has bipolar disorder and a specificity of 83% for correctly classifying a patient who has schizophrenia. The relative prevalences observed in our study were 58% bipolar and 42% schizophrenia, leading to a positive predictive value of 88% and a negative predictive value of 92%.

All five of the subjects with schizophrenia who were classified as bipolar by the neurophysiological measures had a history of mood dysregulation (as demonstrated by the taking of a mood stabilizer or antidepressant at the time of testing or having a history of recurrent mood episodes), compared with 58% of the correctly classified subjects with schizophrenia. These bipolar-like subjects with schizophrenia were first hospitalized at a significantly older age compared with the other subjects with schizophrenia (mean=28.8 years, SD=6.5 versus 20.3 years, SD=4.8; t=3.0, df=15, p=0.009). Of the two subjects with bipolar disorder who were classified neurophysiologically as having schizophrenia, one subject was unmedicated and suffered from symptoms of psychosis throughout his first (and only) episode of mania. The second subject was taking clozapine and also had symptoms of psychosis. Of the remaining 38 subjects with bipolar disorder, 27 (71%) had experienced a psychotic symptom, but only seven (18%) had a history of prominent psychotic symptoms.
Neurophysiology and Classification of Schizoaffective Disorder
Fifty-six percent of subjects with schizoaffective disorder, bipolar type, had a P50 ratio two or more standard deviations above the mean for the comparison group. In a group comparison (F=21.3, df=3, 125, p<0.001), the mean P50 ratio (0.48, SD=0.41) for subjects with schizoaffective disorder, bipolar type, was greater than the mean for the comparison group (p=0.041), no different from the mean for the bipolar group, and less than the mean for the schizophrenia group (p<0.001). For frequency of leading saccades, only 11% of subjects with schizoaffective disorder, bipolar type, were two or more standard deviations above the mean for the comparison group. In the group comparison (F=6.2, df=3, 125, p=0.001), the mean frequency of leading saccades for the schizoaffective disorder group (0.4, SD=1.7) was no different from the means for the comparison, bipolar, and schizophrenia groups. For prosaccade generation during the antisaccade task, 39% had a level of impairment two or more standard deviations above the mean for the comparison group. In the group comparison (F=16.7, df=3, 123, p<0.001), the mean z score (1.7, SD=1.5) for the schizoaffective disorder group was greater than the mean for the comparison group (p<0.001), but no different from the means for the bipolar and schizophrenia groups.
The logistic regression model derived from the subjects with schizophrenia and bipolar disorder was then applied to the 18 individuals with schizoaffective disorder, bipolar type. Figure 2 is a plot of the P50 ratios and leading saccade scores for the affected individuals within each patient group, along with the 68% prediction ellipses for each group. Six of the subjects with schizoaffective disorder, bipolar type, were classified as schizophrenia-like and 12 were classified as bipolar-like. Compared with the bipolar-like subjects, the schizophrenia-like subjects were younger (31.7 years, SD=9.2 versus 45.7 years, SD=9.2; t=3.0, df=16, p=0.009), first hospitalized at a younger age (18.5 years, SD=1.8 versus 27.8 years, SD=10.2; t=3.0, df=11, p=0.013), and somewhat more symptomatic on the day of testing according to PANSS and BDI scores (PANSS score: 24.2, SD=8.1 versus 16.1, SD=5.2; t= –2.6, df=16, p=0.021; BDI score: 19.3, SD=9.7 versus 5.3, SD=4.8; t= –4.2, df=16, p=0.001). There were no significant differences between the two groups for PANSS total score (52.3, SD=18.1 versus 38.9, SD=13.8), positive subscale score (16.7, SD=8.4 versus 12.2, SD=6.4), negative subscale score (11.5, SD=3.3 versus 10.7, SD=3.3), or Young Mania Rating Scale score (9.3, SD=13.2 versus 4.3, SD=4.9).

Discussion
All three of the physiological inhibitory endophenotypes investigated in this study can be abnormal in persons with schizophrenia or bipolar disorder. However, a composite inhibitory endophenotype including just P50 ratio and frequency of leading saccades during smooth pursuit eye movement was able to correctly identify the clinical diagnosis of 90% of our subjects. Thus, for the majority of subjects in this study, the physiological endophenotypes support the nosological separation of the two illnesses. Those subjects not correctly identified by the logistic regression model had clinical features that were highly representative of the other diagnostic group (e.g., affective dysregulation and later age of onset in bipolar-like schizophrenia subjects). Although the small number of schizophrenia-like bipolar subjects limited analysis (N=2), both of the individuals had suffered from prominent symptoms of psychosis.
Consistent with the debate over the categorical validity of schizoaffective disorder, bipolar type, the subjects in this study were split, with one-third displaying schizophrenia-like neurophysiology and the majority displaying bipolar-like neurophysiology. Again, some aspects of clinical course paralleled the neurophysiological classification (e.g., earlier age of onset and higher symptom ratings in schizophrenia-like subjects). However, caution should be used when generalizing these findings, as our logistic regression model was applied to a population with a high prevalence of illness and a ratio of bipolar and schizophrenia subjects similar to the national prevalence of these disorders. When applied to a different population, the positive predictive value of this composite endophenotype might decrease.
Pathological Mechanisms
Subjects with schizophrenia and bipolar disorder both had difficulties with the antisaccade task, a task that relies on the prefrontal cortex for planned inhibition (27 , 28) . A functional imaging study by Al-Mousawi et al. examined both persons with bipolar disorder and schizophrenia and found prefrontal cortex abnormalities, consistent with our findings (29) . In this study, a majority of the subjects with schizophrenia and subjects with bipolar disorder and a history of psychosis had abnormalities in the P50 measures of sensory gating, which relies on an intact auditory system as well as interneuronal integrity and cholinergic modulation within the hippocampus. While structural abnormalities of the temporal lobe, more specifically the superior temporal gyrus or hippocampus, are regularly seen in schizophrenia and not in bipolar disorder (e.g., references 30 , 31) , two studies of subjects with bipolar disorder, the majority of whom either had a history of psychosis or were taking neuroleptics, found reduced hippocampal size (32 , 33) . Many of the subjects with schizophrenia and few of the subjects with bipolar disorder had impaired smooth pursuit performance. The smooth pursuit task requires the coordinated effort of multiple cortical systems, including the frontal, supplementary, and parietal eye fields as well as additional parietal, cingulate, occipital, and cerebellar areas (34) . Subjects with schizophrenia with impaired smooth pursuit eye movements have decreased prefrontal and frontal eye field cortical activation, decreased middle temporal/medial superior temporal cortical activation, and increased hippocampal activation (35 – 37) . Anatomical studies have found more gray matter abnormalities in schizophrenia compared to bipolar disorder (38 , 39) . These global deficits may mean that it is more likely that a person with schizophrenia has smooth pursuit performance impairment.
The results of this study do not have direct clinical applicability. However, the results regarding the neurophysiological endophenotypes support the validity of the clinical distinction between schizophrenia and bipolar disorder. These results also support the utility of the clinically observable measures that clinicians often use to help separate variants of schizoaffective disorder (e.g., age of illness onset).

1. Carlson GA, Goodwin FK: The stages of mania. Arch Gen Psychiatry 1973; 28:221–228Google Scholar
2. Braff DL, Freedman R: Endophenotypes in studies of the genetics of schizophrenia, in Neuropsychopharmacology: The Fifth Generation of Progress. Edited by Davis KL, Charney D, Coyle JT, Nemeroff C. Philadelphia, Lippincott Williams & Wilkins, 2002, pp 703–716Google Scholar
3. Venables PH: Hippocampal function and schizophrenia: experimental psychological evidence. Ann N Y Acad Sci 1992; 658:111–127Google Scholar
4. Adler LE, Pachtman E, Franks RD, Pecevich M, Waldo MC, Freedman R: Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biol Psychiatry 1982; 17:639–654Google Scholar
5. Holzman PS, Proctor LR, Hughes DW: Eye-tracking patterns in schizophrenia. Science 1973; 181:179–181Google Scholar
6. Fukushima J, Fukushima K, Chiba T, Tanaka S, Yamashita I, Kato M: Disturbances of voluntary control of saccadic eye movements in schizophrenic patients. Biol Psychiatry 1988; 23:670–677Google Scholar
7. Louchart-de la Chapelle S, Nkam I, Houy E, Belmont A, Ménard JF, Roussignol AC, Siwek O, Mezerai M, Guillermou M, Fouldrin G, Levillain D, Dollfus S, Campion D, Thibaut F: A concordance study of three electrophysiological measures in schizophrenia. Am J Psychiatry 2005; 162:466–474Google Scholar
8. Olincy A, Martin L: Diminished suppression of the P50 auditory evoked potential in bipolar subjects with a history of psychosis. Am J Psychiatry 2005; 162:43–49Google Scholar
9. Franks RD, Adler LE, Waldo MC, Alpert J, Freedman R: Neurophysiological studies of sensory gating in mania: comparison with schizophrenia. Biol Psychiatry 1983; 18:989–1005Google Scholar
10. Ross RG, Olincy A, Radant A: Amplitude criteria and anticipatory saccades during smooth pursuit eye movements in schizophrenia. Psychophysiology 1999; 36:464–468Google Scholar
11. Fukushima J, Morita N, Fukushima K, Chiba T, Tanaka S, Yamashita I: Voluntary control of saccadic eye movements in patients with schizophrenic and affective disorders. J Psychiatr Res 1990; 24:9–24Google Scholar
12. Crawford TJ, Haeger B, Kennard C, Reveley MA, Henderson L: Saccadic abnormalities in psychotic patients, I: neuroleptic-free psychotic patients. Psychol Med 1995; 25:461–471Google Scholar
13. Sereno AB, Holzman PS: Antisaccades and smooth pursuit eye movements in schizophrenia. Biol Psychiatry 1995; 37:394–401Google Scholar
14. Katsanis J, Kortenkamp S, Iacono WG, Grove WM: Antisaccade performance in patients with schizophrenia and affective disorder. J Abnorm Psychol 1997; 106:468–472Google Scholar
15. Curtis CE, Calkins ME, Iacono WG: Saccadic disinhibition in schizophrenia patients and their first-degree biological relatives: a parametric study of the effects of increasing inhibitory load. Exp Brain Res 2001; 137:228–236Google Scholar
16. Gooding DC, Tallent K: The association between antisaccade task and working memory task performance in schizophrenia and bipolar disorder. J Nerv Ment Dis 2001; 189:8–16Google Scholar
17. Leonard S, Gault J, Hopkins J, Logel J, Vianzon R, Short M, Drebing C, Berger R, Venn D, Sirota P, Zerbe G, Olincy A, Ross RG, Adler LE, Freedman R: Association of promoter variants in the alpha7 nicotinic acetylcholine receptor subunit gene with an inhibitory deficit found in schizophrenia. Arch Gen Psychiatry 2002; 59:1085–1096Google Scholar
18. Ross RG, Olincy A, Mikulich SK, Radant AD, Harris JG, Waldo M, Compagnon N, Heinlein S, Leonard S, Zerbe GO, Adler L, Freedman R: Admixture analysis of smooth pursuit eye movements in probands with schizophrenia and their relatives suggests gain and leading saccades are potential endophenotypes. Psychophysiology 2002; 39:809–819Google Scholar
19. Olincy A, Ross RG, Young DA, Freedman R: Age diminishes performance on an antisaccade eye movement task. Neurobiol Aging 1997; 18:483–489Google Scholar
20. Ross RG, Olincy A, Zerbe G, Radant A: Which duration of postsaccadic slowing identifies anticipatory saccades during smooth pursuit eye movements? Psychophysiology 2001; 38:325–333Google Scholar
21. Ross RG, Harris JG, Olincy A, Radant A, Adler LE, Freedman R: Familial transmission of two independent saccadic abnormalities in schizophrenia. Schizophrenia Res 1998; 30:59–70Google Scholar
22. Fischer B, Weber H: Characteristics of “anti” saccades in man. Exp Brain Res 1992; 89:415–424Google Scholar
23. Ross RG, Olincy A, Harris JG, Radant A, Adler LE, Compagnon N, Freedman R: The effects of age on a smooth pursuit tracking task in adults with schizophrenia and normal subjects. Biol Psychiatry 1999; 46:383–391Google Scholar
24. SAS/STAT User’s Guide, version 9.1. Cary, NC, SAS Institute, 2004Google Scholar
25. Hosmer DW Jr, Lemeshow S: Applied Logistic Regression. New York, Wiley, 1989Google Scholar
26. Nagamoto HT, Adler LE, McRae KA, Huettl P, Cawthra E, Gerhardt G, Hea R, Griffith J: Auditory P50 in schizophrenics on clozapine: improved gating parallels clinical improvement and changes in plasma 3-methoxy-4-hydroxyphenylglycol. Neuropsychobiology 1999; 39:10–17Google Scholar
27. Pierrot-Deseilligny C, Rivaud S, Gaymard B, Müri R, Vermersch AI: Cortical control of saccades. Ann Neurology 1995; 37:557–567Google Scholar
28. Funahashi S, Chafee MV, Goldman-Rakic PS: Prefrontal neuronal activity in rhesus monkeys performing a delayed anti-saccade task. Nature 1993; 365:753–756Google Scholar
29. Al-Mousawi AH, Evans N, Ebmeier KP, Roeda D, Chaloner F, Ashcroft GW: Limbic dysfunction in schizophrenia and mania. Br J Psychiatry 1996; 169:509–516Google Scholar
30. Altshuler LL, Bartzokis G, Grieder T, Curran J, Jimenez T, Leight K, Wilkins J, Gerner R, Mintz J: An MRI study of temporal structures in men with bipolar disorder or schizophrenia. Biol Psychiatry 2000; 48:147–162Google Scholar
31. Hirayasu Y, Shenton ME, Salisbury DF, Dickey CC, Fischer IA, Mazzoni P, Kisler T, Arakaki H, Kwon JS, Anderson JE, Yurgelun-Todd D, Tohen M, McCarley RW: Lower left temporal lobe MRI volumes in patients with first-episode schizophrenia compared with psychotic patients with first-episode affective disorder and normal subjects. Am J Psychiatry 1998; 155:1384–1391Google Scholar
32. Velakoulis D, Pantelis C, McGorry PD, Dudgeon P, Brewer W, Cook M, Desmond P, Bridle N, Tierney P, Murrie V, Singh B, Copolov D: Hippocampal volume in first-episode psychoses and chronic schizophrenia. Arch Gen Psychiatry 1999; 56:133–140Google Scholar
33. Swayze VW, Andreason NC, Alliger RJ, Yuh WTC, Ehrhardt JC: Subcortical and temporal structures in affective disorder and schizophrenia: a magnetic resonance imaging study. Biol Psychiatry 1992; 31:221–240Google Scholar
34. Gaymard B, Pierrot-Deseilligny C: Neurology of saccades and smooth pursuit. Curr Opin Neurol 1999; 12:13–19Google Scholar
35. Blackwood DH, Ebmeier KP, Muir WJ, Sharp CW, Glabus M, Walker M, Souza V, Dunan JR, Goodwin GM: Correlation of regional cerebral blood flow equivalents measured by single photon emission computerized tomography with P300 latency and eye movement abnormality in schizophrenia. Acta Psychiatr Scand 1994; 90:157–166Google Scholar
36. Tregellas J, Tanabe JL, Miller DE, Ross RG, Olincy A, Freedman R: Neurobiology of smooth pursuit eye movement deficits in schizophrenia: an fMRI study. Am J Psychiatry 2004; 161:315–321Google Scholar
37. Ross DE, Thaker GK, Holcomb HH, Cascella NG, Medoff DR, Tamminga CA: Abnormal smooth pursuit eye movements in schizophrenic patients are associated with cerebral glucose metabolism in oculomotor regions. Psychiatry Res 1995; 58:53–67Google Scholar
38. Kubicki M, Shenton ME, Salisbury DF, Hirayasu Y, Kasai K, Kikinis R, Jolesz FA, McCarley RW: Voxel-based morphometric analysis of gray matter in first episode schizophrenia. Neuroimage 2002; 17:1711–1719Google Scholar
39. Zipursky RB, Seeman MV, Bury A, Langevin R, Wortzman G, Katz R: Deficits in gray matter volume are present in schizophrenia but not bipolar disorder. Schizophrenia Res 1997; 26:85–92Google Scholar






