The Pathology of Social Phobia Is Independent of Developmental Changes in Face Processing
Abstract
Objective:
While social phobia in adolescence predicts the illness in adulthood, no study has directly compared the neural responses in social phobia in adults and adolescents. The authors examined neural responses to facial expressions in adults and adolescents with social phobia to determine whether the neural correlates of adult social phobia during face processing also manifest in adolescent social phobia.
Method:
Blood-oxygen-level-dependent (BOLD) responses were compared in 39 medication-free participants with social phobia (25 adults and 14 adolescents) and 39 healthy comparison subjects (23 adults and 16 adolescents) matched on age, IQ, and gender. During fMRI scans, participants saw angry, fearful, and neutral expression stimuli while making a gender judgment.
Results:
Significant diagnosis-by-emotion interactions were observed within the amygdala and the rostral anterior cingulate cortex, as has previously been hypothesized. In these regions, both the adolescent and adult social phobia patients showed significantly increased BOLD responses relative to their respective age-matched comparison subjects, and there was no evidence of age-related modulation of between-group differences. These enhanced responses occurred specifically when viewing angry (rostral anterior cingulate cortex) and fearful (amygdala and rostral anterior cingulate cortex) expressions but not when viewing neutral expressions. In addition, the severity of social phobia was significantly correlated with the enhanced rostral anterior cingulate cortex response in the adults.
Conclusions:
The neural correlates of adult social phobia during face processing also manifest in adolescents. Neural correlates that are observed in adult social phobia may represent the persistence of profiles established earlier in life rather than adaptive responses to such earlier perturbations or maturational changes. These cross-sectional observations might encourage longitudinal fMRI studies of adolescent social phobia.
Although adolescent social phobia predicts adult social phobia (1, 2), which suggests that the neural correlates of social phobia manifest similarly in adolescents and adults, there has been limited research that explores this possibility. In particular, no imaging study has used identical methods to compare adolescents and adults with and without social phobia. It is important to directly compare these groups since the neural correlates of social phobia can be masked by minor variations in task parameters. Moreover, age-related differences manifest between adolescents and adults when comparing the responsiveness of regions implicated in social phobia. Thus, neural correlates of adult social phobia could arise from one of two reasonable scenarios. They could reflect either the persistence of adolescent perturbations or perturbations that manifest differently in adults than they do in adolescents. A direct comparison of data from adolescents and adults with and without social phobia is the first step toward beginning to resolve these two competing scenarios.
In this study, we evaluated the degree to which adolescents and adults with social phobia show similar or dissimilar neural perturbations to morphed expression stimuli relative to age-matched comparison subjects. We used facial expression stimuli because these have been used extensively in studies with healthy individuals and are the most frequently employed stimuli in earlier studies of social phobia (3–13). This previous work provides a rich scientific context in which to embed the present study. Facial expression stimuli also engage relatively automatic responses from participants, which optimizes their utility in developmental work. More complex stimuli, which are also used in studies with adults, rely on contextual information to convey emotional cues, engaging less automatic responses (14–17). Finally, the use of morphed-face stimuli is advantageous because subtly varying the morphing within an emotion class increases stimulus novelty, a factor that has been shown to influence brain response to emotional faces.
Previous studies of adult social phobia reported increased responsiveness across regions involved in facial expression processing in healthy individuals, notably the amygdala but also the anterior cingulate gyrus, insula, and temporal cortical regions. This increased responsiveness appears emotion specific because it occurs consistently for emotional expressions, particularly negative-valence expression (4, 6, 7, 9, 11, 13), but typically not for neutral or positive expressions (4, 6, 9, 11; for contrasting results, see references 3, 8). Given that perturbed emotion-specific processing has been demonstrated relatively consistently across laboratories in adult social phobia, we would expect to observe similar emotion-specific findings in adolescent social phobia if the pathology of the disorder does not vary independently. However, there has been limited research examining emotion-specific processing in adolescent social phobia. Studies have used face-emotion displays in the context of attention-manipulation tasks, and these studies do find increased amygdala responsiveness in various pediatric anxiety disorders (18–20). However, findings from adult studies suggest that atypical responses in social phobia can be distinguished from responses in other anxiety disorders, which emphasizes the need to specifically examine responsiveness in adolescent social phobia (11). The only such functional MRI (fMRI) study in adolescents with social phobia (21) focused on psychological processes more relevant to adolescent than to adult social phobia and failed to examine emotion-specific processing. Thus, the need remains to directly compare neural responses to disorder-specific and relevant social stimuli in adolescents and adults with and without social phobia.
As noted, given that adult social phobia typically begins in adolescence, two scenarios could link neural and symptomatic expressions of social phobia in adolescents and adults. First, similar neural correlates could manifest in adolescents and adults. In this instance, we would expect to find diagnosis-by-emotion interactions in adolescents and adults both with and without social phobia. Specifically, data in adult social phobia suggest that adolescents with social phobia would show increased responsiveness to the fearful and angry expressions but not to neutral expressions when compared with healthy adolescents. We might expect these interactions specifically within the amygdala, the region most frequently associated with hyper responsiveness in adult social phobia, but we would also expect interactions in other regions frequently identified in studies of adults (e.g., anterior cingulate cortex, insula). Second, previous research on face processing indicates differences between adolescents and adults in the responses of these same brain regions (22). Thus, findings in adult social phobia could also either reflect adaptations to perturbations that are present in adolescence or reflect other maturational changes, yielding different neural correlates of the same disorder as manifested in either adolescence or adulthood. In either case, we would expect diagnosis-by-age or diagnosis-by-age-by-emotion interactions, reflecting unique social phobia correlates in the two age groups. The present study tests these alternative possibilities.
Method
Participants
Our study included 39 patients (25 adults and 14 adolescents with current social anxiety disorder) and 39 healthy comparison subjects (23 adults and 16 adolescents), group matched with patients in the same age group, for a total of 78 participants. This yielded two samples of adult and adolescent patients and comparison subjects that did not differ in terms of age, gender, or IQ. Table 1 summarizes these group demographic and clinical characteristics. In addition, there was no significant gender or IQ difference between the adult (N=48) and adolescent (N=30) cohorts. Among the 48 adults, data from 32 of them (15 patients and 17 healthy comparison subjects) were included in a previous report (11). None of the data from the adolescent group have been previously reported.
| Adult Social Phobia Group (N=25) | Adolescent Social Phobia Group (N=14) | Adult Healthy Comparison Group (N=23) | Adolescent Healthy Comparison Group (N=16) | |||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Age | 32.2 | 9.14 | 13.3 | 3.42 | 29.7 | 8.30 | 14.9 | 2.03 |
| IQ | 118.8 | 11.56 | 113.0 | 14.67 | 115.8 | 13.55 | 112.1 | 16.07 |
| Liebowitz Social Anxiety Scale scorea | 73.2 | 20.40 | — | — | — | — | — | — |
| Pediatric Anxiety Rating Scale scorea | — | — | 21.7 | 4.84 | — | — | — | — |
| N | % | N | % | N | % | N | % | |
| Gender (male) | 10 | 40 | 7 | 50 | 13 | 57 | 9 | 56 |
| Race | ||||||||
| Caucasian | 15 | 60 | 12 | 86 | 18 | 78 | 12 | 75 |
| African American | 6 | 24 | 2 | 14 | 3 | 13 | 2 | 13 |
| Asian | 4 | 16 | — | — | 2 | 9 | 1 | 6 |
| Mixed | — | — | — | — | — | — | 1 | 6 |
TABLE 1. Demographic and Clinical Characteristics for Participants in a Study of Face-Emotion Processing in Social Phobia
Adults
The recruitment of adults for this study has been described previously (11, 15). Briefly, all patients met criteria for current generalized social phobia based on the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) (23) and a confirmatory clinical interview by a board-certified psychiatrist (D.S.P.). No patients had any other axis I diagnosis, and all patients indicated a childhood onset of symptoms. All patients were medication free, and the comparison subjects had no history of mental illness. All participants reported no history of substance dependence or current substance abuse, and all screened negative on urine toxicology. We assessed the severity of social anxiety with the Liebowitz Social Anxiety Scale (24). In addition, all participants were in good physical health, as confirmed by a complete physical examination, and all provided written informed consent.
Adolescents
Adolescent participants were also recruited using methods described previously (25). Like adult patients, all adolescent patients met criteria for current generalized social phobia according to DSM-IV criteria. Diagnosis in adolescents was based on the Schedule for Affective Disorders and Schizophrenia for School-Age Children, Present and Lifetime version (K-SADS-PL; 26). Pediatric patients were recruited when the social anxiety for which they had sought treatment persisted after 3 weeks of supportive therapy. This additional inclusion criterion for adolescents but not adults reflected the fact that some forms of pediatric social anxiety can remit with brief interventions, and we wanted to restrict our patient samples to those with severe and persistent anxiety. All of the adolescent patients also demonstrated severe social anxiety on the Pediatric Anxiety Rating Scale (27). As with the adults, none of the adolescent patients had any other axis I diagnoses and all were medication free. Other inclusion and exclusion criteria were identical to those described for the adults.
Task
Participants viewed static gray-scale images of emotionally expressive faces (fearful or angry) in addition to neutral faces from the Pictures of Facial Affect series (28). In designing our paradigm, we wanted to maximize statistical power by devoting as much time as possible to precisely estimating neural responses to a relatively select set of stimuli. Thus, we elected to present only neutral faces and two classes of emotional stimuli plus a baseline stimuli consisting of fixation points.
We selected fearful expressions because we entered the study with a particular interest in amygdala function and selected the expression most consistently shown to elicit amygdala responses in healthy individuals (29–31). The fearful expression is of particular interest since few studies of social phobia have specifically examined responsiveness to this expression. We selected angry expressions because they represent the stimulus class that most consistently has elicited increased responsiveness in social phobia (4, 6, 7, 13).
Inclusion of happy expressions would have allowed us to dissociate valence and arousal effects, since both angry and fearful faces are high-arousal negative stimuli, whereas happy expressions are high-arousal positive stimuli. However, previous work on the amygdala response to happy faces in healthy individuals is inconsistent (31). Moreover, while two studies reported an increase in amygdala responses to happy expressions in adult social phobia (6, 13), three others reported normal responses (3, 4, 9).
A gender judgment task (inferring gender by looking at a face) was selected for three reasons. First, since considerable previous work has relied on this task (11, 32–34), its use in the present study allows our data to be placed within a rich research context. Second, previous work shows that task instructions focusing attention on emotional features influence the neural response to emotional stimuli (32, 34). Gender judgment was selected to minimize the possibility of muting between-group differences by focusing attention on nonemotional aspects of stimuli. Finally, gender judgment has the advantage of being easily performed by a younger subject group because there are a limited number of response options and the concept is simple.
For our stimuli set, 10 different actors were used, and each face was digitally morphed to represent seven different expressions: 50% fear, 100% fear, 150% fear, 50% anger, 100% anger, 150% anger, and 25% happy. Since it is conventional to signal approval in normal social interaction, 100% neutral faces can appear slightly cold and threatening. Thus, the 25% happy face was used to indicate neutral nonemotional facial displays. This generated 70 stimuli (10 actors and seven expressions). We decided to use morphed faces in order to increase variability in emotion displays beyond the prototypical 100% emotional face displays. This reduced the habituation associated with repeated viewing of identical emotional faces. In the data analysis, face-viewing events were categorized using weights that reflected these levels of emotion. This model (weighing the variability in displays of emotion according to intensity) was chosen based on our previous experience analyzing data from this paradigm; power was maximized by modeling the intensity rather than treating each intensity indicator as a separate regressor.
Each face stimulus was presented for 2,500 ms with a 500-ms interstimulus interval. We used an event-related design whereby all events occurred randomly throughout the task. Participants made gender judgments by pressing either the left or right buttons. Responses were monitored with a computer recording of the motor response and by real-time performance observation, as indicated by a light display on a monitor in the scanning room. Participants completed four runs of the task, each consisting of 29 fixation-point trials, 20 neutral face trials, and 10 emotional face trials of each intensity for anger and fear expressions. This resulted in 80 distinct face stimuli and 109 total trials per run.
fMRI Parameters
Whole-brain blood-oxygen-level-dependent (BOLD) fMRI data were acquired using a 1.5-T Siemens MRI scanner. After sagittal localization, functional T2*-weighted images were acquired using an echo-planar single-shot gradient echo pulse sequence with a matrix of 64×64 mm, repetition time of 3,000 ms, echo time of 30 ms, field of view of 240 mm, and voxels of 3.75×3.75×4 mm. Images were acquired in 31 contiguous 4-mm axial slices per brain volume. The functional data were acquired over four runs, each lasting 5 minutes and 27 seconds. In the same session, a high-resolution T1-weighted anatomical image was acquired to aid with spatial normalization (three-dimensional spoiled gradient-recall acquisition in the steady state; repetition time=8.1 msec; echo time=3.2 msec, flip angle=20°; field of view=240 mm, 124 axial slices, thickness=1.0 mm; 256×256 acquisition matrix).
fMRI Analysis
Data were analyzed using the Analysis of Functional Neuroimages software package (35). We performed motion correction, spatially smoothed the data, and normalized the time series data by dividing the signal intensity of a voxel at each time point by the mean signal intensity of that voxel for each run so that regression coefficients represented percent signal change. Regressors for each emotion were created through convolution with gamma-variate hemodynamic response functions for weighted estimates of angry and fearful expressions, reflecting proportions of the emotional prototypes. With this approach, the regressor for each face-emotion type effectively models activation associated with all of the gradations of one or another face-emotion type, generating a beta coefficient and its associated t statistic for each voxel and each regressor.
Voxel-wise group analyses involved transforming single-subject beta coefficients into standard space (36) and submitted these to a 2×2×3 (diagnosis group by age group by face emotion) mixed analysis of variance (ANOVA). This analysis produced statistical maps of the main effects and interactions. Our main interests were in testing the diagnosis-by-emotion interaction, the three-way diagnosis-by-age-by-emotion interaction, and the two-way diagnosis-by-age interaction. These interactions test the degree to which any potential diagnosis-specific response to faces appears similar or different in the two age groups.
As in our previous work, the significance threshold was set through a two-stage procedure. In the initial stage, group maps were generated with significance set at p<0.005 (two-tailed). In the second stage, to correct for multiple comparisons, a spatial-clustering operation was performed using AlphaSim (http://afni.nimh.nih.gov/sscc/gangc/mcc.html) with 1,000 Monte Carlo simulations, to generate a map-wise false positive probability of p<0.05, corrected for multiple comparisons and subsequent region-of-interest-based data. These data then were used in two sets of analyses. First, to facilitate the interpretation of results from our main ANOVA-based analysis, we measured the average percent signal change within each region of interest. This generated mean values for each event type for each participant that could be extracted and submitted to post hoc group-level statistics performed with SPSS, version 17.0 (SPSS, Chicago, 2008). This also clarified the degree to which findings in the ANOVA reflected particularly large or small responses to one or another face-emotion type in one or another participant group. Second, to examine the relationship between symptom severity and neural responses in social phobia, we examined the correlations between the increased responses to emotional expressions identified by the group-by-emotion interaction and the anxiety rating scores listed in Table 1.
Results
Task performance was adequate in the four groups, with low error rates. Among participants with social phobia, the error rates were 1.7% (SD=2.29) for adults and 9.9% (SD=12.25) for adolescents; among healthy comparison subjects, the error rates were 1.6% (SD=1.67) for adults and 5.2% (SD=5.15) for adolescents. Although the adolescent groups committed more overall errors than the adults (F=17.55, df=1, 76, p<0.005), there was no significant difference in error rates between the two adolescent groups or between the patients with social phobia (adults and adolescents) and the healthy comparison subjects (adults and adolescents). Because of equipment malfunction, data for behavioral performance on the gender identification task for five adolescents were not saved.
No regions survived correction for multiple comparisons from the diagnosis-by-emotion-by-age, diagnosis-by-age, or emotion-by-age interaction statistical maps. As noted, our main interest was in statistical tests of diagnosis-by-emotion interactions. Table 2 summarizes the regions showing a significant diagnosis-by-emotion interaction and main effects of diagnosis, age group, and emotion.
| Coordinates | ||||||
|---|---|---|---|---|---|---|
| Region | Brodmann's Area | Volume (mm3) | x | y | z | F |
| Diagnosis-by-emotion interaction | ||||||
| Right anterior cingulate gyrus | 32 | 536 | 7 | 44 | –6 | 8.23 |
| Left amygdala | 147 | –28 | –2 | –18 | 5.55 | |
| Diagnosis main effect | ||||||
| Right occipital/temporal gyrus | 18/19/37 | 14,206 | 10 | –95 | 3 | 16.36 |
| Emotion main effect (angry = fearful > neutral for all) | ||||||
| Right middle frontal gyrus | 6 | 32,201 | 57 | 9 | 38 | 16.76 |
| Right medial frontal gyrus | 6/32 | 4,004 | 5 | 6 | 49 | 9.86 |
| Left amygdala/parahippocampal gyrus | 2,729 | –17 | 0 | –10 | 9.44 | |
| Right amygdala | 29 | 26 | 1 | –14 | 5.93 | |
| Right thalamus | 3,457 | 16 | –28 | 0 | 10.04 | |
| Right middle occipital gyrus | 18 | 59,545 | 35 | –82 | –7 | 55.73 |
| Left inferior occipital gyrus | 18 | 47,082 | –32 | –87 | –5 | 53.23 |
| Right precuneus | 7 | 526 | 27 | –64 | 32 | 6.41 |
| Age main effect (adult > adolescent for all) | ||||||
| Left medial frontal gyrus/orbital frontal cortex | 11 | 1,169 | –7 | 24 | –14 | 20.86 |
| Left inferior frontal gyrus | 45 | 1,775 | –49 | 34 | 7 | 12.82 |
| Left inferior frontal gyrus | 6 | 4,376 | –54 | 3 | 35 | 19.32 |
| Right inferior frontal gyrus | 9 | 1,109 | 60 | 9 | 28 | 14.81 |
| Right cingulate gyrus | 24 | 508 | 0 | 4 | 37 | 11.28 |
| Right postcentral gyrus | 1/3 | 14,206 | 52 | –15 | 49 | 21.28 |
| Right middle occipital gyrus | 19 | 6,115 | 50 | –80 | 3 | 23.58 |
| Left middle temporal gyrus | 19/37 | 3,970 | –63 | –68 | 3 | 18.78 |
| Left culmen | 812 | –15 | –50 | –12 | 13.96 | |
TABLE 2. Significant Areas of Activation for the Diagnosis-by-Emotion Interaction and the Diagnosis, Age, and Emotion Main Effects in a Study of Face-Emotion Processing in Social Phobiaa
Diagnosis-by-Emotion Interaction
Consistent with our predictions, the diagnosis-by-emotion interaction identified the amygdala and the rostral anterior cingulate cortex (see Table 2 and Figure 1). In both regions, social phobia patients showed greater activation to fearful expressions (F=6.31, df=1, 76, p<0.005) relative to healthy comparison subjects (F=10.88, df=1, 76, p<0.005). In addition, within the rostral anterior cingulate cortex, social phobia patients showed greater activation to angry expressions relative to healthy comparison subjects (F=4.77, df=1, 76, p<0.05). However, there were no significant group differences in BOLD responses to the neutral stimuli in either the amygdala or the anterior cingulate cortex (Figure 1).
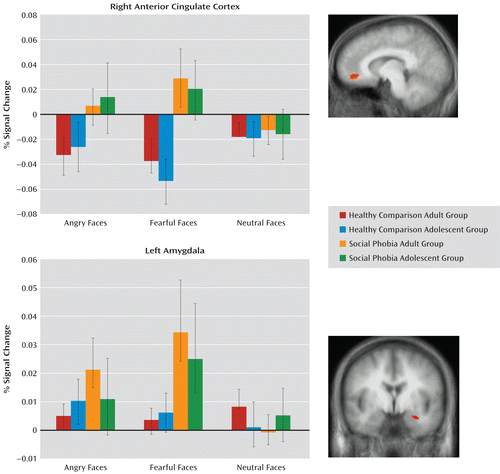
FIGURE 1. Interactions of Diagnosis by Emotion in a Study of Face-Emotion Processing in Social Phobiaa
a Blood-oxygen-level-dependent responses to angry, fearful, and neutral facial expressions in the right anterior cingulate cortex (7, 44, –6) and in the left amygdala (–28, –2, –18). Bars indicate standard errors.
Main Effects
The group main effect identified one large temporo-occipital area (Table 2). In this region, the healthy comparison subjects showed a significantly greater BOLD response relative to the social phobia patients (F=13.31, df=1, 76, p<0.001).
Regions identified by the age main effect included the inferior and medial frontal gyrus; see Table 2 for the full list. In all regions identified by this main effect, adult participants showed a significantly greater BOLD response than did adolescents (F=9.63–24.52, df=1, 76, p<0.001–0.005). None of these regions interacted with social phobia status.
Regions identified by the emotion main effect included the amygdala bilaterally and the inferior, middle, and medial frontal regions (Table 2). In all regions identified by this main effect, the participants showed significantly greater BOLD responses to fearful expressions compared with neutral expressions (F=6.31–31.74, df=1, 77, p<0.05–0.001) and angry expressions relative to neutral expressions (F=9.08–83.66, df=1, 77, p<0.05–0.001).
Correlational Analysis
Using correlational analysis, we examined whether the increased neural responses to angry and fearful expressions in the anterior cingulate cortex and to fearful expressions in the amygdala shown by the patients with social phobia were related to the level of anxiety as reported in Table 1. As shown in Figure 2, for the adult patients, there was a positive correlation between the BOLD response to both angry and fearful expressions and symptom severity score in the anterior cingulate cortex (Pearson's r=0.453 and Pearson's r=0.383, df=25, p<0.05 and p<0.10, respectively). However, the result for neutral expressions was not significant. In contrast, no significant correlation emerged between the BOLD response to fearful expressions and symptom severity in the amygdala. We observed no significant correlations involving adolescents with social phobia.
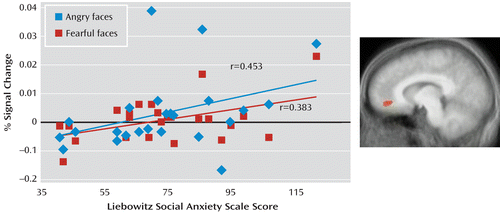
FIGURE 2. Symptom Severity and Blood-Oxygen-Level-Dependent (BOLD) Response in a Study of Face-Emotion Processing in Social Phobiaa
a Correlations between symptom severity in the adult social phobia group and BOLD response to angry and fearful expressions in the anterior cingulate cortex.
Discussion
We compared BOLD responses to fearful, angry, and neutral expressions among adults and adolescents with and without social phobia. The findings appear clear: relative to their respective age-matched healthy comparison groups, both adolescents and adults with social phobia showed a significantly increased response to emotional, but not neutral, facial expressions in the amygdala and the rostral anterior cingulate cortex. Data from some adults examined in this study had been reported previously, and our primary goal was to extend these data to adolescents. The observation of significant diagnosis-by-emotion interactions, but not diagnosis-by-age or diagnosis-by-age-by-emotion interactions, suggests that neural perturbations in adult social phobia, at least to emotional facial expressions, may represent persistent manifestations of abnormalities present in adolescence rather than the result of perturbed development. Longitudinal research should evaluate this possibility more critically.
The results with respect to adult social phobia are broadly consistent with the previous literature. Thus, while studies have implicated a network of emotion-relevant regions in increased responsiveness to emotional facial expression stimuli in adult patients with social phobia, the regions most consistently identified in that work include the amygdala and the anterior cingulate cortex (5, 11, 37), the two regions identified in the present study. With respect to pediatric anxiety disorders, several studies have reported increased amygdala responses (18–20), and one observed increased amygdala responses in adolescent social phobia specifically to peers who were previously rated as undesirable (21). However, no previous study of social phobia in adolescents has used procedures employed in studies of social phobia in adults. The data we present here extend the previous literature by demonstrating comparable neural signatures of social phobia in both adolescents and adults. Two studies have reported enhanced amygdala response to neutral expressions in adult social phobia, emphasizing the importance of contrasting response to emotional and neutral faces (3, 8). However, four other studies (4, 6, 9, 11), like the present study, did not, suggesting that social phobia is not generally associated with heightened responsiveness to face stimuli. In contrast, hyperresponsiveness to angry expressions has been observed with more consistency (4, 6, 7, 9, 13). Finally, only two previous studies of social phobia (4, 11) used fearful faces, the stimulus class most consistently shown to engage the amygdala in healthy adolescents and adults (31), and these studies generated inconsistent findings. However, amygdala hyperresponsiveness to fearful stimuli was observed in the present study in both adults and adolescents with social phobia. More research in both adult and adolescent social phobia is needed to evaluate the degree to which these findings extend to other emotions, such as high-arousal, positive-valence happy faces.
From a developmental perspective, two important features emerge with our data. First, there were significant main effects of age within many regions, where main effects of age, rather than age-by-emotion or age-by-group-by-emotion interactions, emerged. This is consistent with previous imaging (22) and behavioral data (38). Second, regions related to age did not overlap with regions implicated in social phobia. This suggests that the pathophysiology in social phobia operates in adolescence in a relatively functionally independent manner from developmental aspects of face processing.
We observed that the increase in neural responses to angry and fearful expressions in the anterior cingulate cortex, although not in the amygdala, was related to symptom severity as indexed by the Liebowitz Social Anxiety Scale in the adult patients with social phobia. With respect to the adult data, this is consistent with the only previous study to examine the relationship between symptom severity and anterior cingulate cortex responsiveness (5). This did not manifest in adolescent social phobia. This negative finding could either reflect developmental differences in brain-behavior relationships or differing standards for assessing adult and adolescent anxiety. Whereas the Liebowitz Social Anxiety Scale is the accepted standard symptom severity scale for the adults, the Pediatric Anxiety Rating Scale is the clinician-rated scale used in most previous imaging and treatment outcome studies for adolescents (27, 39, 40).
Three caveats should be noted. First, while comparable to other studies involving clinical populations, our sample size for the adolescent groups (N=14 and N=16) was relatively small, which could limit power on tests of interactions with age. Thus, it is possible that a larger sample size would reveal developmental changes to facial expression processing in social phobia not revealed in this study. Second, while the focus of this study was between-group differences, it is worth noting that the healthy comparison subjects did not show an increase in amygdala response to the emotional stimuli in the region of activation identified by our group-by-emotion interaction. However, secondary analyses conducted only on the healthy individuals' data demonstrated the expected increased BOLD responses in other portions of the amygdala to fearful and angry expressions, relative to neutral expressions, affirming the paradigm's suitability for indexing the emotional response in healthy cognition. Third, as noted above, our findings are preliminary because we used a cross-sectional comparison augmented by retrospective data in adults. Social phobia typically begins by adolescence, and the information on onset provided by the adult patients in our study showed that symptoms had uniformly begun by adolescence. However, using such a retrospective approach, it is not possible to precisely and accurately date in these adults the developmental point during which full diagnostic criteria for social phobia were met. Future longitudinal studies will be important in providing definite answers concerning relationships among adolescent social phobia onset, brain function, and symptomatic expressions of social phobia in adulthood.
We employed basic social stimuli that are associated with a relatively automatic emotional response and focused on a narrow, specific stimuli class: facial expressions. Work with adult social phobia has shown that the disorder is associated not only with heightened responsiveness to the more automatic social stimuli but also with heightened medial prefrontal cortex responsiveness to “constructed” social stimuli, such as self-referential social threats and embarrassing situations (14, 15, 17). However, no study has examined whether adolescents with social phobia show anomalously increased medial prefrontal cortex responses to self-referential social threats. If this increased medial prefrontal cortex sensitivity to self-referential social threats is a primary component of the pathology of social phobia, we predict that it will also be seen in adolescents with social phobia. Alternatively, it could be a secondary developmental consequence of the amygdala/anterior cingulate cortex hyperresponsiveness seen in social phobia. In this case, medial prefrontal cortex hyperresponsiveness to self-referential information could be reduced or absent in adolescent social phobia. Future work will need to examine, within a developmental perspective, the relative neural response to more complicated social stimuli, such as self-referential praise and criticism, to determine whether the areas implicated in the processing of such constructed stimuli are also developmentally stable.
1. : The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch Gen Psychiatry 1998; 55:56–64Crossref, Medline, Google Scholar
2. : What do childhood anxiety disorders predict? J Child Psychol Psychiatry 2007; 48:1174–1183Crossref, Medline, Google Scholar
3. : Amygdala activation in the processing of neutral faces in social anxiety disorder: is neutral really neutral? Psychiatry Res 2006; 148:55–59Crossref, Medline, Google Scholar
4. : Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch Gen Psychiatry 2002; 59:1027–1034Crossref, Medline, Google Scholar
5. : Increased activation of the anterior cingulate cortex during processing of disgust faces in individuals with social phobia. Biol Psychiatry 2005; 57:975–981Crossref, Medline, Google Scholar
6. : Common and distinct brain activation to threat and safety signals in social phobia. Neuropsychobiology 2005; 52:163–168Crossref, Medline, Google Scholar
7. : Effect of task conditions on brain responses to threatening faces in social phobics: an event-related functional magnetic resonance imaging study. Biol Psychiatry 2004; 56:921–930Crossref, Medline, Google Scholar
8. : fMRI reveals amygdala activation to human faces in social phobics. Neuroreport 1998; 9:1223–1226Crossref, Medline, Google Scholar
9. : Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biol Psychiatry 2006; 59:424–429Crossref, Medline, Google Scholar
10. : Amygdala reactivity to emotional faces at high and low intensity in generalized social phobia: a 4-Tesla functional MRI study. Psychiatry Res 2007; 154:93–98Crossref, Medline, Google Scholar
11. : Response to emotional expressions in generalized social phobia and generalized anxiety disorder: evidence for separate disorders. Am J Psychiatry 2008; 165:1193–1202Link, Google Scholar
12. : Differential modulation of neural activity throughout the distributed neural system for face perception in patients with social phobia and healthy subjects. Brain Res Bull 2008; 77:286–292Crossref, Medline, Google Scholar
13. : A functional MRI study of amygdala responses to angry schematic faces in social anxiety disorder. Depress Anxiety 2008; 25:496–505Crossref, Medline, Google Scholar
14. : Social norm processing in adult social phobia: atypically increased ventromedial frontal cortex responsiveness to unintentional (embarrassing) transgressions. Am J Psychiatry 2010; 167:1526–1532Link, Google Scholar
15. : Neural response to self- and other referential praise and criticism in generalized social phobia. Arch Gen Psychiatry 2008; 65:1176–1184Crossref, Medline, Google Scholar
16. : Neural mechanisms of cognitive reappraisal of negative self-beliefs in social anxiety disorder. Biol Psychiatry 2009; 66:1091–1099Crossref, Medline, Google Scholar
17. : Atypically reduced modulation of medial prefrontal cortex to self-referential comments in generalized social phobia. Psychiatry Research: Neuroimaging (in press)Google Scholar
18. : Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. Am J Psychiatry 2006; 163:1091–1097Link, Google Scholar
19. : Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Arch Gen Psychiatry 2007; 64:97–106Crossref, Medline, Google Scholar
20. : Amygdala and ventromedial prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry 2008; 65:568–576Crossref, Medline, Google Scholar
21. : Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Arch Gen Psychiatry 2008; 65:1303–1312Crossref, Medline, Google Scholar
22. : A developmental examination of amygdala response to facial expressions. J Cogn Neurosci 2008; 20:1565–1582Crossref, Medline, Google Scholar
23. : Structured Clinical Interview for DSM-IV Axis I Disorders (SCID). Washington, DC, American Psychiatric Press, 1995Google Scholar
24. : Social phobia. Mod Probl Pharmacopsychiatry 1987; 22:141–173Crossref, Medline, Google Scholar
25. : Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Arch Gen Psychiatry 2009; 66:275–285Crossref, Medline, Google Scholar
26. : Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 1997; 36:980–988Crossref, Medline, Google Scholar
27. The Pediatric Anxiety Rating Scale (PARS): development and psychometric properties. J Am Acad Child Adolesc Psychiatry 2002; 41:1061–1069Crossref, Medline, Google Scholar
28. : Pictures of Facial Affect. Palo Alto, Calif, Consulting Psychologists Press, 1976Google Scholar
29. : Dissociable neural responses to facial expressions of sadness and anger. Brain 1999; 122:883–893Crossref, Medline, Google Scholar
30. : A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion 2001; 1:70–83Crossref, Medline, Google Scholar
31. : Functional neuroanatomy of emotions: a meta-analysis. Cogn Affect Behav Neurosci 2003; 3:207–233Crossref, Medline, Google Scholar
32. : Explicit and implicit neural mechanisms for processing of social information from facial expressions: a functional magnetic resonance imaging study. Hum Brain Mapp 2000; 9:93–105Crossref, Medline, Google Scholar
33. : Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. Am J Psychiatry 2008; 165:712–720Link, Google Scholar
34. : An fMRI study investigating cognitive modulation of brain regions associated with emotional processing of visual stimuli. Neuropsychologia 2003; 41:585–596Crossref, Medline, Google Scholar
35. : AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996; 29:162–173Crossref, Medline, Google Scholar
36. : Co-Planar Stereotaxic Atlas of the Human Brain. Stuttgart, Germany, Thieme Medical Publishers, 1988Google Scholar
37. : Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 2007; 164:1476–1488Link, Google Scholar
38. : Development of emotional facial recognition in late childhood and adolescence. Dev Sci 2007; 10:547–558Crossref, Medline, Google Scholar
39.
40. : Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med 2008; 359:2753–2766Crossref, Medline, Google Scholar



