Common Abnormalities and Disorder-Specific Compensation During Implicit Regulation of Emotional Processing in Generalized Anxiety and Major Depressive Disorders
Abstract
Objective:
Anxiety and depressive disorders are both associated with abnormalities in the processing and regulation of emotion. However, little is known about the similarities and differences between anxiety and depression at the neural level. The authors examined emotional conflict processing using a salient stimulus associated with observable and interpretable behavioral outcomes and with activation in limbic and prefrontal regions implicated in anxiety and depression.
Method:
Thirty-two healthy comparison subjects, 18 patients with generalized anxiety disorder only, 14 patients with major depression only, and 25 patients with comorbid generalized anxiety disorder and major depression were studied using functional MRI while they performed an emotional conflict task that involved categorizing facial affect while ignoring overlaid affect label words. The authors used behavioral and neural measures to compare trial-by-trial changes in conflict regulation, a test of implicit regulation of emotional processing.
Results:
Behavioral data indicated that only patients with generalized anxiety (i.e., the anxiety-only and comorbid groups) failed to implicitly regulate emotional conflict. By contrast, deficits in activation and connectivity of the ventral anterior cingulate and amygdala, areas previously implicated in regulating emotional conflict, were found in all patient groups. Depression-only patients, however, compensated for this deficit by also activating the left and right anterior lateral prefrontal cortices, in which activity was correlated with behavioral evidence of successful implicit regulation, thus mediating the disorder-specificity of the behavioral phenotype.
Conclusions:
These data support the existence of a common abnormality in anxiety and depression in the ventral cingulate and the amygdala, which may be related to a shared genetic etiology. Compensatory engagement of cognitive control circuitry in depression illustrates how the complex nature of psychopathology arises from the interaction of deficits and compensation, all of which can occur at an implicit level.
Generalized anxiety and major depressive disorders are highly prevalent and disabling emotional disorders and are frequently comorbid with each other. Their relationship and diagnostic integrity have been the subject of considerable debate (1–5). On the one hand, structural analyses of comorbidity or symptomatology have pointed to anxiety and depression originating from a higher-order common “anxious/misery” factor (6, 7), which is related to the construct of neuroticism (8). Genetic risk for these disorders has also been shown to be similar or identical (9, 10). We refer to this as the “common-disorder” model of anxiety and depression. On the other hand, analyses of symptomatology also support the existence of anxiety- and depression-specific phenotypes (8, 11), and differences exist in the relationship of anxiety and depression to specific stressful life events or triggering events (12, 13). Behavioral studies have provided evidence for automatic attentional biases for threat in anxiety and mood-congruent memory biases in depression, although such studies have rarely compared these patient groups with each other to demonstrate diagnostic specificity (14, 15). We refer to this construct as the “independent-factor” model, in which anxiety and depression reflect separate processes. However, these findings come from complex and multidetermined measures, often lack mechanistic specificity, and are in general causally distal to the abnormalities in emotional processing that are thought to be at the core of anxiety and depressive disorders.
Neurobiological investigations of emotional processing in anxiety and depression also have yet to provide clarity regarding similarities and differences. First, almost all neuroimaging studies contrast a single patient group with healthy comparison subjects, making it difficult to compare between disorders. Second, because most neuroimaging studies either lack behavioral outcome measures or find no differences in behavior, changes in brain activation may reflect core deficits, compensation, or both. Third, anxiety and depression both involve abnormalities in core limbic regions, such as the amygdala, as well as prefrontal regulatory regions, such as the ventral anterior cingulate (16, 17). Thus, while no canonical anxiety- or depression-related neural abnormalities have yet emerged in the literature, these design issues preclude even conclusions about commonalities between the disorders.
Understanding the nature of anxiety and depression therefore represents a paradigmatic example of the opportunities and challenges currently facing psychiatry. Such an understanding could provide insight into vulnerability and resilience factors, related in part to the heritability of these disorders; help shed light on the nosological relationships between mental illnesses; and inform treatment. Anxiety in the context of depression, for example, has been shown to predict worse response to antidepressants in general (18), as well as in the context of specific genetic polymorphisms (19). Yet, unless the experimental design issues mentioned above are dealt with, inferential difficulties will remain. To do so, we turned to an examination of emotional conflict processing, using a salient stimulus associated with observable and interpretable behavioral outcomes and with activation in limbic and prefrontal regions implicated in anxiety and depression (20).
We recently described an emotional conflict task in which study subjects identify the expression of fearful or happy faces while ignoring the overlaid words “fear” or “happy” (21–23). In this task, emotional conflict generated reaction time interference, which was lower when an emotionally incongruent trial followed an incongruent trial than when it followed a congruent trial—an effect termed “emotional conflict adaptation” (21, 22, 24–27) (see Figure S1 in the data supplement that accompanies the online edition of this article). This trial-to-trial adaptation to emotional conflict reflects the operation of an emotional processing regulatory mechanism, activated by previous trial conflict, that improves performance on the current incongruent trial (22, 28–30) and occurs at an implicit (nonconscious) level (23). Unlike healthy subjects, patients with generalized anxiety disorder failed to adapt to emotional conflict (23).
Neuroimaging data in this paradigm were analyzed primarily by comparing the faster postincongruent incongruent trials to the slower postcongruent incongruent trials, drawing on the “conflict monitoring hypothesis,” which is the cognitive model that best accounts for the conflict adaptation effect (24–32). This model distinguishes between neural activity related to the evaluation of conflict, which is higher when conflict is greater (i.e., postcongruent incongruent trials > postincongruent incongruent trials), and dissociates it from neural activity related to the regulation of conflict. This latter function is associated with regions whose activity increases in trials when conflict is minimized through regulation (i.e., postincongruent incongruent trials > postcongruent incongruent trials). We previously examined evaluation- and regulation-related activation in both the emotional conflict task and a complementary nonemotional conflict task (gender identification of the same faces while ignoring gender label words). Regulation of emotional conflict was specifically associated with activation of the pregenual/ventral cingulate and dampening of amygdala reactivity through connectivity with the cingulate (20–22). By contrast, regulation of nonemotional conflict was associated with dorsolateral prefrontal activation and modulation of target-specific processing in the ventral visual cortex (21). Notably, patients with generalized anxiety disorder failed to activate the ventral cingulate and dampen amygdala activity, consistent with their behavioral failure to adapt to emotional conflict (23).
In this article, we extend our previous findings in generalized anxiety disorder, focusing primarily on major depression and its comparison with anxiety by reporting on emotional conflict task data from two additional groups of subjects—those with depression and no generalized anxiety disorder, and those with comorbid anxiety and depression. Specifically, we sought to determine whether results from the emotional conflict task support common-disorder or independent-factor conceptualizations of anxiety and depression, whether the results would produce evidence for behaviorally relevant dissociability of these disorders, and how differences in brain activation during implicit regulation of emotional processing in this task underlie differences in behavior.
Method
Participants
The study included 89 participants, all of whom provided informed consent. Current-episode DSM-IV-based psychiatric diagnoses (33) were determined through both an informal clinical interview and the Mini International Neuropsychiatric Interview (34, 35). All screening and diagnosis of participants were carried out by a single rater (A.E.) applying consistent diagnostic criteria for differentiating among the four groups examined. Exclusion criteria were bipolar, psychotic, substance use, or posttraumatic stress disorders; a history of a neurological disorder, head trauma, or loss of consciousness; claustrophobia; or regular use of benzodiazepines, opioids, or thyroid medications. No patient was taking regular psychiatric medications, and patients who used benzodiazepines on an as-needed basis did not take any within 48 hours of the scan. The anxiety-only (N=18) and depression-only (N=14) groups consisted of patients in which generalized anxiety disorder or major depressive disorder, respectively, was the primary diagnosis without comorbidity with the other; the “comorbid” group (N=25) consisted of patients with comorbid generalized anxiety disorder and major depression. Other disorders are noted in footnotes to Table 1. All healthy comparison subjects (N=32) had no current or past axis I disorders and took no psychiatric medications. All participants completed the questionnaires listed in Table 1 (36–41). Emotional conflict data on 24 of the healthy comparison subjects and 17 of the anxiety-only patients were reported in our previous study (23).
| p Values | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Comparison Group (N=32) | Anxiety-Only Groupa (N=18) | Depression-Only Groupb (N=14) | Comorbid Groupc (N=25) | Common-Disorder Model | Independent-Factor Model | Anxiety-Only Compared With Depression-Only Group | Anxiety-Only Compared With Comorbid Group | Depression-Only Compared With Comorbid Group | |||||
| N | % | N | % | N | % | N | % | ||||||
| Female | 23 | 72 | 11 | 61 | 10 | 71 | 17 | 68 | n.s. | n.s. | n.s. | n.s. | n.s. |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||||
| Age (years) | 35.6 | 11.1 | 31.3 | 9.5 | 32.2 | 11.7 | 33 | 10 | n.s. | n.s. | n.s. | n.s. | n.s. |
| Education (years) | 17 | 2 | 16.5 | 2.2 | 15.4 | 2.1 | 16.2 | 2.5 | n.s. | n.s. | n.s. | n.s. | n.s. |
| State-Trait Anxiety Inventory (36), trait anxiety score | 30.4 | 6.1 | 51.9 | 8.1 | 58 | 11.2 | 62.8 | 8.7 | <0.001 | <0.001d | n.s. | <0.001 | n.s. |
| Penn State Worry Questionnaire score (37) | 32.9 | 8.9 | 61.4 | 8.6 | 53.5 | 10.9 | 61.2 | 11.5 | <0.001 | <0.001d | <0.05 | n.s. | <0.05 |
| Beck Anxiety Inventory score (38) | 3.6 | 3.7 | 22.7 | 11.7 | 14.4 | 6.6 | 26 | 12 | <0.001 | <0.001e | <0.05 | n.s. | <0.001 |
| Beck Depression Inventory score (39) | 3.1 | 3.3 | 14.6 | 8.9 | 27.6 | 8.7 | 32 | 8.2 | <0.001 | <0.001d | <0.001 | <0.001 | n.s. |
| Mood and Anxiety Symptom Questionnaire (40, 41) | |||||||||||||
| Anxious arousal subscore | 18.1 | 1.7 | 25.9 | 7.4 | 23.2 | 3.5 | 33 | 11.6 | <0.001 | <0.001e | n.s. | <0.05 | <0.001 |
| Anhedonic depression subscore | 48.1 | 10 | 69.4 | 12.4 | 87.1 | 11.1 | 88 | 7.7 | <0.001 | <0.001d | <0.001 | <0.001 | n.s. |
TABLE 1. Demographic Characteristics and Clinical Measures for Healthy Comparison Subjects and Patients With Generalized Anxiety Disorder Only, Major Depression Only, or Comorbid Anxiety and Depression
Experimental Paradigm
The emotional conflict task was performed as previously described (21–23). It consisted of 148 presentations of photographs of happy or fearful facial expressions (42), overlaid with the words “FEAR” or “HAPPY.” Stimuli were presented for 1,000 msec, with a varying interstimulus interval of 3,000–5,000 msec (mean=4,000 msec), in a pseudorandom order, counterbalanced across trial types for expression, word, response button, and gender. Participants indicated facial affect with a button-press response.
Functional MRI Data Acquisition
Images were acquired on a 3-T GE Signa scanner using a custom-built head coil. Twenty-nine axial slices (4.0 mm thickness with 0.5 mm gap) were acquired across the whole brain using a T2*-weighted gradient echo spiral pulse sequence (repetition time=2,000 msec, echo time=30 msec, flip angle=80°, 1 interleaf, field of view=22 cm, 64×64 matrix) (43). A high-resolution T1-weighted three-dimensional inversion recovery spoiled gradient-recalled acquisition in the steady state MRI sequence was used with the following parameters: inversion time=300 msec, repetition time=8 msec, echo time=3.6 msec, flip angle=15°, field of view=22 cm; 124 slices in coronal plane; matrix=256×192; number of excitations=2; acquired resolution=1.5×0.9×1.1 mm.
Data Analysis
Functional MRI data were preprocessed using the SPM5 software package (http://www.fil.ion.ucl.ac.uk/spm) implemented in MATLAB (MathWorks, Inc., Natick, Mass.). Images were realigned to correct for motion and were slice timing-corrected, spatially transformed to the Montreal Neurological Institute coordinate system (44), resampled every 2 mm, and smoothed with a 6-mm full-width half-maximum Gaussian kernel. During preprocessing, the effects of global signal were also removed separately for each voxel (45). Separate regressors for the stimulus events (convolved with a canonical hemodynamic response function) were created for postcongruent incongruent trials, postincongruent incongruent trials, postcongruent congruent trials, and postincongruent congruent trials, with error and posterror trials modeled separately. Additional regressors of no interest corresponding to the six motion parameters were also included.
Results from first-level models (46) were submitted to group-level random-effects analyses. Groups were modeled in a 2×2 analysis of variance (ANOVA) using the generalized linear model, with two across-subject factors corresponding to presence of the diagnoses of generalized anxiety disorder or major depressive disorder (columns in the design matrix representing healthy, depression-only, anxiety-only, and comorbid groups). To test the independent-factor model, we created contrasts reflecting the effects of anxiety (anxiety-only and comorbid > healthy and depression-only; [–1, –1, 1, 1]), or depression (depression-only and comorbid > healthy and anxiety-only; [–1, 1, –1, 1]). The same group analysis was used to test the common-disorder model, in which we contrasted healthy comparison subjects with all patient groups ([1, –1/3, –1/3, –1/3]). Behavioral and extracted brain activation data were analyzed in a similar fashion using SPSS (SPSS, Inc., Chicago). We chose not to analyze all groups within a single four-level group factor, as the variance associated with diagnoses for the comorbid group overlaps with that in the anxiety-only and depression-only groups (and hence is not well described in a four-level group factor). Moreover, participant selection across the four groups was explicitly made with respect to the two main effect factors, and each participant could be uniquely identified by a combination of these factors.
Finally, for the analysis of adaptation, we analyzed effects of previous trial separately for current incongruent and congruent trials, because 1) processing during congruent, but not incongruent, trials is potentially and variably confounded by participants switching from labeling faces to labeling words; 2) behavioral work in the laboratory manipulating conflict adaptation has found dissociable effects on incongruent versus congruent trial adaptation (unpublished observations); 3) brain activation is different between these forms of adaptation (unpublished observations); and 4) we previously found deficits in generalized anxiety disorder selectively in incongruent trial adaptation (23).
For the psychophysiological interaction analyses (47), we extracted, for each subject, a deconvolved time course from the ventral cingulate and amygdala clusters defined by the group-level regulation-related postincongruent incongruent trial > postcongruent incongruent trial contrast and the evaluation-related postcongruent incongruent trial > postincongruent incongruent trial contrast, respectively, in the healthy comparison group. Activity in the amygdala was then regressed against the product of the ventral cingulate time course and the vector of the psychological variable of interest, with the physiological and the psychological variables serving as regressors of no interest, along with the six motion parameters. The results were then analyzed using ANOVAs in SPSS as above.
Small-volume corrections were conducted for the ventral cingulate and amygdala regions of interest, which were specified a priori (48) (p<0.05, family-wise error-corrected) using anatomically defined masks. The ventral cingulate region of interest was drawn along the contours defined by a recent diffusion tensor imaging connectivity parcellation of the cingulate (49), thus significantly expanding on our previous cingulate mask (23) to include the entire ventral cingulate (21,320 mm3). The amygdala region of interest corresponded to the left and right amygdala in the WFU (Wake Forest University) PickAtlas (left: 1,264 mm3; right: 1,288 mm3) (50). Results are displayed within these regions of interest only. For the correlation of reaction times with brain activation in the depression-only group, we applied a whole-brain correction for the false discovery rate (q<0.05).
Results
Behavior
Our patient and healthy comparison groups were well matched for age, gender, handedness (all participants were right-handed), and education level (Table 1). As outlined above, we analyzed our data in two parallel ways, either with depression and anxiety as independent and interacting factors (independent-factor model) or with anxiety and depression diagnoses combined into a single “patient” factor (common-disorder model). Overall accuracies were not significantly different using either model (comparison group=94.2% [SD=4.7]; anxiety-only group=93.5% [SD=5.6]; depression-only group=95.9% [SD=3.4]; comorbid group=93.8% [4.6]; all patients=94.2% [SD 4.7]). Average reaction times showed no significant effect of anxiety or depression in the independent-factor model but did show an overall effect of patient status in the common-disorder model; comparison group=766 msec [SD=106]; anxiety-only group=865 msec [SD=235]; depression-only group=900 msec [SD=226]; comorbid group=861 msec [SD=229]; all patients=872 msec [SD=227]; F=6.4, df=1, 85, p<0.05 [partial eta-squared=0.07]).
Emotional conflict, as expected, induced a slowdown in reaction time in all groups (Figure 1A). There were no significant differences in this slowdown as a function of anxiety, depression, or patient status. By contrast, there was a significant deficit in emotional conflict adaptation during incongruent trials, both as a function of anxiety in the independent-factor model (anxiety-only and comorbid groups compared with depression-only and healthy comparison groups; F=8.1, df=1, 85, p<0.01; partial eta-squared=0.087) and as an effect of patient status in the common-disorder model (F=4.8, df=1, 85, p<0.05; partial eta-squared=0.053; see Figure 1B; see also Figure S2 in the online data supplement). The effect of anxiety, but not patient status, remained significant even after controlling for individuals' average reaction times or individuals' scores on scales of anxiety, depression, or worry (p<0.01), indicating a categorical effect of diagnosis rather than a dimensional effect of anxiety or depression.
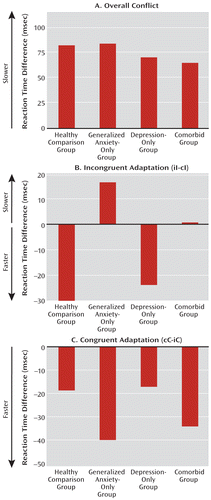
FIGURE 1. Adaptation to Emotional Conflict in an Emotional Conflict Task in Patient and Healthy Comparison Groupsa
a In panel A, reaction time difference scores reflect the overall effect of emotional conflict (incongruent minus congruent trials), showing no difference between groups. Panel B shows facilitation in reaction times during emotional conflict adaptation (postincongruent incongruent trials [iI] faster than postcongruent incongruent trials [cI], resulting in negative reaction time difference scores), showing a deficit as a function of anxiety in the independent-factor model (i.e., in the generalized anxiety disorder-only and comorbid groups). Panel C indicates adaptation on congruent trials (postcongruent congruent trials [cC] faster than postincongruent congruent trials [iC]), showing no group differences. These results show that anxiety is associated with a failure to adapt to emotional conflict.
Furthermore, consistent with the independent-factor model but not the common-disorder model, adaptation during incongruent trials was significantly different between the depression-only group and the combination of the anxiety-only and comorbid groups (F=4.3, df=1, 55, p<0.05; partial eta-squared=0.073). Notably, this effect remained significant after controlling for either average reaction times or individuals' scores on scales of anxiety, depression, or worry (p<0.05). Finally, there were no significant group effects in either the independent-factor or the common-disorder model on adaptation during congruent trials (see Figure 1C), in which reaction times were faster for postcongruent congruent trials than for postincongruent congruent trials. Moreover, all individual groups' one-sample t tests were significant for this measure, suggesting that all the groups showed adaptation during congruent trials, which indicates the specificity of the finding of deficits in adaptation during incongruent trials.
Ventral Cingulate-Amygdala Abnormalities Across Anxiety and Depression
Next, we examined brain activation in the critical contrast examining emotional conflict adaptation during incongruent trials (postincongruent incongruent trials minus postcongruent incongruent trials), focusing on our a priori regions of interest in the ventral cingulate and amygdala. As discussed above, adaptation to emotional conflict is associated with increased activity in the ventral cingulate (postincongruent incongruent trials > postcongruent incongruent trials) and decreased activation in the amygdala (postincongruent incongruent trials < postcongruent incongruent trials)—functions attributed to regulation and evaluation of emotional conflict, respectively (21–23). In both regions, we found significant small volume-corrected group differences as a function of patient status in the common-disorder model and no significant effects of anxiety or depression in the independent-factor model (Figure 2).
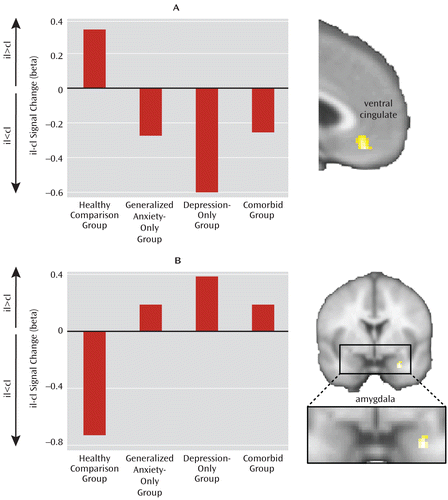
FIGURE 2. Brain Activation in the Ventral Cingulate and the Amygdala in the Contrast Examining Emotional Conflict Adaptation During Incongruent Trials in an Emotional Conflict Task in Patient and Healthy Comparison Groupsa
a Healthy comparison group > all patients contrast for the postincongruent incongruent trial (iI) minus postcongruent incongruent trial (cI) difference within the regions of interest in the ventral cingulate (panel A) and the amygdala (panel B); bar graphs represent each group's data for this contrast extracted for the cluster shown. These results illustrate the inability of all patient groups to activate the ventral cingulate and dampen amygdala activity during emotional conflict adaptation.
The group difference cluster in the ventral cingulate (x=–10, y=28, z=–2; z score=4.09; and x=–4, y=40, z=–16; z score=3.81; 1,008 mm3; partial eta-squared=0.123; see mean cluster signal change for each group in Figure 2A) was driven by a significant conflict regulation-related activity increase in the healthy comparison group (t=3.15, df=31, p<0.005; d=0.55) and the opposite effect in patients (t=2.59, df=56, p=0.01; d=0.34). The group difference cluster in the amygdala (x=28, y=0, z=–28; z score=3.25; 160 mm3; partial eta-squared=0.092; see cluster means in Figure 2B), as expected, involved a significant decrease in signal during adaptation in the healthy comparison group (t=2.19, df=31, p<0.05; d=0.39), whereas in patients no difference was observed. Further breakdown by individual trial types for both the ventral cingulate and amygdala group difference clusters is provided in Figure S3 in the online data supplement. No group differences were observed in either the ventral cingulate or the amygdala for the contrast of postcongruent congruent trials minus postincongruent congruent trials, or when combining across all trial types, indicating that the group differences during adaptation to emotional conflict did not simply reflect generic consequences of reaction time speedup or task-independent deactivations, respectively. The observed group differences for brain activation and behavior were also unaltered when participants with comorbid obsessive-compulsive disorder were excluded or when patients with comorbid dysthymia in the anxiety-only group were excluded (data not shown).
Next, we examined functional connectivity between the ventral cingulate and the amygdala using psychophysiological interaction analyses. We found a blunting of the normally negative functional connectivity between these regions across all patients in the common-disorder model (F=4.4, df=1, 85, p<0.05; partial eta-squared=0.049; see Figure 3A). Finally, within the entire patient cohort, we correlated mean incongruent trial adaptation signal for the ventral cingulate and amygdala clusters and found that they were strongly negatively correlated (r=–0.52, p<0.001; see Figure 3B), which is consistent with a negative regulatory relationship between these regions in patients, even in the context of an overall deficit in their activation. Robust regression confirmed that this relationship was independent of outliers (p<0.0005). In summary, these data demonstrate a broad deficit in cingulate-amygdala activation and connectivity during adaptation in all patient groups, which is consistent with the behavioral adaptation deficits in the anxiety-only and comorbid groups but does not account for the adaptation seen in the depression-only group.
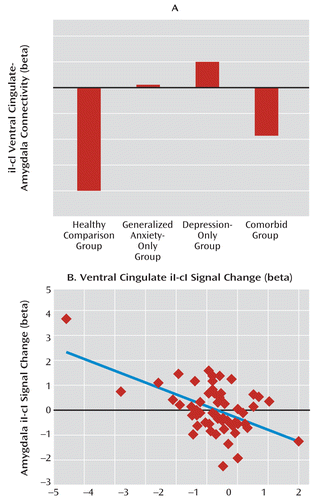
FIGURE 3. Ventral Cingulate-Amygdala Functional Connectivity and Activation Correlations in Patient and Healthy Comparison Groupsa
a In panel A, the normally negative postincongruent incongruent trial (iI) minus postcongruent incongruent trial (cI) functional connectivity between the ventral cingulate and amygdala using psychophysiological interactions is blunted across all patient groups, relative to healthy comparison subjects. Panel B shows a negative correlation between iI-cI activation differences in the ventral cingulate and the amygdala, indicating that greater ventral cingulate activity was associated with less amygdala activity even in the context of overall activation abnormalities.
Compensatory Recruitment of Lateral Anterior Prefrontal Regions in Depression
To identify brain regions that might account for the ability of depressed patients to adapt to emotional conflict, we correlated individuals' reaction time difference scores during incongruent trial adaptation (postincongruent incongruent trials minus postcongruent incongruent trials) with brain activation in the same contrast within the depression-only group. Figure 4 presents results after whole-brain voxelwise correction for multiple comparisons using the false discovery rate (q<0.05). We found that better incongruent trial conflict adaptation (more negative reaction time difference scores) was associated with progressively less activation in the ventral cingulate (i.e., positive correlation) as well as a greater failure to dampen amygdala activation (i.e., negative correlation; see arrows in Figure 4A). Thus, although participants were better able to adapt to emotional conflict, this was associated with a more dysfunctional pattern of activation in the regions associated with emotional conflict adaptation in healthy individuals. By contrast, in the combined anxiety-only and comorbid groups, using a small-volume correction for the amygdala, we found the opposite (and predicted) brain-behavior relationship, in which better adaptation was associated with greater dampening of the amygdala (x=18, y=2, z=–16; z score=3.53; 128 mm3), indicating that the depression-only group is able to adapt by activating a different neural system.
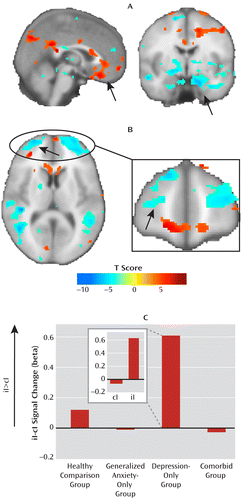
FIGURE 4. Correlation Between Postincongruent Incongruent Trial Minus Postcongruent Incongruent Trial Reaction Time Difference Scores and Brain Activation for the Same Contrasta
a Brain activation is displayed with whole-brain correction for the false discovery rate (q<0.05). In panel A, positive correlations in the ventral cingulate and negative correlations in the amygdala (arrows) suggest a greater deficit in these regions when depression-only patients show better reaction time adaptation. In panel B, negative correlations in the anterior lateral prefrontal cortex suggest regulation-related recruitment of this region with improved adaptation. For reaction time difference scores, more negative indicates more adaptation. Panel C (iI=postincongruent incongruent trial; cI=postcongruent incongruent trial) illustrates activity for the left anterior middle frontal gyrus cluster (arrows in panel B) extracted for the iI-cI contrast for each group, as well as separately for the iI and cI trials (see inset) for the depression-only group; this cluster is activated only in the depression-only group, and this is driven by increased activity in iI trials. The figure shows that engagement of compensatory activation in the anterior lateral prefrontal cortices in the depression-only group is associated with successful adaptation to emotional conflict in this group.
We found three clusters in the frontal lobe, however, in which greater activation in the depression-only group was associated with improved conflict adaptation (i.e., negative correlation; see Figure 4B). These clusters were located in the left superior (x=–22, y=44, z=44; z score=4.89; 3,776 mm3) and middle (x=–22, y=48, z=12; z score=4.83; 3,352 mm3) frontal gyri and in the right middle frontal gyrus (x=30, y=62, z=14; z score=4.45; 8,080 mm3). These correlations were verified to be independent of outliers and remained similarly significant using robust regression (p values <0.001).
We next compared mean activation in each of these clusters (in the postincongruent incongruent trials minus postcongruent incongruent trials contrast) between the depression-only group and the combination of the anxiety-only and comorbid groups, which failed to adapt to emotional conflict. Of the three frontal clusters, only activation in the left anterior middle frontal gyrus significantly differed between the groups (F=7.08, df=1, 55, p=0.01; partial eta-squared=0.114; see Figure 4C), and this difference remained significant after controlling for individuals' scores on scales of anxiety, depression, or worry (p values <0.005). The inset in Figure 4C shows the breakdown by trial types of activity in this cluster for the depression-only group; the breakdown for the other groups is shown in Figure S4 in the online data supplement. Finally, we conducted a mediation analysis to determine whether activation in the left anterior middle gyrus cluster statistically mediated the relationship between group (depression-only group versus anxiety-only and comorbid groups) and ability to adapt to emotional conflict (reaction time difference scores). A significant mediation relationship existed for this cluster (with a=predictor-mediator path, b=mediator-criterion variable path, ab=mediation effect, the following values were obtained: a=0.32, p<0.005; b=–28, p<0.001; ab=–8.9, p<0.05), and it remained significant after controlling for individuals' scores on scales of anxiety, depression, or worry (p values <0.05).
Discussion
In this study, we examined implicit regulation of emotional processing in an emotional conflict task in patients with generalized anxiety disorder, major depression, or both in order to test contrasting conceptualizations of these disorders at the behavioral and neural levels. At the behavioral level, we found evidence supporting the independent-factor model, in which anxiety and depression reflect separate and dissociable processes. That is, failure to implicitly regulate emotional conflict, indexed through reaction time adaptation to emotionally incongruent trials, was perturbed in anxiety and not in depression. This effect was specific to incongruent stimuli, as adaptation to congruent stimuli was robust and intact in all groups.
At the neural level, we found a deficit across all patient groups in the circuitry normally associated with emotional conflict adaptation—an increase in ventral cingulate activity and a dampening of amygdala activity during regulation—which supports the common-disorder model. The depression-only group, however, compensated for this deficit by activating the anterior lateral prefrontal cortex. This compensatory activation accounted for the ability of the depression-only group to regulate emotional conflict. The anxiety-specific independent-factor behavioral deficit therefore arose from a common-disorder-related abnormality in the normally used ventral cingulate-amygdala circuitry, together with an engagement of compensatory lateral prefrontal systems only in nonanxious depression. The specificity of this compensation for nonanxious depression is striking and suggests that it may be related to aspects of depression not shared with anxiety (e.g., anhedonia). Nonetheless, since overall depression levels were the same for the depression-only and comorbid groups, the mere presence of features of depression unrelated to anxiety was insufficient to result in compensation when anxiety was also present, pointing to a conditional relationship between features of depression associated with compensation and anxiety.
To date, few functional neuroimaging studies have compared emotional processing in anxiety and depression, and none have done so in adults. One recent study (51), focusing largely on amygdala activation in adolescents in response to emotionally expressive faces under a variety of attentional conditions, found evidence for both the common-disorder and independent-factor models, which were further moderated by the specific attentional demands of the task. A pediatric study (52), using only one attentional condition during fearful face viewing, found divergent amygdala responses in anxiety and depression. As our data illustrate, parallel investigations of multiple diagnostic groups provide essential and novel information regarding the similarities and differences between disorders, which is not possible through extrapolation from published studies on single-disorder cohorts, and which may allow a reconceptualization of mental illness along dimensions determined by neural and behavioral systems rather than complex symptom expression (53).
Our data indicate that even seemingly simple behavioral measures are themselves determined by several simultaneously operating neural processes, suggesting ultimately that an understanding of anxiety and depression must consider these multiple “layers” of abnormalities that together drive a more complex outcome measure, such as symptoms or diagnosis. One of these neural layers is the shared deficit in ventral cingulate-amygdala activation and functional connectivity. In light of the findings that anxiety and depression share similar genetic risk factors (9, 10), which may be an important aspect of their similarity (5), it is noteworthy that several lines of genetic risk investigations also converge on ventral cingulate-amygdala changes. Polymorphisms in the serotonin transporter gene, for example, have been associated with an elevated risk for depression through an interaction with negative life events (54), are related to the general negative affectivity factor of neuroticism (55), and result in differences in amygdala and cingulate activation, structure, and connectivity (56–59). Similar findings have been reported for polymorphisms in other genes involved in serotonin biosynthesis or signaling, as well as other monoaminergic genes, such as the monoamine oxidase A gene, as well as neurotrophins, such as brain-derived neurotrophic factor (59–62). Individuals who are at risk for depression by virtue of a family history of depression also show abnormalities in amygdala and cingulate activation (63, 64). Finally, a recent structural imaging study (65) of patients with depression, a mixture of anxiety disorders, or comorbid depression and anxiety disorders found common-disorder volumetric reductions across all groups in an anterior cingulate region near our ventral cingulate cluster, although the functional consequences of these abnormalities were unknown because of the nature of structural imaging.
Despite this common ventral cingulate-amygdala deficit, the depression-only group was able to compensate, activating the anterior lateral prefrontal cortices and adapting to emotional conflict. A recent neuroimaging meta-analysis (66) found that these prefrontal regions were very likely to coactivate with a canonical fronto-parietal executive control network across a wide range of cognitive tasks. Aberrant engagement of this cognitive control system suggests that depression-only participants were somehow able to adapt by using a different neural strategy. Indeed, the surprising relationship between ventral cingulate-amygdala activation and adaptation in the depression-only group (better adaptation associated with more neural dysfunction) suggests that compensatory circuitry was engaged at the expense of the normal pathway implicated in adaptation, possibly because the two may normally be in competition. This is striking for several reasons. First, it suggests that intact performance in this task can be achieved despite abnormalities in the core circuitry implicated in conflict adaptation and thus that improvements in performance (e.g., as a consequence of treatment) need not necessarily operate by reversing the baseline deficit. Second, although not formally addressed in these patients, we have reported (23) that participants are unaware of the conflict adaptation effect, indicating that it is an implicit process. Thus, the ability of depressed patients to adapt likely reflects operation of an implicit compensatory process (lateral prefrontal activation). These findings also deepen our understanding of implicit emotion regulation and further emphasize the importance of understanding these processes for investigating psychopathology. In parallel with the present study, we also recently reported compensatory coupling of lateral prefrontal regions with the amygdala in patients with generalized anxiety disorder using resting-state imaging (67). This compensation did not appear to affect that group's performance in this task, and thus it may have functional consequences only when participants process or regulate emotion explicitly, akin perhaps to the attention-demanding process of worry.
Together, our findings bring to greater relief the idea that most, if not all, psychiatric disorders, having their origins years before symptom expression, likely reflect a complex interplay between core deficits and compensatory systems. A more comprehensive understanding of anxiety and depression in that light will require testing the same subjects in multiple tasks that systematically probe implicit and explicit emotional processes, include longitudinal designs or samples across the lifespan whenever possible, and examine the influences of treatment, in order to determine what the consequences of core deficits are and to identify the functional consequences of compensation. Doing so may in turn inform our understanding of the etiology of these disorders and the routes for improving their treatment.
Finally, alternative conceptualizations of anxiety and depressive psychopathology have called for use of a dimensional, instead of a categorical, diagnostic approach (40, 41). As noted, all of our key category-based behavioral findings were independent of individual differences in symptom scale scores, which suggests that these commonly used questionnaires do not adequately capture the behavioral dissociation of anxiety and depression in our task, despite the fact that symptom measures can discriminate between groups (Table 1). Moreover, we suggest that dimensional measures of complex symptoms may provide only limited insight into the nature of psychopathology given the complex layering of deficits and compensations at the neural level, as illustrated in our data.
Several limitations of this study are also important to note. First, this was a cross-sectional study, and thus it is impossible to determine whether symptom changes over time may be associated with dynamic changes, for example, in anterior prefrontal activation. This might mean that loss of compensation may result in comorbidity or that enhancements in compensation may lead to lessening of anxiety in the context of depression. Further longitudinal imaging will be required to test this hypothesis. Second, we did not include in this study patients taking medications. While this likely means that we excluded the more severe cases, inclusion of medicated patients would significantly confound brain activation results, as medications for anxiety and depression alter brain activation in precisely the regions under study. Finally, it might be argued that the intact adaptation in the depression-only group is related to these individuals being less ill or the stimuli being more anxiety- than depression-relevant. However, we find these alternative explanations to be unlikely. The depression-only group was more depressed (but less anxious) than the anxiety-only group and had similar scores on the State-Trait Anxiety Inventory, the Beck Depression Inventory, and the anhedonic depression subscale of the Mood and Anxiety Symptom Questionnaire as the comorbid group (Table 1). Moreover, they were able to adapt not by virtue of being able to engage ventral cingulate-amygdala circuitry, but despite a profound deficit in this circuitry. In summary, our results provide an exciting framework for future work investigating implicit emotion regulation in psychopathology and the nature of anxiety and depression.
1. : Is generalized anxiety disorder an anxiety or mood disorder? considering multiple factors as we ponder the fate of GAD. Depress Anxiety 2008; 25:289–299Crossref, Medline, Google Scholar
2. : Rethinking the mood and anxiety disorders: a quantitative hierarchical model for DSM-V. J Abnorm Psychol 2005; 114:522–536Crossref, Medline, Google Scholar
3. : Towards DSM-V: the relationship between generalized anxiety disorder and major depressive episode. Psychol Med 2008; 38:1671–1675Crossref, Medline, Google Scholar
4. : What is an anxiety disorder? Depress Anxiety 2009; 26:1066–1085Crossref, Medline, Google Scholar
5. : The nosologic relationship between generalized anxiety disorder and major depression. Depress Anxiety 2008; 25:300–316Crossref, Medline, Google Scholar
6. : The structure of common mental disorders. Arch Gen Psychiatry 1999; 56:921–926Crossref, Medline, Google Scholar
7. : The structure and stability of common mental disorders: the NEMESIS study. Arch Gen Psychiatry 2001; 58:597–603Crossref, Medline, Google Scholar
8. : Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. J Abnorm Psychol 1991; 100:316–336Crossref, Medline, Google Scholar
9. : Major depression and generalized anxiety disorder: same genes, (partly) different environments? Arch Gen Psychiatry 1992; 49:716–722Crossref, Medline, Google Scholar
10. : A twin study of generalized anxiety disorder and major depression. Psychol Med 1995; 25:1037–1049Crossref, Medline, Google Scholar
11. : Structural relationships among dimensions of the DSM-IV anxiety and mood disorders and dimensions of negative affect, positive affect, and autonomic arousal. J Abnorm Psychol 1998; 107:179–192Crossref, Medline, Google Scholar
12. : Types of stressful life event and the onset of anxiety and depressive disorders. Psychol Med 1981; 11:803–815Crossref, Medline, Google Scholar
13. : Life events and affective disorder: replications and limitations. Psychosom Med 1993; 55:248–259Crossref, Medline, Google Scholar
14. : Biases of attention and memory in disorders of anxiety and depression. Clin Psychol Rev 1990; 10:589–604Crossref, Google Scholar
15. : Attentional bias in generalized anxiety disorder versus depressive disorder. Cognit Ther Res 2005; 29:29–45Crossref, Google Scholar
16. : Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct 2008; 213:93–118Crossref, Medline, Google Scholar
17. : Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 2007; 164:1476–1488Link, Google Scholar
18. : Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry 2008; 165:342–351Link, Google Scholar
19. : Association of polymorphisms in genes regulating the corticotropin-releasing factor system with antidepressant treatment response. Arch Gen Psychiatry 2010; 67:369–379Crossref, Medline, Google Scholar
20. : Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci 2011; 15:85–93Crossref, Medline, Google Scholar
21. : Dissociable neural systems resolve conflict from emotional versus nonemotional distracters. Cereb Cortex 2008; 18:1475–1484Crossref, Medline, Google Scholar
22. : Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron 2006; 51:871–882Crossref, Medline, Google Scholar
23. : Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. Am J Psychiatry 2010; 167:545–554Link, Google Scholar
24. : Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature 1999; 402:179–181Crossref, Medline, Google Scholar
25. : Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nat Neurosci 2005; 8:1784–1790Crossref, Medline, Google Scholar
26. : Optimizing the use of information: strategic control of activation of responses. J Exp Psychol Gen 1992; 121:480–506Crossref, Medline, Google Scholar
27. : Anterior cingulate conflict monitoring and adjustments in control. Science 2004; 303:1023–1026Crossref, Medline, Google Scholar
28. : Conflict monitoring and cognitive control. Psychol Rev 2001; 108:624–652Crossref, Medline, Google Scholar
29. : Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci 2004; 8:539–546Crossref, Medline, Google Scholar
30. : Conflict-induced behavioural adjustment: a clue to the executive functions of the prefrontal cortex. Nat Rev Neurosci 2009; 10:141–152Crossref, Medline, Google Scholar
31. : Parsing executive processes: strategic vs evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci USA 2000; 97:1944–1948Crossref, Medline, Google Scholar
32. : The neural correlates and functional integration of cognitive control in a Stroop task. Neuroimage 2005; 24:539–547Crossref, Medline, Google Scholar
33.
34. : The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998; 59(suppl 20):22–33Medline, Google Scholar
35. : Reliability and validity of the Mini International Neuropsychiatric Interview (MINI): according to the SCID-P. Eur Psychiatry 1997; 12:232–241Crossref, Google Scholar
36. : Manual for the State-Trait Anxiety Inventory. Palo Alto, Calif, Consulting Psychologists Press, 1970Google Scholar
37. : Development and validation of the Penn State Worry Questionnaire. Behav Res Ther 1990; 28:487–495Crossref, Medline, Google Scholar
38. : Beck Anxiety Inventory Manual. San Antonio, Tex, Psychological Corp, 1993Google Scholar
39. : Manual for Beck Depression Inventory II (BDI-II). San Antonio, Tex, Psychological Corp, 1996Google Scholar
40. : Testing a tripartite model, II: exploring the symptom structure of anxiety and depression in student, adult, and patient samples. J Abnorm Psychol 1995; 104:15–25Crossref, Medline, Google Scholar
41. : Testing a tripartite model, I: evaluating the convergent and discriminant validity of anxiety and depression symptom scales. J Abnorm Psychol 1995; 104:3–14Crossref, Medline, Google Scholar
42. : Pictures of Facial Affect. Palo Alto, Calif, Consulting Psychologists Press, 1976Google Scholar
43. : Self-navigated spiral fMRI: interleaved versus single-shot. Magn Reson Med 1998; 39:361–368Crossref, Medline, Google Scholar
44. : Spatial registration and normalization of images. Hum Brain Mapp 1995; 2:165–189Crossref, Google Scholar
45. : A method for removal of global effects from fMRI time series. Neuroimage 2004; 22:360–366Crossref, Medline, Google Scholar
46. : Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 1995; 2:189–210Crossref, Google Scholar
47. : Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 1997; 6:218–229Crossref, Medline, Google Scholar
48. : A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp 1996; 4:58–73Crossref, Medline, Google Scholar
49. : Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J Neurosci 2009; 29:1175–1190Crossref, Medline, Google Scholar
50. : An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 2003; 19:1233–1239Crossref, Medline, Google Scholar
51. : Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Arch Gen Psychiatry 2009; 66:275–285Crossref, Medline, Google Scholar
52. : Amygdala response to fearful faces in anxious and depressed children. Arch Gen Psychiatry 2001; 58:1057–1063Crossref, Medline, Google Scholar
53. : Research Domain Criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry 2010; 167:748–751Link, Google Scholar
54. : The moderation by the serotonin transporter gene of environmental adversity in the etiology of depression: 2009 update. Mol Psychiatry 2010; 15:18–22Crossref, Medline, Google Scholar
55. : Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 1996; 274:1527–1531Crossref, Medline, Google Scholar
56. : A genetically mediated bias in decision making driven by failure of amygdala control. J Neurosci 2009; 29:5985–5991Crossref, Medline, Google Scholar
57. : Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biol Psychiatry 2008; 63:852–857Crossref, Medline, Google Scholar
58. : 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci 2005; 8:828–834Crossref, Medline, Google Scholar
59. : Imaging genetics of mood disorders. Neuroimage 2010; 53:810–821Crossref, Medline, Google Scholar
60. : Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc Natl Acad Sci USA 2006; 103:6269–6274Crossref, Medline, Google Scholar
61. : Amygdala responsiveness is modulated by tryptophan hydroxylase-2 gene variation. J Neural Transm 2005; 112:1479–1485Crossref, Medline, Google Scholar
62. : A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science 2010; 327:863–866Crossref, Medline, Google Scholar
63. : Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. Am J Psychiatry 2008; 165:90–98Link, Google Scholar
64. : Affective modulation of anterior cingulate cortex in young people at increased familial risk of depression. Br J Psychiatry 2008; 192:356–361Crossref, Medline, Google Scholar
65. : Regional brain volume in depression and anxiety disorders. Arch Gen Psychiatry 2010; 67:1002–1011Crossref, Medline, Google Scholar
66. : Distinct functional connectivity associated with lateral versus medial rostral prefrontal cortex: a meta-analysis. Neuroimage 2010; 53:1359–1367Crossref, Medline, Google Scholar
67. : Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry 2009; 66:1361–1372Crossref, Medline, Google Scholar



