Deficient Emotional Self-Regulation and Adult Attention Deficit Hyperactivity Disorder: A Family Risk Analysis
Abstract
Objective:
A growing body of research suggests that deficient emotional self-regulation (DESR) is prevalent and morbid among patients with attention deficit hyperactivity disorder (ADHD). Family studies provide a method of clarifying the co-occurrence of clinical features, but no family studies have yet addressed ADHD and DESR.
Method:
Participants were 83 probands with and without ADHD and 128 siblings. All were assessed for axis I DSM-IV conditions with structured diagnostic interviews. The authors defined DESR in adult probands and siblings using items from the Barkley Current Behavior Scale. Analyses tested hypotheses about the familial relationship between ADHD and DESR.
Results:
Siblings of ADHD probands were at elevated risk of having ADHD, irrespective of the presence or absence of DESR in the proband. The risk for DESR was elevated in siblings of ADHD plus DESR probands but not in siblings of ADHD probands. ADHD and DESR cosegregated in siblings. The risk for other psychiatric disorders was similar in siblings of the ADHD proband groups.
Conclusions:
The pattern of inheritance of ADHD with DESR preliminarily suggests that DESR may be a familial subtype of ADHD. Our data suggest that DESR is not an expression of other axis I DSM-IV disorders or of nonfamilial environmental factors. The authors cannot exclude contribution of non-axis-I DSM-IV disorders to risk for DESR and cannot determine whether the cosegregation of ADHD in DESR within families is a result of genes or familial environmental risk factors. Further investigation of DESR and its correlates and treatment both in and outside the context of ADHD is warranted.
Adults with attention deficit hyperactivity disorder (ADHD) are at elevated risk for deficient emotional self-regulation (DESR) (1–6). DESR refers to 1) deficits in self-regulating the physiological arousal caused by emotions, 2) difficulties inhibiting inappropriate behavior in response to either positive or negative emotions, 3) problems refocusing attention from strong emotions, and 4) disorganization of coordinated behavior in response to emotional activation (4). DESR traits include low frustration tolerance, impatience, and quickness to anger as well as being easily excited in response to emotional reactions. Although Wender (7) and subsequently others (4, 8, 9) have identified these traits as common in presentations of ADHD in adulthood, in DSM-IV they are not considered diagnostic of the disorder.
Understanding the nature of DESR among ADHD patients is consequential because DESR can be confused with mood disorder symptoms that can be comorbid with ADHD. DESR is distinct from severe mood dysregulation, as described by Leibenluft et al. (10), and also differs from the persistent and severe aggressive irritability often seen in pediatric bipolar disorder (11). Unlike DESR, these latter conditions are not defined by poor self-regulation, and their definition includes features that are not part of DESR (e.g., the other mood symptoms of bipolar disorder and the hyperarousal of severe mood dysregulation).
The difference between DESR associated with ADHD and the mood instability of pediatric bipolar disorder is of particular importance given that these two disorders respond to different pharmacologic treatments. Notably, bipolar disorder is far less prevalent than DESR in ADHD patients, with approximately 15% of clinically referred ADHD adults having a lifetime history of bipolar disorder (12). In contrast, Barkley et al. (3) found that 60% of adults with ADHD in a clinical sample reported traits of DESR, relative to 15% of comparison subjects. Similar symptoms were seen in one-third of adults with ADHD participating in registration trials for atomoxetine (13) and in more than one-half of adult patients in a smaller clinical trial of osmotic-release oral system methylphenidate (2). Barkley and Fischer (14) showed a higher prevalence of DESR in adults with ADHD with persistent symptoms relative to those without persistent symptoms. In their sample, DESR was correlated with functional impairment beyond that accounted for by ADHD. We recently found that 61% of a sample of community-ascertained adults with ADHD reported DESR of greater severity than 95% of comparison subjects (6). DESR was only partially accounted for by other comorbid psychiatric conditions and was associated with significant adverse effect on quality of life.
Because little is known about the mechanisms underlying the association of DESR with ADHD, we sought to use family study data to determine whether familial transmission could clarify the co-occurrence of these conditions, as it has for other disorders (15, 16). Family studies can determine whether two conditions share familial risk factors or whether their co-occurrence is a result of nonfamilial environmental risk factors. Based on models proposed by Pauls et al. (17), we tested five competing hypotheses.
Hypothesis 1 posits that ADHD and DESR are etiologically independent and co-occur as a result of chance. It predicts equally high rates of ADHD in relatives of ADHD probands and ADHD plus DESR probands (relative to comparison probands), but DESR should be increased only among relatives of adults with ADHD plus DESR (relative to comparison probands). Additionally, ADHD and DESR should not cosegregate in families of ADHD plus DESR probands (i.e., the ADHD relatives should not be at increased risk for DESR). Refuting this hypothesis would eliminate any hypothesis suggesting that the association between ADHD and DESR is a result of a referral bias (such as Berkson's bias) in which patients with multiple conditions are overrepresented in referrals because of the greater severity of their clinical picture. Given that there are no population studies of ADHD and DESR to discount such biases, testing this hypothesis is essential to rule out this potential artifact, which could explain the association of the two conditions.
Hypothesis 2 theorizes that ADHD with DESR is a distinct subtype or an entirely separate condition. It predicts the same pattern as hypothesis 1, with the exception that it predicts strong evidence for cosegregation among relatives of ADHD plus DESR adults (i.e., ADHD and DESR should be comorbid among relatives).
Hypothesis 3 postulates that ADHD plus DESR requires more familial transmissible etiologic factors for its expression compared with ADHD without DESR. This would be consistent with the greater severity of ADHD plus DESR observed in prior studies (6, 14). This hypothesis predicts a higher prevalence of both ADHD and DESR among relatives of ADHD plus DESR probands compared with relatives of ADHD probands. However, unlike hypotheses 1 and 2, it predicts that relatives of ADHD probands are at greater risk for DESR than relatives of comparison probands.
Hypothesis 4 speculates that ADHD adults with and without DESR share common familial etiologic factors but differ because of nonfamilial environmental effects. This hypothesis predicts similar rates of ADHD and DESR in the relatives of both subgroups.
Hypothesis 5 posits that DESR among adults with ADHD is a subsyndromal manifestation of another familial disorder such as depression or oppositional defiant disorder. If this is correct, then the relatives of ADHD plus DESR probands should be at higher risk for such disorders compared with the relatives of ADHD probands.
Method
Participants
Probands were men and women between the ages of 18 and 55 years who had children or siblings eligible for participation in the study. We excluded potential probands with major sensorimotor disabilities, psychosis, inadequate command of the English language, or a full-scale IQ <75. No ethnic or racial group was excluded. We recruited ADHD patients and comparison subjects using separate ascertainment methods. The following two ascertainment sources were employed to recruit ADHD probands: referrals to psychiatric clinics at Massachusetts General Hospital and advertisements in the greater Boston area. We recruited potential non-ADHD (comparison) probands through advertisements in the greater Boston area. All available relatives of probands were recruited to participate in the study after contact information from the proband was obtained. Following description of the study to the participants, written informed consent was obtained separately from probands and relatives under procedures approved by the Massachusetts General Hospital Institutional Review Board.
Based on our previous work, we included probands as having ADHD if they met full DSM-IV criteria for the disorder (N=127) or for late-onset ADHD (N=79; participants meeting full DSM-IV criteria for ADHD except for the age at onset criterion). We previously demonstrated that these full and late-onset ADHD groups have similar clinical correlates (12, 18–20). The late-onset patients had onset in adolescence. The remaining participants were defined as not having ADHD (N=123).
To study familial risk in adult relatives, we examined a subset of probands who had information available about both DESR and ADHD for themselves and their siblings. Information on ADHD and DESR was available from 27 probands with ADHD and DESR (ADHD plus DESR probands) and 45 of their adult siblings; from 23 probands with ADHD who did not have DESR (ADHD probands) and 40 of their adult siblings; and from 33 probands without ADHD or DESR (comparison probands) and 43 of their adult siblings.
Assessments
Participants were assessed with the Structured Clinical Interview for DSM-IV Axis I Disorders (21). We used modules from the Schedule for Affective Disorders and Schizophrenia for School-Age Children–Epidemiologic version (22) to assess childhood disorders. A committee of board-certified child and adult psychiatrists who were blind to the ADHD status of participants, referral sources, and all other data resolved diagnostic uncertainties. Diagnoses presented for review were considered positive only when the committee determined that criteria were met to a clinically meaningful degree. The median reliability between individual clinician- and review committee-assigned diagnoses was 0.87. Kappa coefficients for individual diagnoses were as follows: ADHD (1.0), conduct disorder (1.0), major depression (1.0), bipolar disorder (0.78), separation anxiety (0.89), agoraphobia (0.80), panic disorder (0.77), substance use disorder (1.0), and tics/Tourette's syndrome (0.68).
Interviewers were blind to ascertainment group. They had undergraduate degrees in psychology and underwent several weeks of classroom-style training in learning interview mechanics, diagnostic criteria, and coding algorithms. Then, they observed interviews by experienced raters and clinicians and subsequently conducted at least six practice interviews and at least three study interviews while being observed by senior interviewers. Trainees were not permitted to conduct interviews independently until they executed at least three interviews that achieved perfect diagnostic agreement with an observing senior interviewer. Joseph Biederman, M.D., supervised the interviewers throughout the study. We computed kappa coefficients of agreement by having experienced board-certified child and adult psychiatrists as well as licensed clinical psychologists diagnose participants from audio-taped interviews. Based on 500 assessments from interviews of children and adults, the median kappa coefficient was 0.98. Kappa coefficients for diagnoses were as follows: ADHD (0.88), conduct disorder (1.0), major depression (1.0), mania (0.95), separation anxiety (1.0), agoraphobia (1.0), panic disorder (0.95), substance use disorder (1.0), and tics/Tourette's syndrome (0.89).
We used eight items from the self-report Current Behavior Scale, developed by Barkley (8), to assess DESR (Figure 1). The scale asks participants to describe their behavior during the prior 6 months. Responses to each item on this scale range from 0 (never or rarely) to 3 (very often). We defined participants as having DESR if they had a rating on the DESR subscale that was as impaired as or more impaired than the worst 5% of ratings among non-ADHD comparison subjects. Among all probands in our study sample (ADHD and non-ADHD subjects), we previously demonstrated that Cronbach's alpha for the eight scale items was 0.90, indicating high internal consistency, and that DESR, as identified by these items, was associated with greater impairment on validated measures of quality of life and social functioning, suggesting that the scale has external validity (6). Six of the items in the eight-item scale we utilized are identical to items in a seven-item scale whose psychometric properties were previously described by Barkley and Fischer (14) in a population of adults diagnosed with ADHD as children. Factor analysis of responses to the seven-item scale resulted in a single dimension accounting for 72% of the variance, with item loading from 0.75 to 0.91, and self-ratings across two studies correlated well with other ratings in a prior study (r=0.71, p<0.001). Emotional symptoms identified by this seven-item scale were associated with measures of functional impairment beyond the contribution of ADHD symptoms, suggesting external validity.

FIGURE 1. Deficient Emotional Self-Regulation Scale Items
Statistical Analyses
We used chi-square tests to compare the proband groups on demographic variables as well as the prevalence of disorders and cosegregation in relatives. STATA software was utilized for all tests (StataCorp, LP, College Station, Tex. [23]). All tests were two-tailed, and statistical significance was defined at the 5% level.
Results
Familial risk analyses were conducted for 43 siblings of 33 comparison probands, 40 siblings of 23 ADHD probands, and 45 siblings of 27 ADHD plus DESR probands. As seen in Table 1, there were no significant differences in age and gender between the groups. There was also no significant difference in Hollingshead-Redlich socioeconomic status between the siblings of the three proband groups. We therefore did not need to control for differences in these variables in our familial risk analyses.
| Characteristic | Siblings of 33 Comparison Probands (N=43) | Siblings of 23 ADHD Probands (N=40) | Siblings of 27 ADHD Plus DESR Probands (N=45) | Analysis | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | F | df | p | |
| Age (years) of adult sibling | 39.5 | 16.4 | 42.4 | 18.4 | 40.6 | 16.5 | 0.29 | 2, 125 | 0.75 |
| N | % | N | % | N | % | χ2 | df | p | |
| Male adult sibling | 13 | 30 | 14 | 35 | 16 | 36 | 0.33 | 2 | 0.85 |
TABLE 1. Characteristics of Relatives of ADHD Probands, ADHD Plus Deficient Emotional Self-Regulation (DESR) Probands, and Comparison Probands
Familial Risk Analyses
Relative to comparison subjects, ADHD was more prevalent in the siblings of probands with ADHD, irrespective of the presence or absence of DESR. ADHD was present in 47.5% of the siblings of ADHD probands versus 7.0% of the siblings of comparison probands (χ2=17.5, df=1, p<0.001). ADHD was present in 60% of the siblings of ADHD plus DESR probands versus 7.0% of the siblings of comparison probands (χ2=27.5, df=1, p<0.001). The prevalence of ADHD in siblings did not differ significantly between ADHD probands with and without DESR (60% versus 48%, respectively).
Siblings of ADHD plus DESR probands had significantly elevated rates of DESR relative to comparison probands (26.7% versus 0%, respectively; χ2=13.3, df=1, p<0.001), but the siblings of ADHD probands did not (5% versus 0%, respectively). DESR was also significantly more prevalent in the siblings of probands with ADHD plus DESR compared with the siblings of probands with ADHD (26.7% versus 5%, respectively; χ2=7.2, df=1, p<0.01).
We also found that ADHD and DESR cosegregated. The ADHD siblings of ADHD plus DESR probands were at elevated risk for DESR compared with non-ADHD siblings (44.4% versus 0%, respectively; χ2=10.9, df=1, p<0.01).
Nearly all cases of ADHD plus DESR in siblings occurred among siblings of ADHD plus DESR probands (χ2=18.3, df=2, p<0.001 [Figure 2]).
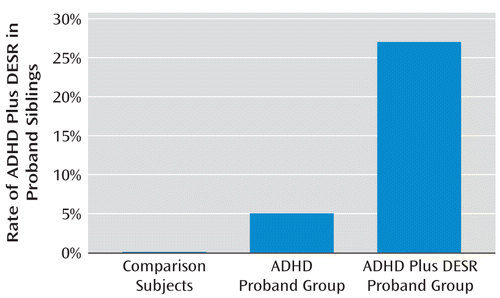
FIGURE 2. Rates of ADHD Plus Deficient Emotional Self-Regulation (DESR) Among First-Degree Relatives in Three Proband Groupsa
a ADHD plus DESR was seen in significantly more siblings of ADHD plus DESR probands (N=12) than in siblings of ADHD probands (N=2; χ2=7.2, df=1, p=0.007) or siblings of comparison probands (N=0; χ2=13.2, df=1, p<0.001).
Table 2 shows the lifetime prevalence of psychiatric disorders in siblings of the three proband groups. Relative to siblings of comparison subjects, siblings of both ADHD plus DESR probands and ADHD probands had significantly higher rates of major depression, oppositional defiant disorder, and alcohol dependence. Siblings of ADHD plus DESR probands additionally had significantly higher rates of lifetime bipolar disorder, social phobia, and generalized anxiety disorder relative to the siblings of comparison probands. There were no significant differences in the rates of any of these psychiatric disorders when comparing the siblings of ADHD plus DESR probands with siblings of ADHD probands.
| Disorder | Siblings of 33 Comparison Probands (N=43) | Siblings of 23 ADHD Probands (N=40) | Siblings of 27 ADHD Plus DESR Probands (N=45) | Analysis | ||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | χ2 (df=2) | p | |
| Major depressive disorder | 13 | 30 | 22* | 55 | 23* | 51 | 6.07 | 0.048 |
| Bipolar disorder | 0 | 3 | 8 | 6* | 13 | 6.00 | 0.05 | |
| Oppositional defiant disorder | 0 | 9** | 23 | 11** | 24 | 12.05 | <0.001 | |
| Conduct disorder | 4 | 9 | 5 | 13 | 8 | 18 | 1.40 | 0.50 |
| Alcohol abuse | 10 | 24 | 17 | 38 | 13 | 33 | 2.02 | 0.36 |
| Alcohol dependence | 3 | 7 | 9* | 23 | 13** | 30 | 7.31 | 0.026 |
| Drug abuse | 4 | 9 | 8 | 20 | 9 | 20 | 2.38 | 0.30 |
| Drug dependence | 2 | 5 | 9* | 23 | 5 | 11 | 6.09 | 0.048 |
| Simple phobia | 5 | 12 | 5 | 13 | 8 | 18 | 0.81 | 0.67 |
| Social phobia | 2 | 5 | 6 | 15 | 12** | 27 | 8.10 | 0.017 |
| Agoraphobia | 5 | 12 | 2 | 5 | 8 | 18 | 3.21 | 0.2 |
| Panic disorder | 4 | 9 | 5 | 13 | 6 | 13 | 0.82 | 0.40 |
| Generalized anxiety disorder | 1 | 2 | 3 | 8 | 7* | 16 | 4.99 | 0.08 |
TABLE 2. Lifetime Prevalence of Psychiatric Disorders in Relatives of ADHD Probands, ADHD Plus Deficient Emotional Self-Regulation (DESR) Probands, and Comparison Probandsa
Conclusions
Our findings support our second hypothesis, which posits that ADHD with DESR represents a distinct familial subtype of ADHD or an entirely separate familial condition. Familial risk analysis revealed that ADHD was transmitted in families irrespective of the presence or absence of DESR, but DESR was only elevated among siblings of adult ADHD probands with DESR. We also found evidence for cosegregation between ADHD and DESR among siblings of ADHD plus DESR probands. This is seen most clearly in Figure 2, which shows that nearly all cases of ADHD plus DESR among siblings were in families of ADHD plus DESR probands.
Although referral biases (such as Berkson's bias) could explain the comorbidity of ADHD and DESR among probands, such biases would not artifactually inflate the comorbidity of ADHD and DESR among relatives. Thus, our cosegregation findings eliminated hypothesis 1. Ideally, population studies of ADHD and DESR, which are lacking, would document this absence of referral bias. In the absence of such studies, our data are the strongest available to show that the high rate of DESR among ADHD probands is unlikely to be an artifact of referral.
The fact that DESR is restricted to families in which DESR and ADHD co-occur suggests that DESR is not simply a secondary manifestation of ADHD or another class of ADHD symptoms. If that were true, then rates of DESR should have been elevated among siblings of ADHD probands, since many of these siblings had ADHD. Because we did not see this elevation of DESR, it suggests that this deficiency does not routinely occur in ADHD patients but only in a subgroup. It is possible that DESR is secondary to ADHD but only in a familial context in which DESR occurs. For example, in some families, intrafamilial factors (e.g., modeling, social learning) may disrupt the normal developmental trajectory of increasing self-regulation with age. Such effects may be stronger in ADHD family members given that their disorder interferes with learning and compromises the self-regulation of cognitions and behavior. Such intrafamilial effects could explain the pattern of findings we observed. Because the prevalence of ADHD was similar between siblings of ADHD and ADHD plus DESR probands, we can rule out hypothesis 3 and conclude that ADHD plus DESR is not caused by having a loading of familial transmissible factors greater than ADHD. If a nonfamilial environmental risk factor accounted for DESR among ADHD probands (hypothesis 4), then we should have found similar rates of ADHD and DESR in the relatives of both subgroups, but we did not.
To identify DESR, we measured traits such as low frustration tolerance, impatience, quickness to anger, and being easily excited. These traits could present clinically in either the presence or absence of other mental health conditions such as mood disorders. If DESR among ADHD probands was an expression of another psychiatric disorder, we would have expected to find a higher prevalence of that psychiatric disorder among siblings of ADHD plus DESR probands compared with ADHD probands (hypothesis 5). In contrast to this prediction, we found no significant difference in the lifetime rate of any psychiatric disorders when comparing these two groups. This suggests that the DESR identified in probands with ADHD is not an expression of any of these familially transmissible disorders. We make this conclusion with caution because it derives from a failure to find significant differences, which could be a result of limited statistical power. However, the suggestion that DESR is not a manifestation of another psychiatric disorder is consistent with our demonstration that current and lifetime axis I conditions do not account for manifestation of DESR in the ADHD probands from the present study, as we previously demonstrated (6).
Our findings have several implications for clinical and research investigation. From a clinical perspective, the assessment of DESR should help identify a relatively homogeneous group of ADHD patients. Prior studies have shown these patients to be at high risk for other psychopathology and severe functional impairments (5, 6). Our study further emphasizes that, in addition to having distinct clinical features, this group of ADHD plus DESR patients may be distinct from other ADHD patients with regard to risk factors. This possibility further suggests that ADHD research would benefit from the stratification of ADHD patients into those with and without DESR, since the former may be a more homogeneous group with regard to treatment response (24), neuroimaging parameters, or predisposing genetic variants (25).
Improvements in the assessment and identification of DESR should help clinicians better differentiate DESR from other forms of comorbid mood disorders associated with ADHD. While many studies show that ADHD is associated with major depression and bipolar disorder, the cardinal feature of these disorders is the experience of strong emotions, not self-regulation (26). These disorders are also associated with nonmood criteria, including somatic and behavioral impairments. For example, a patient in an episode of irritable mania expresses extreme and protracted irritability throughout the episode, not only in response to provoking stimuli. In contrast, ADHD patients with DESR do not have distinct episodes of DESR. Because they cannot self-regulate their emotional response, they respond to a provoking objective stimulus in an exaggerated manner, and thus the response becomes inappropriate in its magnitude and duration. Equally important to differential diagnosis between comorbid mood disorders and DESR is that the expressions of DESR subside relatively rapidly (i.e., “hot temper” or labile mood) and do not form a distinct protracted episode that would qualify for a mood disorder. Thus, ADHD adults with DESR enjoy normal moods much of the time but become easily frustrated or angry with unexpected emotional challenges.
DESR also differs from the mood dysregulation of severe mood dysregulation that is defined in children by 1) markedly increased reactivity in response to emotional negative stimuli (e.g., rages, aggression), 2) abnormal mood between rages in the form of sadness or anger, and 3) hyperarousal (defined by at least three of the following traits: insomnia, agitation, distractibility, racing thoughts or flight of ideas, pressured speech, or intrusiveness) (10). Unlike DESR, severe mood dysregulation must be present at least half of the day on most days. Thus, severe mood dysregulation differs from DESR in the presence of abnormal mood between emotional overreactions and the presence of hyperarousal traits. Finally, although DESR may lead to emotional lability, the latter term broadly refers to emotions that change rapidly and are usually inappropriate. Labile emotions could be caused by poor self-regulation in response to provoking stimuli, but such stimuli are not required, as in the case of dementias.
DESR is therefore distinct from the type of mood dysregulation seen in depression, bipolar disorder, and severe mood dysregulation. We showed this empirically in a prior report of the present sample that found DESR symptoms to be strongly associated with ADHD, independent of both current and lifetime comorbidity with mood and anxiety disorders (6). In the present report, we now show that the familial transmission of DESR is also independent of these other types of mood dysregulation.
Another consideration for research is that DESR could be conceptualized as a quantitative trait, which like neuropsychological functioning shows a continuum of dysfunction not only in ADHD but also for other disorders. Such an approach is consistent with the recently outlined Research Domain Criteria proposed by the National Institute of Mental Health (http://www.nimh.nih.gov/research-funding/nimh-research-domain-criteria-rdoc.shtml). The Research Domain Criteria are dimensional measures ranging from normal to abnormal that cut across current diagnostic boundaries. Given that DESR is independently associated with ADHD and several other disorders, it could be used in this fashion. Future work would need to determine whether the ADHD plus DESR subtype we propose in the present study is simply one expression of DESR, which might also be seen, for example, as depression plus DESR in a family study of depression.
Our results should be interpreted with some limitations in mind. Through analysis of familial risk in the sibling relatives of probands, we have evaluated familial risk for DESR in adulthood and cannot make inferences about risk for DESR in other stages of development. Our sample did not allow exploration of the potential contribution of nonrandom mating to the pattern of inheritance of ADHD with DESR. We also were unable to disentangle the extent to which ADHD among adults with DESR might be secondary to DESR, which would require a sample of probands with DESR who did not have ADHD. It is also possible that our characterization of the familiality of DESR and its relationships with comorbidity were limited by power. It is further possible that intrafamilial factors (e.g., modeling, social learning) could explain familial manifestation of DESR. Thus, we cannot assert with complete confidence that we have accurately differentiated between all models for the coexistence of ADHD and DESR. Our sample was primarily of Caucasian ancestry and thus may not generalize to other ethnic groups.
Despite these limitations, we identified a pattern of familial risk consistent with the hypothesis that ADHD with DESR is a distinct familial subtype or variant of ADHD. Further work with other types of data (e.g., twins, extended relatives) is needed to provide stronger support for this hypothesis. Despite this limitation, we can firmly conclude that DESR is familial in ADHD families and that this familial transmission cannot be accounted for by other disorders. Replication of our findings, as well as further study of the genetic, neuropsychological, neurophysiological, and clinical correlates of ADHD with DESR, could clarify the importance of this putative ADHD phenotype.
Patient Perspectives
An Adult With Attention Deficit Hyperactivity Disorder (ADHD) and Deficient Emotional Self-Regulation (DESR)
A 38-year-old father of three children who works as a computer engineer is frequently angry and frustrated. He and his wife report that display of these feelings is straining his relationships at both work and home. He reports that symptoms of ADHD since early grade school have persisted into adulthood. He underperformed throughout his education relative to his abilities, and his family and friends have learned to expect him not to follow through on their requests. His performance reviews at work note that he is not engaging adequately with his team or completing tasks in an organized, timely way. Although he denies depression or periods of extreme abnormal mood lasting longer than a few hours, he often experiences periods of anger and rage in response to frustrations during a typical day. For example, these reactions occur if he is interrupted when busy, when he cannot quickly troubleshoot a problem that he expected to be straightforward, or when last-minute changes are made in his task list or schedule. His boss has called him in several times in the last few months to discuss coworkers' complaints about his “attitude” in reaction to their requests. At home, his agitation and short fuse have created a tense atmosphere and lead to inconsistency in parenting. He and his wife are both concerned about the effect of his behavior on their children.
An Adult With Attention Deficit Hyperactivity Disorder (ADHD) Without Deficient Emotional Self-Regulation (DESR)
A 42-year-old mother of an 8-year-old boy reports that she wants to be more on top of the demands of her life. In early grade school, she had challenges with listening, organization, and following through as well as with forgetfulness, and she was frequently in trouble for having a messy desk and talking in class. Similar symptoms have persisted throughout her life, and she holds these traits responsible for many disappointments. Despite clear intelligence, she attended three colleges over 8 years without completing a degree—years that were full of last-minute cramming and papers written as all-nighters. She found a job in sales, where she struggled to file expense reports and other paperwork. Recognized for her interpersonal skills and teamwork, she was promoted to a managing position. However, within the year, she was demoted because of poor documentation and errors in detailed work. When she became a mother, the added activities involved in managing her son's life, who was recently diagnosed with ADHD, have compounded the feeling that she is barely on top of the demands of each day. She feels that she would forget many important things without the lists she keeps, although she rarely sticks to them and often loses them. Without her husband's help, she is sure that she would have a stack of overdue bills. She barely stays on top of the demands of daily life through an exhausting degree of effort that leaves her fatigued and, on many days, demoralized. She is particularly worried about her ability to provide an organized environment for her son.
1. : Emotional dysregulation in adult ADHD and response to atomoxetine. Biol Psychiatry 2005; 58:125–131Crossref, Medline, Google Scholar
2. : A double-blind, placebo-controlled, crossover study of osmotic release oral system methylphenidate in adults with ADHD with assessment of oppositional and emotional dimensions of the disorder. J Clin Psychiatry 2007; 68:93–101Crossref, Medline, Google Scholar
3. : ADHD in Adults: What the Science Says. New York, Guilford, 2008Google Scholar
4. : Deficient emotional self-regulation: a core component of attention-deficit/hyperactivity disorder. J ADHD Relat Disord 2010; 1:5–37Google Scholar
5. : Deficient emotional self-regulation in adults with attention-deficit/hyperactivity disorders (ADHD): the relative contributions of emotional impulsiveness and ADHD symptoms to adaptive impairments in major life activities. J ADHD Relat Disord 2010; 1:5–28Google Scholar
6. : Is deficient emotional self-regulation a comorbid or integral feature of attention-deficit/hyperactivity disorder in adults? a controlled study, in Proceedings of the 163rd Annual Meeting of the American Psychiatric Association,
7. : Attention-Deficit Hyperactivity Disorder in Adults. New York, Oxford University Press, 1995Google Scholar
8. : Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull 1997; 121:65–94Crossref, Medline, Google Scholar
9. : An integrative theory of attention-deficit/ hyperactivity disorder based on the cognitive and affective neurosciences. Dev Psychopathol 2005; 17:785–806Crossref, Medline, Google Scholar
10. : Researching the pathophysiology of pediatric bipolar disorder. Biol Psychiatry 2003; 53:1009–1020Crossref, Medline, Google Scholar
11. : How cardinal are cardinal symptoms in pediatric bipolar disorder? an examination of clinical correlates. Biol Psychiatry 2005; 58:583–588Crossref, Medline, Google Scholar
12. : Diagnosing adult attention deficit hyperactivity disorder: are late onset and subthreshold diagnoses valid? Am J Psychiatry 2006; 163:1720–1729; quiz 1859Link, Google Scholar
13. : Emotional dysregulation in adult ADHD and response to atomoxetine. Biol Psychiatry 2005; 58:125–131Crossref, Medline, Google Scholar
14. : The unique contribution of emotional impulsiveness to impairment in major life activities in hyperactive children as adults. J Am Acad Child Adolesc Psychiatry 2010; 49:503–513Crossref, Medline, Google Scholar
15. : The use of multiple thresholds and segregation analysis in analyzing the phenotypic heterogeneity of multifactorial traits. Ann Hum Genet 1979; 42:371–390Crossref, Medline, Google Scholar
16. : Do attention deficit hyperactivity disorder and major depression share familial risk factors? J Nerv Ment Dis 1997; 185:533–541Crossref, Medline, Google Scholar
17. : Gilles de la Tourette's syndrome and obsessive-compulsive disorder: evidence supporting a genetic relationship. Arch Gen Psychiatry 1986; 43:1180–1182Crossref, Medline, Google Scholar
18. : Neuropsychological studies of late onset and subthreshold diagnoses of adult attention-deficit/hyperactivity disorder. Biol Psychiatry 2006; 60:1081–1087Crossref, Medline, Google Scholar
19. : Substance use among ADHD adults: implications of late onset and subthreshold diagnoses. Am J Addict 2007; 16(suppl 1):24–34Crossref, Medline, Google Scholar
20. : Personality traits among ADHD adults: implications of late-onset and subthreshold diagnoses. Psychol Med 2009; 39:685–693Crossref, Medline, Google Scholar
21. : Structured Clinical Interview for DSM-IV Axis I Disorders. Washington, DC, American Psychiatric Publishing, 1997Google Scholar
22. : Schedule for Affective Disorders and Schizophrenia for School-Age Children–Epidemiologic Version, 5th ed (K-SADS-E-5). Ft Lauderdale, Fla, Nova Southeastern University, Center for Psychological Studies, 1994Google Scholar
23.
24. : Emotional dysregulation as a core feature of adult ADHD: its relationship with clinical variables and treatment response in two methylphenidate trials. J ADHD Relat Disord 2010; 1:53–64Google Scholar
25. : The use of emotional dysregulation as an endophenotype for genetic studies in adults with attention-deficit/hyperactivity disorder. J ADHD Relat Disord 2010; 1:29–38Google Scholar
26. : A pilot study of ecological momentary assessment of emotion dysregulation in children. J ADHD Relat Disord 2010; 1:39–52Google Scholar



