Social Norm Processing in Adult Social Phobia: Atypically Increased Ventromedial Frontal Cortex Responsiveness to Unintentional (Embarrassing) Transgressions
Abstract
Objective:
Little is known about the neural underpinnings of generalized social phobia, which is defined by a persistent heightened fear of social disapproval. Using event-related functional MRI (fMRI), the authors examined whether the intent of an event, which mediates the neural response to social disapproval in healthy individuals, differentially affects response in generalized social phobia.
Method:
Sixteen patients with generalized social phobia and 16 healthy comparison subjects group-matched on age, gender, and IQ underwent fMRI scans while reading stories that involved neutral social events, unintentional social transgressions (e.g., choking on food at a party and coughing it up), or intentional social transgressions (e.g., disliking food at a party and spitting it out).
Results:
Significant group-by-transgression interactions were observed in ventral regions of the medial prefrontal cortex. Healthy individuals tended to show increased blood-oxygen-level-dependent responses to intentional relative to unintentional transgressions. Patients with generalized social phobia, however, showed significantly increased responses to the unintentional transgressions. They also rated the unintentional transgressions as significantly more embarrassing than did the comparison subjects. Results also revealed significant group main effects in the amygdala and insula bilaterally, reflecting elevated generalized social phobia responses in these regions to all event types.
Conclusions:
These results further implicate the medial prefrontal cortex in the pathophysiology of generalized social phobia, specifically through its involvement in distorted self-referential processing. These results also further underscore the extended role of the amygdala and insula in the processing of social stimuli more generally in generalized social phobia.
Generalized social phobia typically manifests in early adolescence and predicts risk for depression, substance abuse, and suicide (1–3). At the neural-network level, functional MRI (fMRI) studies of generalized social phobia find atypically increased responses to social stimuli in the amygdala (4–7). In addition, recent studies also find atypical activity in medial prefrontal cortex regions previously implicated in self-relevant processing (8–10; K.S. Blair et al., unpublished 2010 data).
Self-referential processing plays a critical role in regulating interpersonal behavior. Self-referential processing occurs following social transgressions that result in harmless norm violations (e.g., spitting out food at a dinner party). An individual's response to such transgressions reflects their social calculations related to self-reference (i.e., “How will observers react to my behavior?”), and generalized social phobia involves perturbations in such social calculations. These perturbations manifest as heightened fear of social disapproval. Indeed, patients with generalized social phobia show anomalous neural (8; K.S. Blair et al., unpublished 2010 data) and autonomic (11) responses to verbal criticism and during anticipation of negative peer evaluations (4). Moreover, social anxiety is related to elevated levels of self-criticism (12, 13).
No fMRI study of generalized social phobia has examined the response to actions that bring social disapproval. A variable modulating social disapproval is the transgressor's presumed intent (14). Unintentional conventional transgressions (e.g., accidentally choking and consequently spitting out the host's food) lead to displays of embarrassment that, it is argued, serve to indicate the absence of intent and a desire for appeasement (15). In contrast, intentional transgressions (e.g., spitting out the host's unpalatable food) are not associated with embarrassment or other signs of appeasement. Rather, behavior in this situation reflects the transgressor's intent to challenge the social hierarchy (14).
Previous fMRI work with healthy adults has implicated ventromedial prefrontal cortex and temporo-parietal regions in the processing of conventional transgressions (14, 16, 17). These are regions implicated in self-referential processing and representations of the intentional states of others (18–20). In healthy individuals, this region responds to the occurrence of intentional, relative to unintentional, conventional social transgressions (14). This response is thought to reflect heightened representation of the protagonist's intent to challenge the social order (14, 17).
Generalized social phobia involves marked and persistent fear of embarrassment, which might lead those with the disorder to frequently seek appeasement. A tendency to fear embarrassment and seek appeasement in generalized social phobia might be reflected in enhanced neural responses specifically to unintentional, potentially embarrassing, social transgressions (21). In this study, we tested this prediction using an adaptation of Berthoz et al. (14), whereby intentional transgressions, unintentional transgressions, and normative vignettes are presented to patients with generalized social phobia and healthy comparison subjects. Given generalized social phobia-related heightened propensity for embarrassment, coupled with prior imaging work in generalized social phobia, we expected that unintentional social transgressions, when presented to patients, would elicit increased activity in medial prefrontal cortex regions implicated in social cognition (8, 10; K.S. Blair et al., unpublished 2010 data).
The amygdala and insula also appear hyperresponsive in generalized social phobia to a range of negative social stimuli. However, unlike for the medial prefrontal cortex, this hypersensitivity manifests in many contexts (see references 6–8, for example). Thus, we also expected that patients with generalized social phobia would show greater response in these areas. However, unlike the medial prefrontal cortex, which might precisely code context for social transgressions, we expected the amygdala and insula to show hyperresponsivity to social stimuli regardless of context. This would be reflected in a main effect of group, in the absence of group-by-condition interactions.
Method
Participants
Participants were 16 patients with generalized social phobia and 16 healthy comparison subjects, group-matched on age, gender, and IQ (Table 1). Participants were recruited from advertisements approved by the NIMH Institutional Review Board.
| Characteristic | Patients With Generalized Social Phobia (N=16) | Healthy Comparison Subjects (N=16) | p | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Male | 9 | 56.3 | 7 | 43.8 | ns |
| Race | ns | ||||
| –Caucasian | 13 | 81.3 | 14 | 87.5 | |
| –African American | 3 | 18.8 | 2 | 12.5 | |
| Mean | SD | Mean | SD | ||
| Age | 35.1 | 9.60 | 30.0 | 8.37 | ns |
| IQ | 118.6 | 8.54 | 117.2 | 9.74 | ns |
| Liebowitz Social Anxiety Scale-Self Report | 67.1 | 23.43 | 16.2 | 11.65 | <0.001 |
| Inventory of Depressive Symptomatology-Self Report | 11.0 | 7.20 | 5.1 | 3.50 | <0.05 |
| Global Assessment of Functioning Scale | 61.3 | 5.34 | |||
TABLE 1. Demographic and Clinical Characteristics of Participants in a Study of Social Norm Processing in Adult Social Phobia
Participants with generalized social phobia had to meet DSMIV criteria for the disorder based on the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) (22) and a confirmatory clinical interview by a board-certified psychiatrist (D.S.P.). No participant with generalized social phobia had any other axis I diagnoses; all had been medication free for at least 6 months. Healthy comparison subjects had no history of any psychiatric illness. All participants were in good physical health, as confirmed by a complete physical examination, and all provided written informed consent.
As part of the assessment, all participants completed the Liebowitz Social Anxiety Scale-Self Report (23) and the Inventory of Depressive Symptomatology-Self Report (24). In addition, for the patients with generalized social phobia, the level of overall social, occupational, and psychological functioning was assessed by the Global Assessment of Functioning Scale. Scores on these measures characterized the generalized social phobia group as having moderate levels of social anxiety with mild associated impairment in functioning (Table 1).
Behavioral Task
Participants read stories (e.g., Joanna is invited for dinner at a friend's house, she has a bite of the first course, chews…) that could either involve a neutral social event (…and swallows the food), an unintentional transgression (…chokes and coughs up the food), or an intentional transgression (…dislikes it and spits out the food). Twenty-six different stem stories were used, each presented with the three different types of ending. Thus, a total of 78 endings were used. The three different types of ending were matched on number of letters and words, and care was taken to ensure that the framing of the comments was consistent across the three ending types. Prior to scanning, participants were told that they would read different stories and were instructed to imagine what they would feel like if they were in the situation described. For each story, participants were simply required to press a button with their left hand when they had read the story. Each stem comment was presented on its own for 3000 msec. The ending of the story would then appear underneath the stem, and the stem and ending (that is, the full story) would be shown on the screen together for 6000 msec. For each experimental run, 22 fixation points of 3000 msec each were presented between the stimuli (five at beginning of run, five at end of run, and 12 randomized throughout the run), serving as an implicit baseline. Participants completed three randomly presented runs.
After echo-planar imaging (EPI) acquisition, participants rated each of the 78 individual stories, presented in a randomized order across participants, on a 5-point Likert scale according to how embarrassing they thought the behaviors were (1=not at all embarrassing; 3=somewhat embarrassing; and 5=extremely embarrassing). In addition, they rated the stories according to how inappropriate they thought the behaviors were (1=not at all inappropriate; 3=somewhat inappropriate; 5=extremely inappropriate).
fMRI Parameters
Whole-brain blood-oxygen-level-dependent (BOLD) fMRI data were acquired using a 1.5-T GE MRI scanner. After sagittal localization, functional T2*-weighted images were acquired using an echo-planar single-shot gradient echo pulse sequence (matrix=64×64 mm, repetition time=3000 msec, echo time=30 msec, field of view=240 mm, voxels=3.75×3.75×4 mm). Images were acquired in 31 contiguous 4-mm axial slices per brain volume, with each run lasting 4 minutes 54 seconds. In the same session, a high-resolution T1-weighed anatomical image was acquired to aid with spatial normalization (three-dimensional spoiled gradient-recall acquisition in the steady state; repetition time=8.1 msec, echo time=3.2 msec, flip angle=20°; field of view=240 mm, 124 axial slices, thickness=1.0 mm; 256×256 acquisition matrix).
Data were analyzed within the framework of the general linear model using AFNI (25). Both individual and group-level analyses were conducted. The first four volumes in each scan series, collected before equilibrium magnetization was reached, were discarded. Motion correction was performed by registering all volumes in the EPI data set to a volume collected close to acquisition of the high-resolution anatomical data set.
The EPI data sets for each participant were spatially smoothed (isotropic 6-mm kernel) to reduce variability among individuals and generate group maps. Next, the time-series data were normalized by dividing the signal intensity of a voxel at each time point by the mean signal intensity of that voxel for each run and multiplying the result by 100, producing regression coefficients representing percent signal change. Regressors for the three comment categories (transgression: intentional, unintentional, none) were created by convolving the train of stimulus events with a gamma-variate hemodynamic response function. Linear regression modeling was performed using these regressors plus regressors for a first-order baseline drift function. This produced for each voxel and each regressor a beta coefficient and its associated t statistic.
Voxel-wise group analyses involved the transformation of single-subject beta coefficients into the standard coordinate space of Talairach and Tournoux (26). Subsequently, a 2×3 (group-by-transgression) analysis of variance (ANOVA) was performed to produce statistical maps of the main effect of group and transgression and group-by-transgression interaction (p<0.005). To correct for multiple comparisons for the whole-brain analysis at p<0.005, we performed a spatial clustering operation using Alpha-Sim (http://afni.nimh.nih.gov/afni/doc/manual/AlphaSim) with 1,000 Monte Carlo simulations taking into account the entire EPI matrix. This procedure yielded a minimum cluster size with a map-wise false positive probability of p<0.05, corrected for multiple comparisons. We report results primarily for brain regions that survived this whole-brain correction procedure. Nevertheless, previous results for specific brain regions suggest regionally specific a priori hypotheses. Accordingly, we also report results from secondary analyses using a lower threshold for the insula and the medial prefrontal cortex.
After observing hypothesized group differences, post hoc analyses were performed to facilitate interpretations. For these analyses, average percent signal change was measured across all voxels within each region of interest generated from the functional mask, and data for main effects and interactions were unpacked and analyzed using appropriate follow-up tests, principally one-way repeated-measures ANOVAs, in SPSS.
Results
Behavioral Ratings Data
The behavioral data pertaining to the ratings of embarrassment and inappropriateness were analyzed using two separate 2×3 (group-by-transgression) ANOVAs. Subsequent one-way repeated-measures ANOVAs were used to unpack significant interactions. Here we first consider the embarrassment ratings. There was a significant group-by-transgression interaction (F=3.15, df=2, 60, p<0.05); whereas the patients with generalized social phobia rated the unintentional transgressions as being significantly more embarrassing compared to the healthy volunteers (F=11.32, df=1, 30, p<0.005), the two groups did not differ significantly in their ratings of neutral or intentional behaviors (Table 2). There was also a significant main effect of group; overall, the patients with generalized social phobia rated the behaviors as significantly more embarrassing compared to the healthy volunteers (F=4.26, df=1, 30, p<0.05).
| Embarrassment | Inappropriateness | |||||||
|---|---|---|---|---|---|---|---|---|
| Patients With Generalized Social Phobia | Healthy Comparison Subjects | Patients With Generalized Social Phobia | Healthy Comparison Subjects | |||||
| Transgression | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Unintentional | 3.48 | 0.57 | 2.81 | 0.55 | 2.71 | 0.77 | 2.02 | 0.74 |
| Intentional | 3.20 | 1.03 | 2.90 | 0.79 | 4.40 | 0.53 | 3.92 | 0.58 |
| None (neutral) | 1.13 | 0.11 | 1.15 | 0.11 | 1.15 | 0.13 | 1.09 | 0.11 |
TABLE 2. Embarrassment and Inappropriateness Ratings for Three Transgression Types in a Study of Social Norm Processing in Adult Social Phobia
There was also a significant group-by-transgression interaction for the inappropriateness ratings (F=3.50, df=2, 60, p<0.05); the patients with generalized social phobia rated the unintentional and intentional transgressions as being significantly more inappropriate compared to the healthy volunteers (F=6.57, df=1, 30, p<0.05 and F=5.85, df=1, 30, p<0.05, respectively), although the two groups did not differ significantly in their ratings of neutral behaviors (Table 2). As with the embarrassment ratings, there once again was a significant main effect of group; overall, the patients with generalized social phobia rated the behaviors as significantly more inappropriate compared to the healthy volunteers (F=9.32, df=1, 30, p<0.05).
EPI Data
The BOLD response data were analyzed by a 2×3 (group-by-transgression) ANOVA. Our goal was to determine whether patients with generalized social phobia showed a particularly elevated activation to unintentional (embarrassing) transgressions relative to healthy comparison subjects. Two statistical maps were critical to our predictions: group-by-transgression and group.
The analysis for group-by-transgression interactions identified two regions: the left ventromedial prefrontal cortex and a small region of the more dorsal right medial pre-frontal cortex, although this second region did not survive correction for multiple comparisons (Table 3). In line with predictions, patients with generalized social phobia showed significantly greater BOLD responses in both regions to unintentional relative to intentional transgressions (F=12.80, df=1, 15, p<0.005 and F=6.43, df=1, 15, p<0.05). In contrast, the healthy volunteers showed a trend in both regions for significantly greater activation to intentional relative to unintentional transgressions (F=3.29, df=1, 15, p=0.09 and F=3.00, df=1, 15, p=0.10), as well as an overall reduction in response to stories. Notably, group differences in activation were particularly marked for unintentional transgressions (F=20.90, df=1, 30, p<0.001, compared to F=14.36, df=1, 30, p<0.005 for intentional transgressions and F=5.67, df=1, 30, p<0.05 for neutral transgressions) (Figure 1).
| Region | Brodmann's Area | Volume (mm3) | Coordinatesb | F | df | p (Uncorrected) | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Group-by-transgression interaction | 1, 30 | |||||||
| Left ventromedial prefrontal gyrus | 10 | 1,008 | −7 | 59 | 13 | 8.74 | <0.001 | |
| Right medial prefrontal gyrus | 9 | 364 | 5 | 58 | 38 | 7.74 | 0.001 | |
| Group main effectc | 2, 60 | |||||||
| Left ventromedial prefrontal gyrus | 10 | 12,603 | −13 | 60 | 16 | 33.90 | <0.001 | |
| Left dorsomedial prefrontal gyrus | 9 | 644 | −23 | 32 | 34 | 19.32 | <0.001 | |
| Left insula | 13 | 623 | −37 | −14 | 0 | 18.23 | <0.001 | |
| Right insula | 13 | 305 | 26 | 16 | −6 | 13.67 | <0.001 | |
| Right amygdala/parahippocampal gyrus | 1,088 | 41 | −8 | −18 | 14.07 | <0.001 | ||
TABLE 3. Significant Areas of Activation for the Group-by-Transgression Interaction in a Study of Social Norm Processing in Adult Social Phobiaa
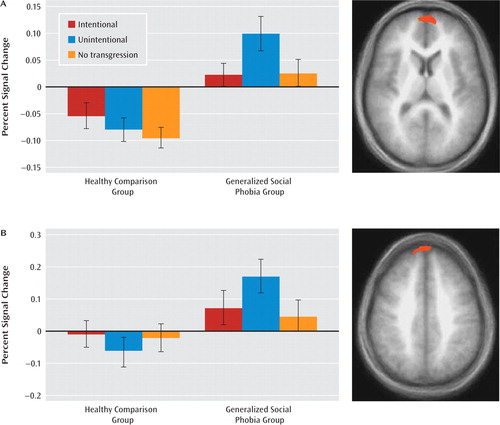
FIGURE 1. Group-by-Transgression Interactions in Individuals With Generalized Social Phobia and Healthy Comparison Subjects in a Study of Social Norm Processinga
a Blood-oxygen-level-dependent responses in the left medial prefrontal cortex (panel A) (x, y, z=−7, 59, 13) and the right medial prefrontal cortex (panel B) (x, y, z=5, 58, 38) to behaviors involving intentional, unintentional, and no social transgressions for the two groups.
There were also significant main effects of group in both a large region of the ventromedial prefrontal cortex and a slightly lateral region of the dorsal medial prefrontal cortex as well as the insula bilaterally and the right amygdala (Table 3). In all these regions, patients with generalized social phobia showed significantly greater BOLD responses across transgressions relative to the healthy volunteers (F values ranged from 12.50 to 32.69, all p values <0.001).
Although major depression was an exclusion criterion for participation in this study, patients with generalized social phobia did report a higher level of depressive symptoms on the Inventory for Depressive Symptomatology-Self Report than did the healthy volunteers (F=8.70, df=1, 30, p<0.05; Table 1). To exclude the possibility that the higher depression scores in the patient group influenced results, we used depression score as a covariate in the follow-up analyses on the extracted average percent signal change from the two main regions of interests, the medial prefrontal cortex regions identified by the group-by-transgression interaction. This inclusion of depression scores as a covariate did not change the significant results (F=5.28, df=1, 30, p=0.008 and F=5.77, df=1, 30, p=0.005, respectively), suggesting that level of depression did not significantly contribute to the results.
Correlational Analysis
Using correlational analysis, we examined whether the increased responses to unintentional transgressions in the medial prefrontal cortex in generalized social phobia were related to symptom severity. We tested whether there was a significant relationship between severity of social anxiety symptoms, as indexed by the Liebowitz Social Anxiety Scale-Self Report, and activation to unintentional transgression in the two medial prefrontal cortex regions identified by the group-by-transgression interaction. There was a trend toward a significant positive correlation for one of the two regions. However, neither test reached two-tailed significance (Pearson's r values were 0.48 and 0.19, respectively, with p values of 0.059 and 0.487).
Discussion
We examined the neural responses in generalized social phobia to intentional and unintentional embarrassing social transgressions. In line with predictions, we observed a significant group-by-transgression interaction in both the behavioral and medial prefrontal cortex neural response patterns. Patients with generalized social phobia rated unintentional, but not intentional, transgressions as significantly more embarrassing than did the healthy comparison subjects. Moreover, healthy individuals showed greater activation to intentional relative to unintentional transgressions in the ventromedial prefrontal cortex, consistent with previous research (14). Patients with generalized social phobia, in contrast, showed the opposite: significantly greater activation to unintentional relative to intentional transgressions. In addition, and again in line with predictions, patients with generalized social phobia showed increased responses relative to the comparison subjects in an extensive region of the ventromedial prefrontal cortex as well as in the amygdala and insula bilaterally to the social transgressions regardless of whether they were intentional or unintentional.
Social cognition research on the medial prefrontal cortex suggests a functional distinction between the ventral and the more dorsal medial prefrontal cortex. It has been argued that the ventromedial prefrontal cortex is particularly implicated in self-referential processing, that is, the processing of stimuli that are experienced as strongly related to one's own person (27). In contrast, the dorsal medial prefrontal cortex has been implicated in the representation of the mental states of others (19, 28–34). It is notable that in this study patients with generalized social phobia showed an atypically increased response to unintentional, embarrassing social transgressions in the ventromedial prefrontal cortex, the region particularly implicated in self-referential processing. (Although there was also a significant activation within a more dorsal region of the medial prefrontal cortex, this region did not survive correction for multiple comparisons.) We would suggest that this is because they judge such transgressions as significantly more self-relevant than do healthy individuals. Indeed, healthy individuals show a very different pattern of activity in this region, showing in this and previous work (14) greater activity to intentional rather than unintentional transgressions. It is argued that intentional transgressions have greater significance for healthy individuals and lead to generally increased activity in regions engaged in social cognition. This is because a social transgression, unmarked by embarrassment and instead demonstrating intent, marks a challenge to the social hierarchy. Hence, we speculate that these results (together with those of K.S. Blair et al., unpublished 2010 data) indicate a fundamental reorganization in self-relevant processing in patients with generalized social phobia. Evaluations of the self in generalized social phobia primarily focus on potentially embarrassing events. In healthy individuals, in contrast, evaluations of the self focus more closely on potential status challenges. Notably, these data indicate that the pathophysiology of generalized social phobia has, perhaps developmentally, extended beyond a heightened amygdala response to social threat.
In addition to the significant group-by-transgression interaction in the ventromedial prefrontal cortex, we observed a highly significant main effect of group within a large region of the ventromedial prefrontal cortex, as well as a slightly lateral region of the dorsal medial prefrontal cortex, together with the amygdala and insula bilaterally. The amygdala and the insula have been frequently identified in imaging studies involving social phobia (7), suggesting that hyperresponsiveness in those regions in generalized social phobia is not specific to the anomalous processes underlying self-referential processing in this disorder but rather extends to the processing of social stimuli more generally.
One aspect of the data from this study that should be evaluated particularly carefully—and that might suggest avenues for future work—is that for some brain regions, the presence of a group effect in the absence of a group-by-condition interaction reflects greater responsiveness in generalized social phobia to all the social stimuli, regardless of their meaning, context, or intent. Hence, findings in this study differentiate regions showing greater responsiveness in patients with generalized social phobia to social stimuli more generally (e.g., the amygdala and insula) from regions showing a greater responsiveness to social stimuli occurring specifically in self-referential contexts (e.g., the medial prefrontal cortex). Nevertheless, the design of this study allows only an initial comparison of findings across these brain regions, on a relatively restricted class of event types. Future work might employ larger collections of stimuli extending across broader classes of events, including nonsocial control stimuli. The inclusion of such stimuli could allow assessment of the possibility that generalized social phobia involves heightened generalized responsiveness in some regions to any form of emotionally salient stimulus, including both social and nonsocial stimuli.
In summary, we found that in the ventromedial prefrontal cortex, and in contrast to healthy comparison subjects, patients with generalized social phobia showed greater responses to unintentional (embarrassment-mediated) relative to intentional transgressions. Behaviorally, patients with generalized social phobia assigned the unintentional transgressions higher embarrassment ratings than did comparison subjects. These results underscore the importance of the ventromedial prefrontal cortex as part of the pathophysiology of generalized social phobia which, we speculate, may be related to distorted self-referential processing in this disorder. In addition, we observed greater amygdala and insula activation across both intentional and unintentional transgressions in the patients with generalized social phobia, further implicating those two regions in the pathophysiology of the disorder.
1. : Comorbidity of mood and anxiety disorders. Depress Anxiety 2000; 12(suppl 1):69–76Crossref, Medline, Google Scholar
2. : The impairments caused by social phobia in the general population: implications for intervention. Acta Psychiatr Scand Suppl 2003; 417:19–27Crossref, Google Scholar
3. : Incidence of social anxiety disorder and the consistent risk for secondary depression in the first three decades of life. Arch Gen Psychiatry 2007; 64:903–912Crossref, Medline, Google Scholar
4. : Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Arch Gen Psychiatry 2008; 65:1303–1312Crossref, Medline, Google Scholar
5. : Response to emotional expressions in generalized social phobia and generalized anxiety disorder: evidence for separate disorders. Am J Psychiatry 2008; 165:1193–1202Link, Google Scholar
6. : Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch Gen Psychiatry 2002; 59:1027–1034Crossref, Medline, Google Scholar
7. : Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 2007; 164:1476–1488Link, Google Scholar
8. : Neural response to self- and other referential praise and criticism in generalized social phobia. Arch Gen Psychiatry 2008; 65:1176–1184Crossref, Medline, Google Scholar
9. : Functional neuroimaging of mentalizing during the trust game in social anxiety disorder. Neuroreport 2009; 20:984–989Crossref, Medline, Google Scholar
10. : Neural mechanisms of cognitive reappraisal of negative self-beliefs in social anxiety disorder. Biol Psychiatry 2009; 66:1091–1099Crossref, Medline, Google Scholar
11. : Elevated fear conditioning to socially relevant unconditioned stimuli in social anxiety disorder. Am J Psychiatry 2008; 165:124–132Link, Google Scholar
12. : Self-criticism in generalized social phobia and response to cognitive-behavior treatment. Behav Ther 2002; 33:479–491Crossref, Google Scholar
13. : Self-criticism and social phobia in the US National Comorbidity Survey. J Affect Disord 2004; 82:227–234Crossref, Medline, Google Scholar
14. : Neural correlates of violation of social norms and embarrassment. Brain 2002; 125:1696–1708Crossref, Medline, Google Scholar
15. : Embarrassment: its distinct form and appeasement functions. Psychol Bull 1997; 122:250–270Crossref, Medline, Google Scholar
16. : Brain activation associated with evaluative processes of guilt and embarrassment: an fMRI study. Neuroimage 2004; 23:967–974Crossref, Medline, Google Scholar
17. : Caught in the act: the impact of audience on the neural response to morally and socially inappropriate behavior. Neuroimage 2006; 33:414–421Crossref, Medline, Google Scholar
18. : Modulation of cortical midline structures by implicit and explicit self-relevance evaluation. Soc Neurosci 2009; 4:197–211Crossref, Medline, Google Scholar
19. : Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron 2006; 50:655–663Crossref, Medline, Google Scholar
20. : Development and neurophysiology of mentalizing. Philos Trans R Soc Lond B Biol Sci 2003; 358:459–473Crossref, Medline, Google Scholar
21. : New theoretical conceptions of social anxiety and social phobia. Clin Psychol Rev 1989; 9:19–35Crossref, Google Scholar
22. : Structured Clinical Interview for DSM-IV Axis I Disorders (SCID). Washington, DC, American Psychiatric Press, 1997Google Scholar
23. : Screening for social anxiety disorder with the self-report version of the Liebowitz Social Anxiety Scale. Depress Anxiety 2009; 26:34–38Crossref, Medline, Google Scholar
24. : The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med 1996; 26:477–486Crossref, Medline, Google Scholar
25. : AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996; 29:162–173Crossref, Medline, Google Scholar
26. : Co-Planar Stereotaxic Atlas of the Human Brain. Stuttgart, Germany, Thieme, 1988Google Scholar
27. : Self-referential processing in our brain: a meta-analysis of imaging studies on the self. Neuroimage 2006; 31:440–457Crossref, Medline, Google Scholar
28. : Other minds in the brain: a functional imaging study of “theory of mind” in story comprehension. Cognition 1995; 57:109–128Crossref, Medline, Google Scholar
29. : Reading the mind in cartoons and stories: an fMRI study of “theory of mind” in verbal and nonverbal tasks. Neuropsychologia 2000; 38:11–21Crossref, Medline, Google Scholar
30. : A PET investigation of the attribution of intentions with a nonverbal task. Neuroimage 2000; 11:157–166Crossref, Medline, Google Scholar
31. : The link between social cognition and self-referential thought in the medial prefrontal cortex. J Cogn Neurosci 2005; 17:1306–1315Crossref, Medline, Google Scholar
32. : Neuroanatomical evidence for distinct cognitive and affective components of self. J Cogn Neurosci 2006; 18:1586–1594Crossref, Medline, Google Scholar
33. : Neural correlates of individual ratings of emotional salience: a trial-related fMRI study. Neuroimage 2004; 21:768–780Crossref, Medline, Google Scholar
34. : Cortical activations during judgments about the self and an other person. Neuropsychologia 2004; 42:1168–1177Crossref, Medline, Google Scholar



