Prefrontal Cortical Deficits and Impaired Cognition-Emotion Interactions in Schizophrenia
Abstract
Objective:
Despite schizophrenia patients' reports of diminished experience of emotion in interviews and self-report measures, their emotional experience in the presence of emotional stimuli and in daily life (“in the moment”) appears largely intact. To examine emotion-cognition interactions, the authors tested the hypothesis that schizophrenia patients have unimpaired in-the-moment emotional reactivity but have a deficit in prefrontal cortical mechanisms needed to maintain and report on experience following exposure to emotional stimuli.
Method:
Using a slow event-related functional MRI paradigm, the authors examined the brain activity of 23 schizophrenia patients and 24 healthy comparison subjects during trials in which they viewed an affective picture and, after a delay, reported their emotional experience while viewing it.
Results:
The patients' self-reports of emotional experience differed from those of the healthy subjects when they rated their experience on dimensions inconsistent with the stimulus valence but not when the dimension was consistent with it. In the presence of emotional stimuli, brain activity in the patients was similar to that of the comparison subjects. During the delay, however, patients showed decreased activation in a network of brain structures, including the dorsolateral prefrontal cortex and other prefrontal, limbic, and paralimbic areas. In patients, the delay-related response of the dorsolateral prefrontal cortex to pleasant stimuli correlated negatively with an anhedonia measure.
Conclusions:
These results suggest that schizophrenia is characterized by a failure of prefrontal circuitry supporting the link between emotion and goal-directed behavior and that the failure of this mechanism may contribute to deficits in processes related to emotion-cognition interaction.
Disturbances of emotional experience, and in particular a decrease in capacity to experience pleasure (anhedonia), have long been regarded as characteristic features of pathology in schizophrenia (1), and they have been associated with functional impairment (2). However, recent research on emotional disturbances in schizophrenia has raised fundamental questions about the nature of these deficits. Schizophrenia patients report experiencing lower levels of pleasure in their daily lives than nonpatients on measures of trait social and physical anhedonia (3). In contrast, the patients' immediate (or “in the moment”) ratings of experienced emotion are intact, in both laboratory experiments and real life, and reflect the same two-dimensional structure (valence and arousal) as that of healthy subjects (4).
A possible mechanism underlying the patients' deficits in trait reports of emotional experience can be formulated when one considers a model of cognitive control (5) in which the dorsolateral prefrontal cortex provides top-down “guided activation” that biases representations toward context-appropriate responding and away from automatic but inappropriate ones. Together with data from affective science (6), this model points toward integration of emotion and cognitive control when people make decisions based on affective states. Cognitive control processes supported by the dorsolateral prefrontal cortex have indeed been found to modulate the prediction, generation, interpretation, or regulation of emotions (7) and to translate affectively valenced motivational states into goal-oriented behavior (8). Thus, reporting on one's experienced emotion outside the presence of an eliciting stimulus, as is often the case with clinical interviews or anhedonia measures, likely relies on cognitive control processes that depend on the dorsolateral prefrontal cortex, such as establishing and maintaining representations that link and integrate affective states with task-relevant responses. Consequently, the patients' deficits as reflected in clinical interviews or anhedonia measures may stem in part from an impaired capacity to build and maintain such representations, which are necessary for generation and maintenance of emotional responses in the face of competing ones. This possibility is consistent with previous behavioral findings (e.g., as in references 9 and 10) and functional magnetic resonance imaging (fMRI) results (such as in references 11–13) indicating that schizophrenia patients exhibit impairments of dorsolateral prefrontal cortex functioning necessary for maintaining task-relevant informa-tion needed to override prepotent responses. It also accords with findings that better working memory performance in patients is associated with a smaller discrepancy between in-the-moment and delayed reports of experienced emotion (14).
fMRI studies examining brain activation during presentation of emotion-eliciting stimuli in schizophrenia (15–17) have generated variable findings, including limbic and prefrontal increases and decreases in activity in patients relative to healthy subjects. To our knowledge, no fMRI study has specifically examined the neural correlates of cognitive control processes related to maintaining and reporting on emotional experience in schizophrenia. We used event-related fMRI to explore brain activity during trials in which subjects viewed affective images and, after a delay, rated how “positive,” “negative,” and “energizing” the emotion was while they were viewing the image. Performing this task required controlled processes depending on the dorsolateral prefrontal cortex, such as active maintenance of affective representations and effective manipulation of valenced aspects of the emotional experience in order to accurately rate it on dimensions both consistent and inconsistent with its overall valence. This design allowed us to test the hypothesis that schizophrenia patients' in-the-moment emotional reactivity is spared but that they have a deficit in cognitive control processes needed to maintain and report on emotional experience after the eliciting stimulus is removed. In support of this hypothesis, we sought evidence of 1) robust brain activation during the presence of emotional stimuli, in particular in limbic and paralimbic structures, consistent with an intact ability to generate an emotional response in the presence of an emotional stimulus, and 2) altered delayed ratings of experienced emotion and delayed brain activation, in particular in areas critical for active implementation of cognitive control (e.g., dorsolateral prefrontal cortex). In contrast, the alternative hypothesis (that schizophrenia patients are characterized by a fundamental impairment in emotion generation) would be supported by impaired brain activation even during the presence of the emotional stimuli in structures involved in affective representations.
Method
Subjects
The participants were 23 patients and 24 healthy comparison subjects. Exclusion criteria for patients included 1) age above 50 years or below 18 years, 2) diagnosis of substance abuse or dependence (except one involving nicotine or caffeine) in the 6 months before testing, 3) a score less than 70 on the Wechsler Abbreviated Scale of Intelligence, 4) color blindness, 5) diagnosis of neurological disorder, and 6) pregnancy. Patient diagnoses of schizophrenia (N=21) and schizoaffective disorder (N=2) were confirmed by using the Struc-tured Clinical Interview for DSM-IV-R, administered by trained research personnel. The Brief Psychiatric Rating Scale (BPRS), the Scale for the Assessment of Positive Symptoms (SAPS), and the Scale for the Assessment of Negative Symptoms (SANS) were used to measure symptom severity. Six patients were not medicated with neuroleptics at the time of testing. The other 17 were being treated with atypical antipsychotics (risperidone, N=3; olanzapine, N=2; aripiprazole, N=8; quetiapine, N=1; ziprasidone, N=1; clozapine, N=1; perphenazine, N=1) and a typical agent (haloperidol, N=1), with one patient receiving two neuroleptics.
The groups did not differ in age, handedness, or parental education (Table 1). After data from three patients and four comparison subjects were excluded because of excessive movement during the scanning (five subjects) and visible artifacts in the functional images (two subjects), data from 20 patients (five women, 14 medicated) and 20 healthy subjects (seven women) were included in subsequent imaging analyses. These subgroups also did not differ in age, handedness, or parental education (Table 1).
| Characteristic | Full Group | Imaging Analysis Subgroup | ||||||
|---|---|---|---|---|---|---|---|---|
| Patients With Schizophrenia (N=23)a | Comparison Subjects (N=24) | Patients With Schizophrenia (N=20)b | Comparison Subjects (N=20) | |||||
| N | % | N | % | N | % | N | % | |
| Demographic | ||||||||
| Male gender | 17 | 74 | 17 | 71 | 15 | 75 | 13 | 65 |
| White race | 9 | 39 | 11 | 46 | 8 | 40 | 10 | 50 |
| Right-handed | 22 | 96 | 21 | 88 | 19 | 95 | 17 | 85 |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 29.4 | 9.6 | 29.3 | 8.1 | 28.8 | 9.6 | 28.7 | 8.0 |
| Education (years) | 13.4 | 2.0 | 15.6 | 2.0 | 13.4 | 2.1 | 15.7 | 2.2 |
| Parental education (years) | 14.2 | 2.8 | 14.8 | 2.9 | 14.3 | 2.9 | 14.8 | 3.0 |
| Clinical | ||||||||
| Brief Psychiatric Rating Scale (BPRS) score | 38.04 | 6.87 | — | — | 37.30 | 6.64 | — | — |
| Scale for the Assessment of Negative Symptoms score | 4.13 | 3.20 | — | — | 3.75 | 3.16 | — | — |
| Scale for the Assessment of Positive Symptoms score | 3.48 | 2.02 | — | — | 3.30 | 2.00 | — | — |
TABLE 1. Demographic and Clinical Characteristics of Patients With Schizophrenia and Healthy Comparison Subjects in Study of Cognition-Emotion Interactions
Healthy comparison subjects were recruited from the community. Additional exclusion criteria used for comparison subjects included 1) history of axis I disorder, 2) first-degree relative with a psychotic disorder, and 3) having been treated with any psychotropic medication within the past 6 months.
All procedures, including written informed consent, were part of a protocol approved by the institutional review board of the University of California, Davis.
Emotional Experience Task
The subjects viewed 72 picture stimuli (24 pleasant, 24 unpleasant, 24 neutral) selected from the International Affective Picture System (18) (see supplemental text in online data supplement). The stimuli were pseudorandomly presented by projector, in three runs of 24 trials each, and viewed in a mirror attached to the head coil. Each 45-second trial (Figure 1) included a picture, a delay, and three rating questions. The questions (presented in counterbalanced order relative to picture valence) asked subjects to rate the valence of the emotion experienced (“positive” and “negative”) and their arousal level (how “energized” they felt) during the presentation of the previous picture, on a 5-point scale. Subjects' responses were recorded online by means of a five-button MRI-compatible response unit attached to the dominant hand. The rare ratings with a latency below 300 msec were considered the result of guessing or late responses to the preceding scale, and therefore they were not included in the behavioral analyses.

a The picture shown is an example of a neutral stimulus. Each picture was presented for 5 seconds, followed by a 12.5-second maintenance period, during which subjects viewed a fixation point. After the maintenance period, subjects were instructed to rate how “positive,” how “negative,” and how “energized” they felt during the previously viewed picture, on a 5-point scale. The three scales (“positive,” “negative,” “energized”) were present on the screen for 5 seconds each, and their order was counterbalanced relative to stimulus valence (pleasant, unpleasant, or neutral). Following the three rating scales, a fixation point was presented as an intertrial interval of 12.5 seconds. Thus, the delay between two consecutive pictures was 40 seconds. The long delay between the stimulus and ratings and the long intertrial interval allowed for a good estimate of the baseline activity without the need for separate fixation trials. On the graphical timeline at the top of the figure, the arrows mark the onset of the regressors used to model the events in the multiple regression analysis of the fMRI data (in order: stimulus, delay, first scale, second scale, third scale).
Functional Imaging
Functional T2* magnetic resonance volumes were acquired by using a 1.5-T GE Signa scanner (spiral “in-out” sequence, 32 oblique coronal 6-mm-thick slices, 64×64 matrix, 220-mm field of view, TR=2.5 sec, TE=35 msec, flip angle=70°). After slice time correction and motion correction, data were excluded if the mean total movement exceeded 1 voxel or if the mean scan-to-scan movement exceeded 1 mm. For subjects included in the imaging analyses, the motion parameters were not different across groups, according to a multivariate analysis of variance (MANOVA) (group-by-parameter interaction: p>0.3). For these subjects, the data were filtered for low-frequency drift and differences in mean signal across runs, resliced to 2-mm3 voxels, spatially normalized (with the Montreal Neurological Institute refer-ence brain, 90-parameter nonlinear warping algorithm [19]), and spatially smoothed (8-mm full width at half maximum three-dimensional Gaussian filter).
Statistical Analyses
The statistical tests were conducted in SPM5 (http://www.fil.ion.ucl.ac.uk/spm) by using a multiple regression model that included 10 regressors. Three were for picture onset (one per valence), three were for delayed activity (6 seconds after the end of picture presentation, capturing activity during the last 2–3 seconds of the delay [20]), and three were for the rating scales (“positive,” “negative,” and “energized”). The other one marked excluded events, either ratings with reaction times less than 300 msec or entire trials in which the reaction time was less than 300 msec for the scale matching the stimulus valence, i.e., the “positive” scale for pleasant pictures, the negative scale for unpleasant pictures, and the energized scale for neutral pictures. For most subjects, fewer than 10% of the trials were excluded (data from four patients and one healthy subject lost 11%–18% and 24% of the trials, respectively). This did not result in a statistically significant combined effect of stimulus valence across groups in the proportion of excluded trials (one-way between-group MANOVA with pleasant, unpleasant, and neutral trials as dependent variables: F=0.51, df=3, 36, p=0.10, Wilks's lambda=0.84).
The parameter estimates obtained from fitting this omnibus model were used to compute within-subjects contrasts (e.g., delay activity after pleasant picture versus baseline activity), which were used in group-level random-effects analyses. Given previous reports of altered brain activity in response to neutral pictures in patients (15), we explored the brain responses to the three types of stimuli individually, rather than as pleasant versus neutral and unpleasant versus neutral subtractions, which can obscure complex interactions driven by group differences in the subtracted condition (i.e., neutral). Consequently, the alpha levels used for group-level multiple comparisons were Bonferroni corrected to account for separate examinations of the three types of stimuli (that is, a group-level contrast was considered corrected only if it reached a corrected alpha level of 0.016 or less, i.e., approximately 0.05/3). Since testing our spe-cific hypothesis required investigating a network of regions specified by previous results, we used the set level alpha of <0.016 within a volume of search (created by using the Wake Forest PickAtlas toolbox of SPM5) that included the dorsolateral prefrontal cortex, medial prefrontal cortex, orbitofrontal cortex/basal ganglia, thalamus, hippocampal formation, amygdala (21, 22), and fusiform face area (as a positive control for stimulus-related nonspecific activity).
For the schizophrenia patients, we computed correlations between individual differences in brain activity (as indexed by each subject's beta weight of a given regressor, averaged across all the voxels of a region of interest) and a clinical rating of anhedonia, computed as the average of the anhedonia items from the SANS anhedonia/asociality subscale (i.e., recreational interests and sexual interest/activity) (23).
Results
Delayed Experience Ratings
Three separate 2×3 analyses of variance (ANOVAs) were conducted for the “positive,” “negative,” and “energized” ratings, with group as a between-subjects factor (patient, comparison subject) and stimulus valence as a within-subjects factor (pleasant, unpleasant, neutral) (Figure 2). For each rating type, the only significant main effect was that of the stimulus valence, indicating that all subjects' experience was most positive in response to pleasant stimuli, most negative in response to unpleasant ones, and more energizing in response to pleasant and unpleasant pictures than to neutral pictures. The interactions between group and stimulus valence were significant for the “positive” and “energized” ratings but did not reach significance for negative ratings. These interactions appeared to be driven by group differences in ratings of feelings that were inconsistent with the stimulus (i.e., how positive subjects felt while viewing an unpleasant picture and how negative they felt when exposed to a pleasant picture). To examine this effect more closely, we grouped the stimuli in terms of their degree of match with the rating type. This resulted in two straightforward groupings of stimuli, namely one for “positive” ratings (for which pleasant pictures were a match and for which unpleasant or neutral pictures were a mismatch) and one for “negative” ratings (for which unpleasant pictures were a match and pleasant or neutral pictures were a mismatch). The results of these ANOVAs (Figure 2B) confirmed that the patients differed from the healthy subjects in the experience of mismatch stimuli: the patients reported more positive emotion in response to unpleasant or neutral pictures and more negative emotion in response to pleasant or neutral pictures than did the comparison subjects.
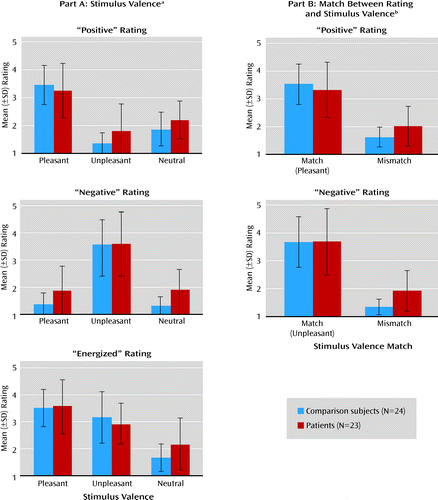
a Data are displayed according to the type of rating (how “positive,” “negative,” or “energized” the subject felt while viewing the preceding picture) and valence of the stimulus (pleasant, unpleasant, or neutral picture). The difference in mean ratings across stimulus valence illustrates higher “positive” ratings for pleasant stimuli than for unpleasant or neutral ones, higher “negative” ratings for unpleasant stimuli than for pleasant or neutral ones, and higher “energized” ratings for pleasant and unpleasant stimuli than for neutral ones. The main effect of stimulus valence was significant in the “positive” ratings (F=83.03, df=2, 44, p<0.001), “negative” ratings (F=83.03, df=2, 44, p<0.001), and “energized” ratings (F=78.92, df=2, 44, p<0.001). The group-by-valence interactions were significant for the “positive” ratings (F=3.28, df=2, 44, p=0.05) and “energized” ratings (F=3.88, df=2, 44, p=0.03) but not for the “negative” ratings (F=2.02, df=2, 44, p=0.14).
b Data for “positive” and “negative” ratings are displayed according to the degree of match between the type of rating and stimulus valence. For “positive” ratings, pleasant pictures were considered matching stimuli while unpleasant or neutral ones were mismatches; for “negative” ratings, unpleasant pictures were matching stimuli, while pleasant or neutral ones were mismatches. Group differences were reflected in significant group-by-match interactions for “positive” ratings (F=5.20, df=1, 45, p=0.03) and “negative” ratings (F=3.88, df=1, 45, p=0.05). Analyses of simple main effects confirmed that these interactions were driven by differences between the patients and comparison subjects in the ratings when the stimulus valence was a mismatch relative to the type of experience being rated (p=0.02 and p=0.001 for “positive” and “negative” ratings, respectively) but not when the stimulus valence matched the rating (p>0.39 in both cases).
Note that all of the condition-related and between-group differences just described were present, albeit less robustly, in the subgroup of subjects included in the fMRI analyses (see Figure S1 in the online data supplement).
fMRI Results
We explored the brain responses to each type of stimulus (pleasant, unpleasant, neutral) separately (15) (see Method section), but the pleasant versus neutral and the unpleasant versus neutral subtractions were also examined post hoc, and these were consistent with the differences noted across valences (see Tables S2 and S3 in the online data supplement).
Stimulus-related activity.
The direct group contrasts of the activity during presentation of pleasant, unpleasant, and neutral stimuli showed no evidence of differential activity in patients relative to comparison subjects (the uncorrected statistical maps are provided as Figure S2 in the online data supplement). Within-group analyses conducted separately in the patient and comparison groups confirmed that this lack of group differences was likely due to robust, extensive activation by both groups in response to all types of stimuli (Figure 3). In both groups, these activations were located in several regions predicted by our model, including dorsolateral prefrontal areas (Brodmann's areas 8, 9, 46), the orbitofrontal and ventromedial prefrontal cortex, the basal ganglia, and the amygdalae. The complete list of activations identified by these analyses and the coordinates of local maxima are provided as Table S2 in the online data supplement. While the patients appeared to show less extensive activation in the presupplementary motor and supplementary motor areas on the medial frontal wall, these differences were not statistically significant in the between-group contrasts (see discussion and Table S2 and Figure S2 in the supplemental material).
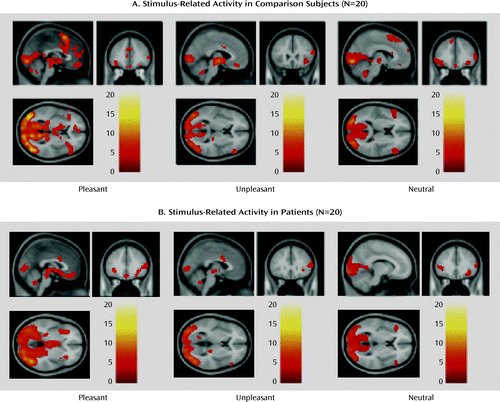
a Whole-brain statistical parametric maps of brain activity during presentation of pleasant, unpleasant, and neutral pictures, obtained from separate analyses of each group. Significant activation was noted in a similar set of brain structures, many of which were included in the predefined volume of the search mask, which included the lateral, medial prefrontal, and orbitofrontal cortices, basal ganglia, amygdalae, and fusiform face areas. Activation in the medial prefrontal presupplementary motor/supplementary motor areas during presentation of pleasant and neutral stimuli appeared more extensive in the healthy subjects, but this difference was not statistically significant (see Figure S2 in online data supplement).
Delay-related activity.
In notable contrast to the stimulus-related analysis, the direct group contrast of delay-related activity revealed several brain areas with stronger activity in the comparison subjects than in the patients (Figure 4A), including several prefrontal foci, both dorsolateral and orbitofrontal/ventromedial. These group differences were confirmed by the two separate within-group analyses: these revealed robust activation in comparison subjects in an extensive network of dorsal prefrontal, limbic, and paralimbic regions (Figure 4B); the same analyses in the patient group showed very little activity, which did not survive the correction for multiple comparisons. The online data supplement includes the complete list of statistically significant activations (Table S3 in online data supplement) and a figure of the uncorrected statistical maps generated by the within-group analysis in the schizophrenia patients (supplemental Figure S3).
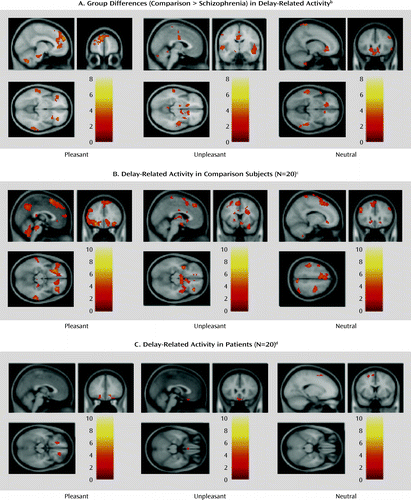
a Whole-brain statistical parametric maps of brain activity during the delay phase of an emotional experience task featuring pictures with pleasant, unpleasant, and neutral valence.
b Several of these regions fell within the predefined volume of search and survived the criterion of correction for multiple comparisons. These regions included the dorsolateral, medial, and ventrolateral prefrontal cortices for pleasant stimuli, medial frontal (supplementary motor area) and basal ganglia for unpleasant stimuli, and dorsolateral, ventromedial, and ventrolateral prefrontal cortices for neutral stimuli.
c For each type of stimulus, significant activation was generally noted in a supraset of the brain structures that showed greater activity for the healthy subjects than for the patients in the direct group contrast for the corresponding stimulus type.
d The few suprathreshold clusters identified (all shown in the figure) did not survive the correction for multiple comparisons. Uncorrected maps (p<0.05) are presented in Figure S3 in the online data supplement.
Correlations with clinical measures and task performance.
In order to explore the hypothesized inverse relationship between dorsolateral prefrontal functioning and severity of negative symptoms, we computed the correlation between the clinical rating of anhedonia and measures of brain activity in the left and right dorsolateral prefrontal cortex after the delay in trials with pleasant stimuli (Figure 5). The activity of the dorsolateral prefrontal cortex in the left hemisphere was nega-tively correlated with the anhedonia rating (r=−0.47, df=18, p=0.04), and the correlation with activity on the right ap-proached statistical significance (r=−0.33, df=18, p=0.16). However, correlations between brain activity and overall sever-ity of symptoms (indexed by the total BPRS score) were not statistically significant (p>0.3 in both cases). We also conducted post hoc analyses and found that the correlation between activity in the right orbitofrontal cortex and the anhedonia rating was significant for the patients. Moreover, the correlation between delay-related brain activity and task performance (indexed by the difference between matched and mismatched ratings of emotional experience) was positive and stronger in the dorsolateral prefrontal cortex in healthy subjects than in patients, but it was similar across groups in the parahippocampal gyrus and orbitofrontal cortex (see additional text in the online data supplement).

a The regions of interest in the dorsolateral prefrontal cortex were obtained by isolating two clusters of voxels that showed greater activity in comparison subjects than in patients during the delay phase of task trials with pleasant stimuli. The left region of interest was a cluster of 50 voxels isolated from a cluster with a local maximum peak at coordinates −18, 52, 34 in the Montreal Neurological Institute system, while the right region of interest included 41 voxels with the peak at 48, 16, 34. Both correlation coefficients were negative (−0.47 and −0.33, respectively), although only the one for activity on the left reached statistical significance (df=18, p=0.04 and p=0.16, respectively, two-tailed).
Discussion
The main findings of this study were 1) in the presence of stimuli with affective content, the brain activity of schizophrenia patients was similar to that of comparison subjects in a network of prefrontal, limbic, and paralimbic structures, 2) during the delay between stimulus offset and emotion ratings (when maintenance and evaluation of past emotions required significant support from cognitive control), patients showed less activation in this network, which included the dorsolateral prefrontal cortex, and furthermore, 3) the patients' ratings of emotional experience showed evidence of impairment when the emotion was rated on dimensions incongruent with the stimulus valence, and 4) the delay-related activity elicited in the patients' dorsolateral prefrontal cortex by pleasant stimuli correlated negatively with a clinical measure of anhedonia. Taken together, these results suggest that schizophrenia may be characterized by a failure of the prefrontal circuitry supporting the critical link between affect and goal-directed behavior.
These results help shed new light on the precise mechanism behind disturbances of emotion-cognition interactions in schizophrenia, and they may provide a parsimonious account of the variability noted in previous studies. The experimental design used here allowed for a clearer separation than in previous studies between the immediate emotional experience and the control processes necessary for ratings of that experience. This has allowed us to detect robust in-the-moment affect-related brain activity in patients, as in another recent study (24), followed by prominent hypoactivity during the delay in much of the same affect-related circuitry, as well as in control-related structures (necessary for the maintenance and reporting of affective experience after the stimulus offset). The significant negative correlation between delay-related dorsolateral prefrontal cortex activity and anhedonia supports the idea that much of the disconnect between emotional experience and behavior observed in patients, at least in laboratory contexts, may be related to dysfunctional top-down support from cognitive control processes (14, 25). Furthermore, the variability in previous results may be accounted for, at least in part, by disturbances in the patients' neural substrates of cognitive control. It is likely that these deficits may have contributed, by means of dynamic interactions with affect-related systems (7, 26), to manifestations of group differences in emotion-related brain activity, especially since the majority of previous studies used designs that led to significant overlap between brain activity related to experiencing emotion and that elicited by cognitive control (24).
The translation of affective-motivational states into goal-directed behavior is thought to depend critically on effective implementation of top-down controlled processes (8, 27). Therefore, deficits in cognitive control may also contribute to impaired representations of the value of outcomes (28) and may account for a hypothesized decoupling of affect from motivated behavior (29). In our study, the patients' dorsolateral prefrontal hypoactivity was weakly correlated with ratings of avolition, but the correlation did not reach statistical significance. However, since deficits in striatal responses to primary reinforcers correlated with avolition in other studies (30), the specific role of cognitive control dysfunction in schizophrenia patients' avolition symptoms needs further exploration.
The precise nature of the dorsolateral prefrontal-dependent control processes supporting delayed reporting of emotional experience cannot be unequivocally resolved by this experiment. For instance, the timing of control processes may vary across subjects, although it is unlikely that the two groups differed in this respect. Notably, post hoc analyses of correlations between brain activation and task performance (Table S1 in the online data supplement) provided indirect evidence consistent with a critical role of sustained dorsolateral prefrontal cortex activity in delayed ratings of emotional experience, in particular for ratings that did not match the valence of the stimulus (i.e., mismatch ratings). Furthermore, the patients' abnormalities in the delayed mismatch ratings appeared related primarily to dysfunction of the dorsolateral prefrontal cortex, rather than other brain areas previously proposed to be dysfunctional in schizophrenia, such as the hippocampus, or other structures involved in affective representations, such as the orbitofrontal cortex (see “Comparison of DLPFC and Other Brain Areas…” in online supplemental text file). Considering the numerous previous reports that dysfunction of the same prefrontal area (Brodmann's area 9) correlates with failure of cognitive function in schizophrenia, even when emotional states are irrelevant to task performance, this dorsolateral prefrontal functional deficit is probably not valence specific or even emotion related. Instead, it is likely that prefrontal representations are the actual contextual link between affective states and task-appropriate responses, and these representations appear to degrade faster in patients, similar to previously described deficits in task context processing (11). This may also explain why the patients' performance was similar to that of the healthy subjects when such representations were more readily available (i.e., for ratings matching the stimulus valence). Nevertheless, these mechanisms remain to be definitively characterized by more studies involving systematic manipulations of the affective aspects and the cognitive demands of the task.
Another potential limitation is that most patients were chronically ill and, consequently, medicated. It is unlikely that medication status can fully account for our findings, as previous results have shown emotional responding not to be strongly affected by medication (31) and deficits in prefrontal cognitive processes have been documented in first-episode and never-medicated schizophrenia patients (11, 12). Nevertheless, future studies of individuals at risk for developing schizophrenia or of medication-naive first-episode patients would be helpful in understanding what, if any, role medication or illness duration has in this type of task.
It is interesting that in the within-group statistical maps of stimulus-related activity, some activations in the presupplementary motor and supplementary motor areas appeared to be more extensive in healthy subjects than in patients (Figure 3), and these areas have been implicated in various aspects of motor control (32). While this apparent difference was not statistically significant, it could, theoretically, reflect a real but small effect. Indeed, such an effect would be consistent with the hypothesized reduced cognitive control in schizophrenia patients. Another aspect worth noting is that the delay between stimulus offset and experience rating was evidently short enough so that the patients and comparison subjects did not differ in their matched ratings, a finding that has been repeatedly reported when ratings were not delayed (15, 25, 33, 34). However, differences did emerge in ratings of experience that did not match the stimulus. These findings extend previous reports of stronger aversion reported by patients in response to positive and neutral stimuli (35), as well as stronger arousal in response to neutral stimuli (4), but their precise mechanisms remain elusive. Previous results suggest that encoding of the valence of emotional stimuli, at least negative stimuli, depends on controlled processes supported by the dorsolateral prefrontal cortex (36). Thus, rating emotional experiences on dimensions inconsistent with the stimulus valence may require support from control processes in order to overcome prepotent responding to a valence that is a match between stimulus and rating type. Our additional correlational analyses support this idea (see Table S1 in the online data supplement). Nevertheless, further research is necessary to confirm that this mechanism contributes to the higher mismatch ratings in patients (see also reference 35).
In conclusion, the results presented here suggest that a disturbance of prefrontal cognitive control processes may account to a greater degree than previously thought for the hypothesized dysfunctional interaction of cognition and emotion in schizophrenia. As such, they provide a basis for formulating and testing hypotheses in future research aimed at investigating the nature of neural deficits in this disorder, their clinical correlates, and even possible therapeutic approaches.
1. : Dementia Praecox or the Group of Schizophrenias (1911). Translated by Zinkin J. New York, International Universities Press, 1950Google Scholar
2. : Affect and social functioning in schizophrenia, in Handbook of Social Functioning in Schizophrenia. Edited by Mueser KTarrier N. Needham Heights, Mass, Allyn & Bacon, pp 181–196Google Scholar
3. : Anhedonia, positive and negative affect, and social functioning in schizophrenia. Schizophr Bull 1998; 24:413–424Crossref, Medline, Google Scholar
4. : Emotional response deficits in schizophrenia: insights from affective science. Schizophr Bull 2008; 34:819–834Crossref, Medline, Google Scholar
5. : An integrative theory of prefrontal cortex function. Annu Rev Neurosci 2001; 24:167–202Crossref, Medline, Google Scholar
6. : Affective neuroscience and psychophysiology: toward a synthesis. Psychophysiology 2003; 40:655–665Crossref, Medline, Google Scholar
7. : The cognitive control of emotion. Trends Cogn Sci 2005; 9:242–249Crossref, Medline, Google Scholar
8. : Distinguishing expected negative outcomes from preparatory control in the human orbitofrontal cortex. Brain Res 2008; 1227:110–119Crossref, Medline, Google Scholar
9. : Context-processing deficits in schizophrenia: diagnostic specificity, 4-week course, and relationships to clinical symptoms. J Abnorm Psychol 2003; 112:132–143Crossref, Medline, Google Scholar
10. : Intact attentional control of working memory encoding in schizophrenia. J Abnorm Psychol 2006; 115:658–673Crossref, Medline, Google Scholar
11. : Specificity of prefrontal dysfunction and context processing deficits to schizophrenia in never-medicated patients with first-episode psychosis. Am J Psychiatry 2005; 162:475–484Link, Google Scholar
12. : Association of dorsolateral prefrontal cortex dysfunction with disrupted coordinated brain activity in schizophrenia: relationship with impaired cognition, behavioral disorganization, and global function. Am J Psychiatry 2008; 165:1006–1014Link, Google Scholar
13. : Dorsolateral prefrontal cortex activity during maintenance and manipulation of information in working memory in patients with schizophrenia. Arch Gen Psychiatry 2005; 62:1071–1080Crossref, Medline, Google Scholar
14. : Anhedonia and the experience of emotion in individuals with schizophrenia. J Abnorm Psychol 2007; 116:30–42Crossref, Medline, Google Scholar
15. : Medial frontal hyperactivity in reality distortion. Biol Psychiatry 2007; 61:1171–1178Crossref, Medline, Google Scholar
16. : Differential hemodynamic brain activity in schizophrenia patients with blunted affect during quetiapine treatment. J Clin Psychopharmacol 2005; 25:367–371Crossref, Medline, Google Scholar
17. : An fMRI study of differential neural response to affective pictures in schizophrenia. Neuroimage 2004; 22:1247–1254Crossref, Medline, Google Scholar
18. : International Affective Picture System (IAPS): Instruction Manual and Affective Ratings, in Technical Report A-4. Gainesville, University of Florida, Center for Research in Psychophysiology, 1999Google Scholar
19. : Automated image registration, II: intersubject validation of linear and nonlinear models. J Comput Assist Tomogr 1998; 22:153–165Crossref, Medline, Google Scholar
20. : The neural substrate and temporal dynamics of interference effects in working memory as revealed by event-related functional MRI. Proc Natl Acad Sci USA 1999; 96:7514–7519Crossref, Medline, Google Scholar
21. : Detecting activations in PET and fMRI: levels of inference and power. Neuroimage 1996; 4(3, pt 1):223–235Crossref, Medline, Google Scholar
22. : Impaired prefrontal cortical function and disrupted adaptive cognitive control in methamphetamine abusers: a functional magnetic resonance imaging study. Biol Psychiatry 2009; 65:706–709Crossref, Medline, Google Scholar
23. : Anhedonia in schizophrenia: a review of assessment strategies. Schizophr Bull 2006; 32:259–273Crossref, Medline, Google Scholar
24. : Anhedonia and emotional experience in schizophrenia: neural and behavioral indicators. Biol Psychiatry 2010; 67:902–911Crossref, Medline, Google Scholar
25. : What aspects of emotional functioning are impaired in schizophrenia? Schizophr Res 2008; 98:239–246Crossref, Medline, Google Scholar
26. : Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biol Psychiatry 2007; 61:198–209Crossref, Medline, Google Scholar
27. : Motivation and cognitive control in the human prefrontal cortex. Nat Neurosci 2009; 12:939–945Crossref, Medline, Google Scholar
28. : Reward processing in schizophrenia: a deficit in the representation of value. Schizophr Bull 2008; 34:835–847Crossref, Medline, Google Scholar
29. : Patients with schizophrenia demonstrate dissociation between affective experience and motivated behavior. J Abnorm Psychol 2007; 116:268–278Crossref, Medline, Google Scholar
30. : Patients with schizophrenia have a reduced neural response to both unpredictable and predictable primary reinforcers. Neuropsychopharmacology 2009; 34:1567–1577Crossref, Medline, Google Scholar
31. : Schizophrenic patients show facial reactions to emotional facial expressions. Psychophysiology 1999; 36:186–192Crossref, Medline, Google Scholar
32. : Role of the human medial frontal cortex in task switching: a combined fMRI and TMS study. J Neurophysiol 2002; 87:2577–2592Crossref, Medline, Google Scholar
33. : Does anhedonia in schizophrenia reflect faulty memory for subjectively experienced emotions? J Abnorm Psychol 2006; 115:496–508Crossref, Medline, Google Scholar
34. : Flat affect in schizophrenia does not reflect diminished subjective experience of emotion. J Abnorm Psychol 1993; 102:507–517Crossref, Medline, Google Scholar
35. : Emotional experience in patients with schizophrenia revisited: meta-analysis of laboratory studies. Schizophr Bull 2010; 36:143–150Crossref, Medline, Google Scholar
36. : Two routes to emotional memory: distinct neural processes for valence and arousal. Proc Natl Acad Sci USA 2004; 101:3310–3315Crossref, Medline, Google Scholar



