Chronic Insomnia
Ms. F, a 42-year-old divorced woman, presents for evaluation of chronic insomnia. She complains of difficulty falling asleep, often 30 minutes or longer, and difficulty maintaining sleep during the night, with frequent awakenings that often last 30 minutes or longer. These symptoms occur nearly every night, with only one or two “good” nights per month. She typically goes to bed around 10:00 p.m. to give herself adequate time for sleep, and she gets out of bed around 7:00 a.m. on work days and as late as 9:00 a.m. on weekends. Her nighttime sleep problems result in daytime irritability and difficulty focusing and organizing her thoughts, which subjectively impair her work as an administrative assistant, although her performance evaluations have been satisfactory. She says that she has “no energy for anything extra,” that her house is a mess, and that she routinely declines invitations to join social and even family activities. Her insomnia began approximately 5 years ago during a period of increased life stress related to a difficult divorce and a job change. At that time she was diagnosed with major depression and was started on a successful trial of escitalopram, which she continues at a dose of 10 mg/day. Her current symptoms are distinct from those that were associated with her episode of major depression. She denies pervasive sadness or loss of interest, but she is very frustrated with her inability to function more effectively, which she attributes to her insomnia. In fact, she believes that her cognitive difficulties and irritability are most noticeable after nights of particularly poor sleep. Her medical history is unremarkable other than a past history of Graves’ disease. She has been treated with levothyroxine for the past 15 years. How should Ms. F be evaluated? What medical testing, if any, would be appropriate? What factors should be considered in formulating a treatment plan? What treatments would be appropriate?
Insomnia Definitions
“Insomnia” is used to refer to both a symptom and a disorder. The symptom of insomnia is defined as a subjective complaint of difficulty falling asleep, difficulty staying asleep, or poor quality sleep. In DSM-IV-TR (1) , insomnia symptoms are included among the diagnostic criteria for several other mental disorders, including major depressive disorder and generalized anxiety disorder. Insomnia disorders are characterized by insomnia symptoms accompanied by significant distress or impairment. In DSM-IV-TR the specific diagnosis of primary insomnia is further defined by a duration of at least 1 month and by symptoms that do not occur exclusively during the course of another sleep disorder, mental disorder, or medical disorder or result from use of substances or medications. Finally, DSM-IV-TR includes diagnoses of “secondary” insomnia disorders, that is, insomnia that causes significant distress or impairment or warrants independent clinical attention but is believed to be directly related to a coexisting mental disorder or medical disorder or to the effects of substances or medications.
Sleep medicine specialists use the International Classification of Sleep Disorders, Second Edition (ICSD-2) (2) , to diagnose sleep disorders. ICSD-2 describes general criteria that are common to all insomnia disorders ( Table 1 ) as well as eight specific insomnia disorders (in addition to “unspecified” and “not otherwise specified” categories), each of which meets the general criteria along with more specific diagnostic criteria. For most purposes, the general insomnia criteria proposed in ICSD-2 serve as a useful basis for discussing insomnia as a clinical disorder.
Other classification schemes for insomnia have also been used. For instance, symptom-based classifications distinguish between sleep-onset and sleep-maintenance insomnia. However, longitudinal studies suggest that these specific symptoms have limited stability over time (3) ; patients who present with sleep-onset insomnia at one time may present with frequent awakenings at a later time. Moreover, a majority of patients present with some combination of symptoms. Duration-based classifications (acute, short-term, and chronic) have also been suggested (4) . Such classifications may provide clues to the cause of insomnia. For instance, acute and short-term insomnia are more often related to life stresses, acute illnesses, or medications, whereas chronic insomnia is more likely to be related to behavioral factors or the effects of chronic mental or medical disorders. However, the majority of patients in clinical trials and in clinical practice have chronic symptoms, which is the focus of this review.
Distinctions have also been drawn between primary and secondary insomnia disorders. The underlying rationale is that secondary insomnia is caused by another disorder, whereas primary insomnia has no other identifiable cause. However, as noted in the 2005 National Institutes of Health (NIH) State of the Science Conference statement on the Manifestations and Management of Chronic Insomnia in Adults (5) , such distinctions may not be helpful clinically. Insomnia often has multiple causes, and distinguishing when another condition “causes” insomnia can be difficult. For instance, the course of insomnia may not follow the course of the other disorder, and the two conditions may require different treatments. For this reason, the NIH conference commended the term “comorbid insomnia” as a preferable alternative to the term “secondary insomnia.”
Epidemiology
The prevalence of insomnia differs depending on the specific case definition used and the population assessed. However, whatever definition is used, insomnia remains the most prevalent sleep disorder in the population. Insomnia symptoms—that is, the complaint in the absence of specific duration or distress criteria—occur in some 30%–40% of adults, and specific insomnia disorders—that is, the complaint together with meeting duration and impairment criteria—occur in 5%–10% of adults (6) . The latter is most relevant to clinical practice, since the majority of individuals with insomnia symptoms do not present for medical evaluation or treatment unless they have significant distress or impairment. Rates of insomnia are higher in medical and psychiatric care settings than in the general population.
A number of consistent risk factors for insomnia have been identified (6 , 7) . The strongest of these is concurrent depressive symptoms. Female sex is also consistently associated with insomnia, with a ratio of approximately 1.4:1. Increasing age, comorbid medical disorders, and comorbid psychiatric disorders are other consistent risk factors. Additional evidence also suggests that being separated or divorced, having lower income, lower socioeconomic status, increased chronic life stress, and black race are also associated with insomnia prevalence.
Insomnia often runs a chronic course. Longitudinal studies suggest that approximately 50% of individuals with insomnia continue to have symptoms after follow-up periods of 1 year or longer, and most cross-sectional studies of insomnia patients report a duration of several years (8 – 10) . Although the number of true longitudinal insomnia studies is small, evidence suggests that improvement in medical and psychiatric conditions is associated with improvement in insomnia (11) . As noted above, cross-sectional associations between insomnia and psychiatric disorders are strong, but additional evidence suggests that insomnia is a risk factor for the development of psychiatric disorders and for poor outcomes in these disorders. For instance, approximately a dozen longitudinal studies have demonstrated that insomnia is an independent risk factor for subsequent development of depression (12) . This relationship has been observed from adolescence to later adulthood and is maintained after adjustment for concurrent depressive symptoms. In addition, insomnia is one of the most common persistent symptoms in individuals treated for depression, and its presence is a risk factor for nonresponse to depression treatment and for recurrence following remission (13 – 15) .
Evaluation
A thorough clinical history is the cornerstone of evaluation for chronic insomnia (16) . The evaluation should focus on the description of current symptoms, including not only the type of sleep disturbance at night but also sleep habits and patterns. In particular, the clinician should inquire about times of going to bed and getting out of bed, variability in sleep timing from day to day, and emotional, cognitive, and physical states surrounding sleep. Symptoms of other specific sleep disorders should also be considered. These include loud snoring and witnessed breathing pauses, which might suggest sleep apnea, and motor restlessness and involuntary leg movements, which might suggest restless legs syndrome.
Daytime consequences associated with insomnia should also be evaluated. The most common complaints include mood disturbance (typically described as irritability and mood lability, rather than depression or anxiety), fatigue, and complaints of cognitive inefficiency or difficulty concentrating. The majority of patients with chronic insomnia do not actually complain of daytime sleepiness, that is, the tendency to fall asleep in inappropriate situations. Rather, insomnia appears to be associated with difficulty sleeping at any time during the 24-hour day.
The evaluation of insomnia should also include careful consideration of comorbid psychiatric and medical disorders as well as medications and substances that might interfere with sleep ( Table 2 ). Virtually any psychiatric disorder can be associated with insomnia, but depressive and anxiety disorders are especially common. Likewise, a wide range of medical and neurologic conditions can be associated with insomnia. Particular attention should be given to those conditions that are associated with pain, breathing difficulty, and impaired mobility. Medications that affect any CNS neurotransmitter can be associated with insomnia. Common examples include high doses of caffeine, alcohol, and antidepressants, particularly selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and atypical antidepressants.
It is often useful to view the insomnia history in terms of predisposing, precipitating, and perpetuating factors (17) . These factors can help identify potential causes of insomnia and treatment targets. Predisposing factors include family/genetic diathesis and chronic mental or medical disorders that increase the likelihood of insomnia symptoms. Precipitating factors are acute events or experiences that may push vulnerable individuals over the threshold into acute insomnia. Perpetuating factors include behavioral patterns (e.g., spending excessive time in bed) and medical or medication factors that maintain insomnia symptoms over time.
The clinical history can be usefully supplemented by collection of a 2-week sleep-wake diary. This is a prospective charting of a patient’s actual sleep hours and habits, and it can usefully identify variability in sleep patterns and specific daytime correlates that may provide targets for subsequent intervention ( Figure 1 ).

a In a graphic sleep diary, each day is displayed on one horizontal line, with times of day along the horizontal axis. In this example, midnight is displayed in the middle of the page, and midnight and 8:00 a.m. have been circled. The patient indicates the time of going to bed with a down arrow, the time of getting out of bed with an up arrow, and estimated actual sleep time with a horizontal line. In the far right column, the patient rates sleep quality on a scale of 1–10. This example shows day-to-day regularity in times for going to bed and getting up, with interrupted sleep in between.
Specific laboratory testing has limited utility in the diagnosis and assessment of insomnia. However, in specific situations, general metabolic panels, CBC, or endocrine testing (e.g., thyroid hormone) may be useful. Overnight sleep studies with polysomnography are not routinely indicated in the evaluation of chronic insomnia. However, in specific situations, such testing may be useful. For example, individuals with a high index of suspicion for sleep apnea should be referred for polysomnographic testing. Risk factors include obesity, loud snoring, witnessed pauses in breathing, craniofacial abnormalities, or factors that might lead to a central dysregulation of breathing, such as congestive heart failure and stroke. Insomnia patients with unusual behaviors during sleep, such as violent behavior, should also be considered for polysomnography. Finally, failure to respond to usual insomnia treatments may warrant referral to a sleep specialist and, in some cases, polysomnography.
Differential Diagnosis
As noted above, distinctions between primary and secondary forms of insomnia are of limited clinical utility because of the difficulty in establishing causality. ICSD-2 lists eight specific insomnia diagnoses, each distinguished by specific clinical features. However, in general practice, it is most useful to identify an insomnia disorder using the general criteria listed in Table 1 , and then to distinguish between comorbid and noncomorbid (primary) forms of insomnia. Common comorbidities, as noted above, include psychiatric disorders, general medical and neurologic disorders, and certain sleep disorders, such as sleep apnea and restless legs syndrome. The importance of identifying comorbid insomnia is that it should lead to appropriate treatment of both the insomnia disorder and the comorbid conditions. Treatment of primary insomnia, on the other hand, focuses only on the sleep problems and their daytime correlates.
Pathophysiology
The etiology and pathophysiology of chronic insomnia are unknown. However, converging lines of evidence from psychological and physiological studies suggest that some form of “hyperarousal” is common to many individuals with insomnia (18) . The cognitive and affective hyperarousal in insomnia has been demonstrated with both symptom reports and results of experimental cognitive paradigms. Physiological indicators of increased arousal include elevated cortisol/ACTH in the evening hours, increased heart rate and alterations in heart rate variability, and increased whole body metabolic rate. EEG markers of increased arousal include increased activity in frequency ranges from 16 to 50 Hz. Evidence from positron emission tomography (PET) studies also supports the hypothesis of hyperarousal: individuals with primary insomnia have elevated whole brain metabolism during both sleep and wakefulness and regional activation in affective and arousal centers during non-REM sleep (19) .
Psychological and Behavioral Treatment
Both psychological-behavioral treatments and pharmacologic treatments have demonstrated efficacy in the treatment of chronic insomnia. A variety of psychological and behavioral techniques have been evaluated in well-designed controlled clinical trials. Specific techniques include sleep restriction therapy, stimulus control therapy, relaxation approaches, and multimodal cognitive-behavioral treatment for insomnia (20) . The general features of these treatments are summarized in Table 3 . Qualitative and quantitative analyses of treatment studies using these techniques have indicated robust treatment effects on a variety of self-report sleep outcome measures and a smaller number of objective sleep outcome measures using polysomnography (21) . Published treatment manuals and self-help books describe the specific techniques for these treatments, which are simple enough to implement in routine clinical practice (22 – 25) .
The various psychological and behavioral treatments for insomnia share several common elements that have been combined into briefer forms of treatment. Common elements to many of these treatments include 1) education regarding sleep, sleep needs, and physiological regulation of sleep; 2) the establishing of more regular sleep hours, with particular emphasis on the time of arising in the morning; 3) limitation of time in bed to more closely match the individual’s actual sleep hours; 4) reinforcement of the bed and bedroom as a stimulus for sleep rather than for wakefulness and frustration about sleep. The mechanisms of psychological-behavioral treatments are unknown, but these common elements suggest the potential importance of augmenting homeostatic sleep drive, providing regular sleep-wake (and dark-light) cues for the circadian timing system, and reducing cognitive-affective arousal.
Psychological-behavioral treatments for chronic insomnia may be provided by psychologists and other behavioral medicine specialists with adequate training. In addition, certification in behavioral sleep medicine is provided by the American Academy of Sleep Medicine. More basic forms of psychological-behavioral treatments may be provided by other health professionals, including nurses as well as physicians.
Pharmacologic Treatment
Numerous prescription and nonprescription agents have been used to treat insomnia. Agents currently approved by the U.S. Food and Drug Administration (FDA) for treatment of insomnia include eight benzodiazepine receptor agonists (BzRAs) and one melatonin receptor agonist. Although several barbiturate and miscellaneous nonbarbiturate drugs (e.g., chloral hydrate, ethchlorvynol) are also approved as sedative-hypnotics, they are not recommended for clinical use given their low therapeutic index. Approved BzRAs include a set of drugs that are true benzodiazepines as defined by their chemical structure and a set of drugs that are not benzodiazepines but appear to share a common mechanism of action. BzRAs act at a specific recognition site on g-aminobutyric acid type A (GABA A ) receptors in the CNS; this recognition site is distinct from the GABA recognition site itself (26) . Benzodiazepines have affinity for several subtypes of GABA A receptors, which are defined by the specific composition of five protein subunits. Some nonbenzodiazepine BzRAs have greater selectivity for GABA receptors containing an alpha 1 protein subunit. The sedating and amnestic effects of BzRAs both appear to be related to affinity for alpha 1 -containing GABA A receptors, but the clinical significance of drug specificity in vivo is uncertain.
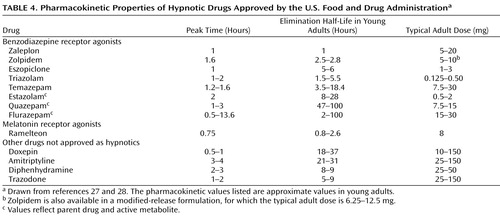
BzRAs differ from each other mainly in terms of pharmacokinetic properties, and particularly in terminal elimination half-life ( Table 4 ). Available agents have terminal elimination half-lives ranging from 1 to 120 hours. The duration of action of a drug is most strongly, although not exclusively, related to half-life. Therefore, selection of a particular drug often depends on the ideal duration of action for a particular patient, rather than on any specific distinctions between drugs.
BzRA drugs as a class reduce sleep onset latency and increase total sleep time; depending on the specific pharmacokinetics of the drug in question, decreased wakefulness after sleep onset may also result. Qualitative and quantitative analyses of clinical trials with BzRAs demonstrate their efficacy as judged by self-report and objective outcomes (29 , 30) . However, as is the case with psychological and behavioral treatments, few studies have paid attention to functional outcomes or to the broader insomnia syndrome.
BzRAs also share a similar set of adverse effects, which include anterograde amnesia, postural instability, and sleepiness (16 , 31) . The presence of such side effects may be related to the specific drug’s half-life; impaired memory and daytime sedation are more likely with agents having long half-lives. A range of more serious behavioral adverse consequences, including sleep-related eating, sexual behavior, and even driving, have been reported anecdotally and in the lay press. Although most of these reports have occurred in association with zolpidem, which is the most widely prescribed drug in this class, it seems likely that similar adverse effects are possible with any agent in this class.
BzRAs may also be associated with rebound insomnia, withdrawal, tolerance, and dependence (16 , 31) . Rebound insomnia has been demonstrated predominantly with short-acting drugs, and it can be minimized by gradual dose tapering and even by alternate-day dosing after the dose has been reduced. Withdrawal symptoms can also be minimized by dose and frequency tapering. Abuse of BzRA drugs as agents of first choice is not common. However, individuals who abuse these drugs commonly abuse other substances, and the use of higher-than-prescribed doses or requests for early prescription refills should raise concern. Hypnotic dependence has both physiological and psychological determinants. Physiological aspects of dependence are related to the discontinuation effects noted above. Psychological aspects of dependence are related to the fear or belief that good sleep will not be possible without medications. This may lead some individuals to continue taking medications for extended periods, even in the absence of clear therapeutic benefits.
BzRAs have also been shown to be useful for individuals with comorbid insomnia, including those with comorbid depression (32) . They also reduce insomnia in perimenopausal women with hot flashes (33) . Both nightly dosing and intermittent (e.g., three times per week) dosing have been shown to be efficacious (34) . Although most clinical trials have been conducted for 1 week or less, recent evidence from double-blind studies shows continued evidence for efficacy for up to 6 months of nightly use (35) .
One melatonin receptor agonist, ramelteon, has been shown to be efficacious for treatment of insomnia (36) . Its primary effect appears to be on sleep onset latency, with little effect on wakefulness during the middle of the night. Its side effect profile appears to be relatively benign, although increased blood levels have been observed in older adults, and some hormonal alterations (e.g., reduced testosterone) have been observed.
A wide variety of drugs that have not been approved by the FDA for the treatment of insomnia have been used in clinical practice. These include sedating antidepressant drugs, sedating antipsychotic drugs, and sedating anticonvulsant drugs, often used in low doses. Small clinical trials have supported the efficacy of low doses of trazodone and sedating tricyclic drugs for treatment of insomnia (16) . Their role in the routine treatment of chronic insomnia has not been defined, but they are likely to be most useful in patients who have a history of substance use and those who have a contraindication or poor response to BzRAs. Important side effects of these drugs include orthostatic hypotension and anticholinergic effects, which are of particular concern in the elderly. Even greater concerns are raised by the use of sedating antipsychotic drugs, which may have metabolic and neurologic side effects. Therefore, their use for treatment of sleep disturbance is recommended only in cases of insomnia that is comorbid with psychiatric disorders that independently warrant their use, such as severe bipolar disorder.
Alcohol and antihistamines are the most commonly used self-treatments for insomnia. Alcohol may help promote sleep initially, but it disrupts sleep for the remainder of the night and raises the additional issues associated with chronic alcohol use. Very little evidence is available supporting the efficacy or safety of diphenhydramine or other antihistamines for the treatment of insomnia. In addition, their anticholinergic properties raise safety questions, particularly in older people. Finally, naturopathic remedies such as valerian (37) have been assessed in some clinical trials. However, substantial difficulties arise in the standardization of these products, and their overall efficacy is marginal at best.
Treatment Implementation
Before initiating any treatment, the clinician should determine whether the patient’s insomnia symptoms warrant treatment, as indicated by the severity of sleep symptoms, daytime symptoms, and distress. Reasonable treatment goals should also be discussed. For instance, patients should recognize that some degree of night-to-night variability in sleep quality is natural and may be experienced even after treatment. Their sleep problems should become more manageable with treatment, however, and their daytime function can improve. Treatment outcomes should focus not only on the quantitative sleep parameters, such as sleep latency, wakefulness after sleep onset, and sleep duration, but also on qualitative aspects of the individual’s sleep and daytime function.
Psychological and behavioral treatments are appropriate in almost all cases of chronic insomnia. A simplified approach focusing on education and behavioral principles, as outlined above, may result in substantial improvement in some cases. Referral to individuals with specific training in behavioral sleep medicine or the use of cognitive-behavioral approaches may also be appropriate.
Pharmacologic treatment may be appropriate when the insomnia causes significant symptoms, distress, or impairment. Medication effects and side effects should be discussed with the patient. In most cases, initial treatment with a short-acting BzRA is a reasonable first step. Depending on initial treatment response, a second BzRA with either a shorter or longer half-life may be tried. In the event of nonresponse, or in the case of individuals with a history of substance abuse, a trial of a sedating antidepressant at a low dose may be a reasonable approach.
Unresolved Issues
Although a great deal has been learned about the epidemiology, pathophysiology, and treatment of chronic insomnia, other questions remain to be answered. First, the optimal method of combining behavioral and pharmacologic treatments remains to be determined. Some evidence suggests that psychological-behavioral treatment alone may provide superior long-term outcomes, but the combination may provide better short-term outcomes (38) . A second unresolved issue is the length of treatment, particularly pharmacologic treatment. Although previous assumptions about a rapid development of tolerance have not been borne out in recent double-blind placebo-controlled studies, it is a common clinical observation that patients say the drug is “no longer working.” In addition, discontinuation of long-term treatment with hypnotics often results in further improvements in sleep (39) . Third, the benefits of intermittent or as-needed medication use, as opposed to nightly treatment, remain to be defined. Finally, it has not been clearly demonstrated whether treating insomnia leads to consistent improvements in comorbid conditions or other more general aspects of daytime functioning. Early evidence suggests that treatment of insomnia may improve outcomes of comorbid depression (40 , 41) , but further controlled trials are needed.
Summary and Recommendations
Chronic insomnia should be carefully evaluated, beginning with a thorough clinical history, including sleep habits and patterns. The possibility of other sleep disorders, comorbidity with another medical or psychiatric disorder, and effects of substances and medications should be considered in patients with insomnia. Treatments for insomnia include psychological and behavior treatments and pharmacotherapy, which can be used in combination.
Ms. F’s physician ordered further medical testing, including assessment of thyroid function tests, which showed normal T4, T3, and thyroid-stimulating hormone levels. Given that Ms. F’s insomnia had a course independent of her prior major depression, a diagnosis of primary insomnia was made. Initial discussion with the patient focused on sleep education, including the need for an adequate duration of wakefulness each day to promote sleep drive at night. The variability of the patient’s sleep hours and her excessively long time in bed were also discussed. A treatment plan was agreed upon using the four general rules discussed above, based on sleep restriction and stimulus control principles; the patient was given a “prescription” of going to bed at 11:00 p.m. and getting out of bed no later than 6:00 a.m. each day. She was also asked to keep a sleep diary. At follow-up 4 weeks later, the sleep diary showed improved consolidation of her nighttime sleep, although Ms. F still felt tired during the day. She thought that her cognitive function was mildly improved. A trial of a short-acting hypnotic, zolpidem, was then initiated, with the patient maintaining the same sleep hours. Subsequent follow-up in another month demonstrated further improvements in sleep consolidation at night, but with some feelings of morning sedation, occurring approximately three nights out of the week. The patient felt more in control of her sleep problem. It was agreed that intermittent use of zolpidem might be appropriate. Thus, the patient began a trial of zolpidem intermittently, while maintaining attention to her sleep behaviors. She was advised to use her medication if she had several bad nights in a row, if she felt particularly stressed, or if she needed the certainty of consolidated sleep before important activities the following day. Several months later, she reported general improvement in her sleep, to a tolerable level. Her mood also remained stable, with no evidence of reemergence of depression.
1. American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed, Text Revision. Washington, DC, American Psychiatric Association, 2000Google Scholar
2. American Academy of Sleep Medicine: The International Classification of Sleep Disorders, 2nd ed: Diagnostic and Coding Manual. Westchester, Ill, American Academy of Sleep Medicine, 2005Google Scholar
3. Hohagen F, Kappler C, Schramm E, Riemann D, Weyerer S: Sleep onset insomnia, sleep maintaining insomnia, and insomnia with early morning awakening: temporal stability of subtypes in a longitudinal study on general practice attenders. Sleep 1994; 17:551–554Google Scholar
4. National Institutes of Health: Drugs and Insomnia: The Use of Medications to Promote Sleep: NIH Consensus Development Conference. National Institutes of Health Consensus Development Conference Summary 1984; 4:1–19Google Scholar
5. National Institutes of Health State of the Science Conference Statement on Manifestations and Management of Chronic Insomnia in Adults, June 13–15, 2005. Sleep 2005; 28:1049–1057Google Scholar
6. Ohayon MM: Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev 2002; 6:97–111Google Scholar
7. Buysse DJ, Germain A, Moul DE: Diagnosis, epidemiology, and consequences of insomnia. Prim Psychiatry 2005; 12:37–44Google Scholar
8. Buysse DJ, Angst J, Gamma A, Ajdacic V, Eich D, Rössler W: Prevalence, course, and comorbidity of insomnia and depression in young adults. Sleep 2008; 31:473–480Google Scholar
9. Foley DJ, Monjan A, Simonsick EM, Wallace RB, Blazer DG: Incidence and remission of insomnia among elderly adults: an epidemiologic study of 6,800 persons over three years. Sleep 1999; 22(suppl 2):S366–S372Google Scholar
10. Moul DE, Nofzinger EA, Pilkonis PA, Houck PR, Miewald JM, Buysse DJ: Symptom reports in severe chronic insomnia. Sleep 2002; 25:553–563Google Scholar
11. Katz DA, McHorney CA: Clinical correlates of insomnia in patients with chronic illness. Arch Intern Med 1998; 158:1099–1107Google Scholar
12. Perlis ML, Smith LJ, Lyness JM, Matteson SR, Pigeon WR, Jungquist CR, Tu X: Insomnia as a risk factor for onset of depression in the elderly. Behav Sleep Med 2006; 4:104–113Google Scholar
13. Buysse DJ, Tu XM, Cherry CR, Begley AE, Kowalski J, Kupfer DJ, Frank E: Pretreatment REM sleep and subjective sleep quality distinguish depressed psychotherapy remitters and nonremitters. Biol Psychiatry 1999; 45:205–213Google Scholar
14. Nierenberg AA, Keefe BR, Leslie VC, Alpert JE, Pava JA, Worthington JJ, Rosenbaum JF, Fava M: Residual symptoms in depressed patients who respond acutely to fluoxetine. J Clin Psychiatry 1999; 60:221–225Google Scholar
15. Reynolds CF III, Frank E, Houck PR, Mazumdar S, Dew MA, Cornes C, Buysse DJ, Begley A, Kupfer DJ: Which elderly patients with remitted depression remain well with continued interpersonal psychotherapy after discontinuation of antidepressant medication? Am J Psychiatry 1997; 154:958–962Google Scholar
16. Buysse DJ, Germain A, Moul D, Nofzinger EA: Insomnia, in Sleep Disorders and Psychiatry. Edited by Buysse DJ. Review of Psychia, vol 24. Arlington, Va, American Psychiatric Publishing, 2005, pp 29–75Google Scholar
17. Spielman AJ, Anderson MW: The clinical interview and treatment planning as a guide to understanding the nature of insomnia: the CCNY Insomnia Interview, in Sleep Disorders Medicine: Basic Science, Technical Considerations, and Clinical Aspects. Edited by Chokroverty S. Boston, Butterworth-Heinemann, 1999, pp 385–416Google Scholar
18. Perlis ML, Smith MT, Pigeon WR: Etiology and pathophysiology of insomnia, in Principles and Practice of Sleep Medicine. Edited by Kryger MH, Roth T, Dement WC. Philadelphia, Elsevier Saunders, 2005, pp 714–725Google Scholar
19. Nofzinger EA, Buysse DJ, Germain A, Price JC, Miewald JM, Kupfer DJ: Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry 2004; 161:2126–2129Google Scholar
20. Morin CM: Psychological and behavioral treatments for primary insomnia, in Principles and Practice of Sleep Medicine. Edited by Kryger MH, Roth T, Dement WC. Philadelphia, Elsevier Saunders, 2005, pp 726–737Google Scholar
21. Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, Lichstein KL: Psychological and behavioral treatment of insomnia: an update of recent evidence (1998–2004). Sleep 2006; 29:1398–1414Google Scholar
22. Glovinsky P, Spielman A: The Insomnia Answer: A Personalized Program for Identifying and Overcoming the Three Types of Insomnia. New York, Perigee Books, 2006Google Scholar
23. Morin CM: Insomnia: Psychological Assessment and Management. New York, Guilford, 1993Google Scholar
24. Morin CM, Espie CA: Insomnia: A Clinical Guide to Assessment and Treatment. New York, Kluwer Academic/Plenum Publishers, 2003Google Scholar
25. Perlis ML, Smith MT, Jungquist C, Posner D: Cognitive Behavioral Treatment of Insomnia: A Session-by-Session Guide. New York, Springer-Verlag, 2005Google Scholar
26. Bateson AN: The benzodiazepine site of the GABA A receptor: an old target with new potential? Sleep Med 2004; 5(suppl 1):S9–S15 Google Scholar
27. Drug Facts and Comparisons: Pocket Version 2008. Baltimore, Lippincott Williams & Wilkins, 2007Google Scholar
28. Brunton LL, Lazo JS, Parker KL: Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 11th ed. New York, McGraw-Hill, 2006Google Scholar
29. Nowell PD, Mazumdar S, Buysse DJ, Dew MA, Reynolds CF, Kupfer DJ: Benzodiazepines and zolpidem for chronic insomnia: a meta-analysis of treatment efficacy. JAMA 1997; 278:2170–2177Google Scholar
30. Smith MT, Perlis ML, Park A, Smith MS, Pennington J, Giles DE, Buysse DJ: Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry 2002; 159:5–11Google Scholar
31. Walsh JK, Roehrs T, Roth T: Pharmacologic treatment of primary insomnia, in Principles and Practice of Sleep Medicine. Edited by Kryger MH, Roth T, Dement WC. Philadelphia, Elsevier Saunders, 2005, pp 748–760Google Scholar
32. Fava M, McCall WV, Krystal A, Wessel T, Rubens R, Caron J, Amato D, Roth T: Eszopiclone co-administered with fluoxetine in patients with insomnia coexisting with major depressive disorder. Biol Psychiatry 2006; 59:1052–1060Google Scholar
33. Dorsey CM, Lee KA, Scharf MB: Effect of zolpidem on sleep in women with perimenopausal and postmenopausal insomnia: a 4-week randomized, multicenter, double-blind, placebo-controlled study. Clin Ther 2004; 26:1578–1586Google Scholar
34. Perlis ML, McCall WV, Krystal AD, Walsh JK: Long-term, non-nightly administration of zolpidem in the treatment of patients with primary insomnia. J Clin Psychiatry 2004; 65:1128–1137Google Scholar
35. Krystal AD, Walsh JK, Laska E, Caron J, Amato DA, Wessel TC, Roth T: Sustained efficacy of eszopiclone over 6 months of nightly treatment: results of a randomized, double-blind, placebo-controlled study in adults with chronic insomnia. Sleep 2003; 26:793–799Google Scholar
36. Erman M, Seiden D, Zammit G, Sainati S, Zhang J: An efficacy, safety, and dose-response study of ramelteon in patients with chronic primary insomnia. Sleep Med 2006; 7:17–24Google Scholar
37. Taibi DM, Landis CA, Petry H, Vitiello MV: A systematic review of valerian as a sleep aid: safe but not effective. Sleep Med Rev 2007; 11:209–230Google Scholar
38. Morin CM, Wooten V: Psychological and pharmacological approaches to treating insomnia: critical issues in assessing their separate and combined effects. Clin Psychol Rev 1996; 16:521–542Google Scholar
39. Morin CM, Bastien C, Guay B, Radouco-Thomas M, Leblanc J, Vallières A: Randomized clinical trial of supervised tapering and cognitive behavior therapy to facilitate benzodiazepine discontinuation in older adults with chronic insomnia. Am J Psychiatry 2004; 161:332–342Google Scholar
40. Krystal A, Fava M, Rubens R, Wessel T, Caron J, Wilson P, Roth T, McCall WV: Evaluation of eszopiclone discontinuation after cotherapy with fluoxetine for insomnia with coexisting depression. J Clin Sleep Med 2007; 3:48–55Google Scholar
41. Smith MT, Huang MI, Manber R: Cognitive behavior therapy for chronic insomnia occurring within the context of medical and psychiatric disorders. Clin Psychol Rev 2005; 25:559–592Google Scholar