Neural Stimulation Successfully Treats Depression in Patients With Prior Ablative Cingulotomy
Surgical treatment of major depression has been performed for the past several decades using precise stereotactic targeting to lesion select sites in the frontal lobes and in various deeper subcortical structures. These procedures have been reserved for only the most severely depressed and otherwise unresponsive patients. Targets for intervention have included the anterior capsule, the substantial innominata/subcaudate white matter and the caudal anterior cingulate gyrus. Of these, cingulotomy has seen the greatest application, with over 500 procedures reported (1 – 4) .
In the past several years, there has been increasing enthusiasm for the application of deep brain stimulation to the treatment of major depression. Here, too, multiple targets have been suggested, including the subgenual cingulate gyrus (5) , the inferior thalamic peduncle (6) , the anterior capsule (7) , and the subthalamic nucleus (8) . Deep brain procedures hold several principal advantages. First, the treatment is nonablative and therefore reversible if it is performed without complication. Furthermore, the parameters of stimulation can be adjusted, and stimulation can be increased or decreased over time. This is of particular importance in treating a disease such as depression in which the disease anatomy is still poorly understood and the symptoms are not easily quantified or measured and may respond over months rather than minutes.
In this article, we describe the case of a patient who, under the direction of a multidisciplinary case committee, underwent both an ablative cingulotomy and subsequent bilateral deep brain stimulation placement in the Cg25 area. Review of this case provides a means of comparing the two techniques and of illustrating what we feel are some important advantages to using stimulation in the treatment of major depression. This report also provides potential insight into common and unique mechanisms mediating the two procedures.
Case Presentation
“Ms. A,” a 55 year-old woman, was seen with a history of severe major depression. She had a family history significant for major depression in her brother, father, and paternal grandfather. Her own depressive history began at age 9 and continued intermittently during her adolescence. The condition became more severe at age 17, and she continued to have fluctuating symptoms from that time forward. The diagnosis of depression was made by her referring psychiatrist, based on DSM clinical criteria (Structured Clinical Interview for DSM-IV [SCID, 9 ]: major depressive disorder, chronic, severe, without psychotic features). Her psychiatrist had been treating her since 1981. Upon referral to our center, she underwent a standardized diagnostic interview in the form of the SCID. Her condition had been characterized by feelings of misery and by somatic complaints of low energy, fatigability, and muscle tension in the context of her depression. She was not determined to have a somatization disorder. She had a remission taking bupropion from ages 36 to 48, at which time she worked as a letter carrier for the postal service. From that time forward, she experienced severe depression, which had not remitted for the 7 years prior to her referral. She was incapacitated by her illness and unable to work for that time. She had been treated and had limited clinical benefit from trials adequate in dose and duration of each of the following antidepressants: nomifensine, maprotiline, amitriptyline, clomipramine, desipramine, trimipramine, trazodone, phenelzine, moclobemide, nefazodone, citalopram, escitalopram, fluoxetine, fluvoxamine, and bupropion. She had also been treated with trials of adequate dose and duration of the following augmentation and adjunctive strategies: lithium carbonate, l -tryptophan, tri-iodothyronine, methylphenidate, buspirone, amoxapine, flupenthixol, carbamazepine, topiramate, and valproic acid. All had failed to provide any sustained benefit. Ms. A had also tried venlafaxine and pramipexole but was unable to tolerate these medications before conventional therapeutic doses were reached. Additionally, she had undergone a course of eight bilateral ECT treatments without benefit.
Ms. A was referred for surgical management of her depression. Her 17-item Hamilton Depression Rating Scale (17-item HAM-D) score at that time was 22. Her case was reviewed by a multidisciplinary committee, which meets regularly for the express purpose of overseeing the surgical management of psychiatric disease. The conference includes individuals from neurosurgery, psychiatry, neurology, and neuropsychology at the University of Toronto. This group reviews and discusses the merits of each case being considered for surgical treatment of psychiatric disease. Ms. A was deemed a good candidate for either cingulotomy or for participation in a novel trial of deep brain stimulation of the subgenual cingulate. She was offered these options. After discussion with her original psychiatrist, she declined the untested deep brain stimulation therapy in favor of a lesional cingulotomy. This procedure was approved by the same committee.
Ms. A underwent a bilateral stereotactic ablative cingulotomy. The procedure, which is described below, was performed without complication. Afterward she reported subjective improvement in her depression and improvements in her symptoms of fatigue and hypersomnia. She stated that she felt better than she had in years. Her 17-item HAM-D score improved to 8 one month after surgery and further to 5 when she was tested 6 weeks after surgery. In the weeks following her surgery, her psychiatrist was able to decrease her bupropion dose considerably. Ms. A remained depression free for a period of 6 months with a sustained 17-item HAM-D score of 5. She gradually noted a worsening of her depression, both in her symptoms of melancholia and in her fatigue. Her antidepressant doses, previously reduced, were restored to her presurgery doses. Despite this, her 17-item HAM-D score increased to 9 that month, 15 the next month, and was 19 two months later. A magnetic resonance imaging (MRI) scan was obtained to review the extent of her prior cingulotomies. The lesions were felt to be appropriate in size and location. The option of a repeat cingulotomy was considered less promising for this reason.
Ms. A was again presented at the multidisciplinary case conference. At this time, our center had just completed the pilot study assessing the benefit of deep brain stimulation of the Cg25 region for refractory depression. It was felt that this procedure might offer a greater probability of benefit to her refractory condition. Ms. A was sent for additional neuropsychiatric testing to ensure that her prior cingulotomy had not diminished her cognitive reserve in a way that might confound interpretation of the effects of the deep brain stimulation procedure. After an extensive discussion with her psychiatrist, the conference was convened and recommended that the patient be offered deep brain stimulation surgery to stimulate the subgenual cingulate. The option of vagal nerve stimulation was not seriously considered for several reasons. The definitive blind study on vagal nerve stimulation for depression was not published until September 2005, 4 months after Ms. A underwent deep brain stimulation surgery (10 , 11) . The procedure did not receive Food and Drug Administration approval until July 2005, and we did not have an ongoing vagal nerve stimulation trial at our center at the time Ms. A was treated. The initial open study of vagal nerve stimulation therapy showed that ECT response predicted vagal nerve stimulation outcome (12) . Ms. A had failed to respond to ECT so, based on the best available evidence, we would have been unlikely to recommend vagal nerve stimulation therapy had it been available.
The deep brain stimulation procedure was scheduled, based on compassionate use of the new procedure. Just over a year after Ms. A’s cingulotomy, placement of deep brain stimulators in the subgenual cingulate was performed. Her 17-item HAM-D score just before surgery was 19 and had been stable for 4 months. The surgical procedure is described in greater detail below. Intraoperative assessment was conducted as done with the deep brain stimulation pilot study with patient self-report, analogue mood rating, and changes in the Positive and Negative Affect Schedule (13) to assess the effect of stimulation at the various contacts of the deep brain stimulation electrode. Notably, during intraoperative stimulation, Ms. A reported alleviation of the ever-present sensation of heaviness described as a surrounding “black cloud.” She also expressed immediate interest in returning to activities avoided since her relapse. This effect was contact-specific and reversible.
Stimulation was begun on the day after surgery. Initial screens with the Positive and Negative Affect Schedule were performed to determine the most effective contacts for stimulation. Parameters of stimulation were a rate of 130 Hz, a pulse width of 60, and an amplitude of 4.5 V. Slight adjustments were made over the next several months to maximize Ms. A’s response. At the 1-month evaluation, she reported a subjective improvement in her mood. Her 17-item HAM-D score had decreased to 11 when she was tested 3 months after surgery. Her level of activity increased, and she was able to resume volunteer work, similar to what had occurred after her cingulotomy. Subsequent 17-item HAM-D scores were 8 at 6 months and 7 at 1 year. She continues to have a sustained remission to ongoing Cg25 stimulation, with a 17-item HAM-D score of 7 at 30 months after her deep brain stimulation surgery ( Figure 1 ).
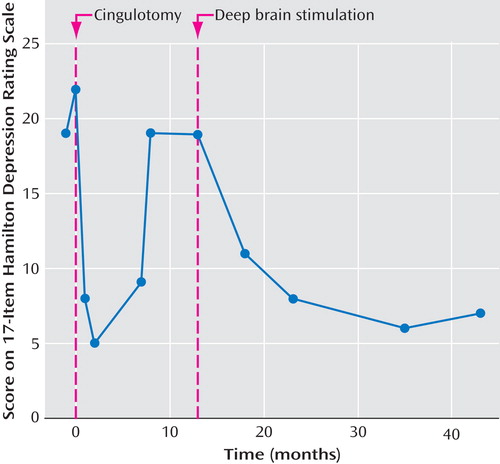
Since her deep brain stimulation procedure, Ms. A reports marked and sustained amelioration of the following symptoms: suicidal ideation, depressed mood, guilty ruminations, appetite, anxiety symptoms, and initiating and maintaining sleep. Lesser improvements have been reported in energy, libido, and concentration. She has also been noted on assessment to have a bright affect and an increased range of emotional expression. She is no longer demonstrating signs of psychomotor retardation. No signs or symptoms of mania or psychosis are present. She continues to work full-time 6 days a week as a volunteer driving patients to and from a local hospital. She has not missed a day since her return after deep brain stimulation surgery. The relative degree and longevity of benefit that the patient received from the cingulotomy and deep brain stimulation procedures are compared in Table 1 .
Surgical Interventions
Cingulotomy
A Leksell G stereotactic head frame (Elekta, Inc., Atlanta) was applied under local anesthesia and an MRI was obtained. Images were fused in an imaging workstation (FrameLink 4.1 software, StealthStation, Medtronic SNT, Minneapolis) and used for the stereotactic targeting. A coronal slice 2 cm to 2.5 cm posterior to the anterior tip of the frontal horn was identified, and a point was selected in the center (in the coronal plane) of the cingulate gyrus on this image. The stereotactic coordinates of this point were calculated, and microelectrodes were passed on a trajectory through this point.
The details of the microelectrode recording mapping procedures and equipment used in our service have been previously published (4 , 14) . Microelectrode recording mapping procedures were used to delineate cell-rich areas interpreted as the gray matter of the upper and lower banks of the cingulate gyrus. Areas devoid of somatodendritic action potentials were electrophysiologically silent and corresponded to the cingulate bundle and the corpus callosum. After verifying the depth of the corpus callosum, the microelectrodes were removed, and a radiofrequency electrode (1.2 mm wide with a 5-mm exposed tip) (Owl Universal RF system, Diros Technology, Toronto) was inserted just above the corpus callosum. Test stimulation (RF Generator/Stimulator Model URF-1 or RFS-1, Diros Technology) was performed at 100 Hz and up to 5 V through the macroelectrode. Because no adverse effects were noticed, three stacked lesions were created by applying radiofrequency current to the electrode to a temperature of approximately 90°C for 90 seconds. The electrode was withdrawn 5 mm, and after another test stimulation, the process was repeated with the same parameters. The electrode was withdrawn another 5 mm, and the procedure was repeated a third time. Then the electrode was withdrawn completely, and three lesions were created 1 cm anterior to this track with a similar process. The same process was repeated in the contralateral hemisphere ( Figure 2 ).

Deep Brain Stimulation
The placement of the stereotactic frame and the imaging procedures for the insertion of deep brain stimulation electrodes were similar to that employed for cingulotomy. However, rather than targeting the cingulate gyrus above the body of the corpus callosum, we targeted the subgenual cingulum. For that, a line was traced from the most anterior aspect (genu) of the corpus callosum to the anterior commissure and the midpoint was selected (5) . The coronal section correspondent to that plane was identified, and the coordinates of the transition between the gray and white matters of area 25 were calculated. Microelectrode recording mapping procedures were conducted in this case to identify the location of the area of transition between gray and white matter of area 25, characterized, respectively, by the recording of neuronal activity and cell-sparse areas. Thereafter, deep brain stimulation quadripolar electrodes (Medtronic 3387, Medtronic, Inc., Minneapolis) were implanted bilaterally such that the tip of the electrode array sat at the inferior gray matter-white matter junction, and the four contacts spanned the full gyrus. Each of the four electrode contacts was tested for adverse effects and clinical benefits. The electrodes were then connected to a pulse generator (Kinetra, Medtronic, Inc.) that was implanted in the infraclavicular region under general anesthesia.
After several adjustments, stimulation parameters were selected with monopolar stimulation from contacts 2 and 5 (third contact from the bottom on the right and second contact from the bottom on the left). The anterior commissure-posterior commissure coordinates of these contacts are the following—right side: x=5.1, y=33.7, z=–4.4; and left side: x=3.8, y=34.3, z=–4.9. Stimulation was performed at a rate of 130 Hz, a pulse width of 60 Hz, and an amplitude of 4.5 V. These settings have not been substantially changed during Ms. A’s subsequent therapy.
Discussion
Our experience with this single patient is valuable for the insight it can provide on ablative and novel stimulatory therapies for major depression. The mechanisms by which these procedures affect mood is a matter that currently draws much study and interest. The individual therapies are discussed below, followed by some conjectures on their interrelation in this novel case. Like all case studies, it is limited in its scope. It is hoped that ongoing trials and research to clarify the role of the Cg25 region will eventually elucidate the role of this region in major depression.
Lesional Cingulotomy
Whitty and colleagues first reported cingulectomy in 1952. Based on observations by Friedman and Watts that transection of cingulate fibers decreased psychic tension and were an important component of successful leucotomies for affective disorders, they performed open bilateral cingular resections (15) . Ballantine refined the procedure considerably by performing stereotactic lesioning of the cingulum guided by air ventriculography. Between 1962 and 1982, he performed cingulotomies on 465 patients for severe and treatment-resistant psychiatric disorders and for intractable pain. In 1987, he and his colleagues reported the results on 120 of these patients who had undergone cingulotomy for major affective disorders. Of this group, 64% were substantially improved at 2–9 years of follow-up. Sixteen percent demonstrated complete remissions and were able to discontinue all medications. An additional 12%, however, committed suicide in the years after their surgery. Notably, assessments were not blind, and for ethical reasons, random assignment was not possible (3) . Also, evaluations with standardized rating scales were not performed. Instead, patients were placed in one of seven categories that graded their status from suicidal to in remission. This makes interpretation and comparison with more recent studies difficult.
A later study from the same institution evaluated the use of MRI frame-based guidance and estimated that 33% of the patients treated for major depression were substantially improved. They also reported that many patients in this protocol required numerous repeat surgeries to expand the lesions anteriorly and posteriorly before benefit was achieved. Forty-five percent of the patients in this study required a repeat procedure. It is supposed that broader lesions of the cingulum are more effective (1) . A later study used the somewhat more objective Beck Depression Inventory (BDI) as the primary outcome measure. In a group of 13 patients, only four of 13, or 31%, demonstrated a decrease of 50% on their BDI. This group of patients also underwent positron emission tomography (PET) imaging prior to their procedure. A subsequent correlational analysis relating percent change in the BDI to baseline levels of regional cerebral metabolic rate for glucose demonstrated two regions of significant correlation. These were the subgenual cingulate cortex and the posterior thalamus, where higher baseline activity correlated with better outcome to cingulotomy (16) . Of interest, both areas have been independently demonstrated to have decreased activity in depressed patients and then to normalize with successful antidepressant medication treatment (17 – 19) . These modern assessments of cingulotomy as a treatment for major depression suggest that it is effective but imperfect. An efficacy rate of 31% in a population resistant to all modes of pharmacologic therapy and to ECT would represent a considerable success, although it has not been tested in blind randomized fashion. Furthermore, the fact that response correlates with activity in the thalamus and in a distinctly separate portion of the cingulate gyrus, which is not immediately adjacent to fibers targeted for ablation, suggests that the treatment succeeds by disrupting a segment of a more complex network.
Deep Brain Stimulation for Major Depression
As noted above, there are considerable data from functional neuroimaging that implicate the subgenual cingulate in the physiology of major depression (16 , 17 , 20) . Several studies have identified increased metabolism in the subgenual cingulate (Brodmann’s area 25) as an important correlate of depression (21) . Based on the supposition that deep brain stimulation focally suppresses activity in this area and might directly reverse the aberrant physiology of depression, a series of patients at our institution have undergone electrode placement bilaterally in the subgenual cingulate to treat their refractory depression. A pilot study of six patients who underwent the procedure demonstrated substantial improvement in four of six patients (66%) with decreases of 50% or greater in their HAM-D scores (5) . The successful application of deep brain stimulation to treat psychiatric disease would represent a major breakthrough in modern functional neurosurgery. The high incidence of these disorders in the general population and the large percentage that are refractory despite medical treatment make this an important area for research and clinical development.
This case report is of interest because it demonstrates the potential advantages of the newer technique of deep brain stimulation surgery over the more extensively reported ablative cingulotomy. This patient, who underwent both procedures in succession, was able to gain prolonged benefit from Cg25 deep brain stimulation despite no improvement from previous ablative therapy. Cingulotomy produced remission for only 6 months. The subsequent deep brain stimulation procedure continues to be beneficial at 30 months after implantation.
There are several possible causes for the patient’s improvement. The patient was not blind to the procedure or to subsequent stimulation settings. As such, it is possible that the derived benefit is secondary to placebo effect, either from the surgery or from the periodic visits and adjustments that are necessary for programming the deep brain stimulation system. Indeed, the patient had a significant, but unsustained, benefit from her initial ablative surgery, which might also have been a placebo effect. We are, however, encouraged that benefit after deep brain stimulation surgery has continued for 2.5 years, significantly longer than after her previous surgery. She had also failed to have similar transient benefit from either ECT or from any of the nearly 20 medications with which she had been treated in the last decade. Her response, as measured on the 17-item HAM-D, has also improved progressively over the past year. A pure placebo response might be expected to have the greatest effect shortly after surgery and then progressively return to baseline. The opposite has been evidenced.
As noted in the studies above, a large percentage of cingulotomy patients relapse after their first surgery and have found benefit with further lesioning of the caudal anterior cingulum. Indeed, the timing of her initial failure is similar to that suggested by prior investigators performing cingulotomy. It is possible that this patient’s deep brain stimulation procedure has accomplished the same effect simply by expanding the portion of the cingulum that is functionally lesioned. A second cingulotomy was considered in this patient; however, because the first procedure included lesioning at multiple positions bilaterally and because the postoperative imaging demonstrated that large areas had been lesioned, we did not consider that further ablation of tissue in that region would be of benefit. Whether stimulation in the Cg25 region simply expands the area of effective lesion or acts by a separate mechanism remains a matter for research.
The Cg25 region seems to play a role both with the traditional cingulotomy and with the new deep brain stimulation procedure. As noted above, elevated activity in the Cg25 region preoperatively was predictive of success in a series of cingulotomy patients. The same increase of activity in Cg25 noted in depressed patients more broadly was the principal impetus for suggesting deep brain stimulation treatment of this target. The presumption in both cases is that ablation of the caudal anterior cingular gyrus or direct electrical stimulation of the Cg25 region suppresses activity, by direct or indirect means, of the Cg25 region and thus restores a more normal physiology. Although there are direct connections between the subgenual cingulate and mid-cingulate regions through the cingulum bundle, their patterns of cortical connectivity are quite distinct. The subgenual cingulate is connected by unique monosynaptic pathways to the orbitofrontal cortex, striatum, amygdala, hypothalamus, and brainstem. These areas are not directly affected by the caudal cingulotomy procedure. These differences may help to explain transient but unsustained effects with cingulotomy and more sustained effects with deep brain stimulation because the latter procedure may more directly affect regions that typically demonstrate abnormal metabolism in depression. Further studies are needed to fully characterize the necessary and sufficient pathway effects mediating a sustained antidepressant response.
As in deep brain stimulation therapy more broadly, there remains uncertainty on the mechanism of high-frequency stimulation. The use of deep brain stimulation in the globus pallidus or subthalamic nucleus is typically presumed to create a functional lesion of the nucleus. This is presumed only because the global effect of high-frequency stimulation in movement disorder surgery is similar to the effect demonstrated when the nucleus is lesioned due to the similarity of its effect that was obtained in lesioning the same nuclei. This matter is still hotly debated. The substructure of the globus pallidus or subthalamic nucleus is certainly quite different from that of the subgenual cingulate gyrus. Thus we can presume little about the effect of high-frequency stimulation on the myriad connections to and from this region.
Although PET scanning was not performed on this patient, in the first cohort of Cg25 stimulation patients, there were increases in the mid-cingulate after chronic stimulation of the Cg25, whereas the subgenual cingulate itself showed decreased activity. This again suggests an important connectivity between these two areas of the cingulate cortex and supports the idea that the two procedures have worked synergistically. This case report is perhaps most interesting in that it demonstrates that the two procedures can work in concert to control a patient’s depression.
The deep brain stimulation procedure does, however, have some advantages over cingulotomy. If performed without complication, it is a reversible procedure that does not involve the permanent destruction of brain tissue. The effect is also titratable. Thus, the degree of stimulation or the region of effect (altered by changing electrode contacts) can be adjusted, if clinically indicated, without further surgery. If the results of the deep brain stimulation pilot study and the evidence provided by this patient are substantiated by further evidence supporting the effectiveness of Cg25 deep brain stimulation, we expect the procedure to see greater application in the coming years.
This case report suggests that patients who have achieved suboptimal antidepressant effects after a cingulotomy may expect a similar degree of antidepressant efficacy after Cg25 deep brain stimulation as patients who have not received an ablative procedure.
1. Spangler WJ, Cosgrove GR, Ballantine HT, Cassem EH, Rauch SL, Nierenberg A, Price BH: Magnetic resonance image-guided stereotactic cingulotomy for intractable psychiatric disease. Neurosurgery 1996; 38:1071–1106Google Scholar
2. Ballantine HT, Cassidy WL, Flanagan NB, Marino R: Stereotaxic anterior cingulotomy for neuropsychiatric illness and intractable pain. J Neurosurg 1967; 26:488–495Google Scholar
3. Ballantine HT, Bouckoms AJ, Thomas EK, Giriunas IE: Treatment of psychiatric illness by stereotactic cingulotomy. Biol Psychiatry 1987; 22:807–819Google Scholar
4. Richter EO, Davis KD, Hamani C, Hutchison WD, Dostrovsky JO, Lozano AM: Cingulotomy for psychiatric disease: microelectrode guidance, a callosal reference system for documenting lesion location, and clinical results. Neurosurgery 2004; 54:622–628Google Scholar
5. Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH: Deep brain stimulation for treatment-resistant depression. Neuron 2005; 45:651–660Google Scholar
6. Jiménez F, Velasco F, Salin-Pascual R, Hernández JA, Velasco M, Criales JL, Nicolini H: A patient with a resistant major depression disorder treated with deep brain stimulation in the inferior thalamic peduncle. Neurosurgery 2005; 57:585–593Google Scholar
7. Kopell BH, Greenberg B, Rezai AR: Deep brain stimulation for psychiatric disorders. J Clin Neurophysiol 2004; 21:51–67Google Scholar
8. Bejjani BP, Damier P, Arnulf I, Thivard L, Bonnet AM, Dormont D, Cornu P, Pidoux B, Samson Y, Agid Y: Transient acute depression induced by high-frequency deep-brain stimulation. N Engl J Med 1999; 340:1476–1480Google Scholar
9. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for Axis I DSM-IV Disorders—(SCID), Patient Edition, Version 2.0. New York, Biometrics Research Department, New York Psychiatric Institute, 1996Google Scholar
10. Rush AJ, Marangell LB, Sackeim HA, George MS, Brannan SK, Davis SM, Howland R, Kling MA, Rittberg BR, Burke WJ, Rapaport MH, Zajecka J, Nierenberg AA, Husain MM, Ginsberg D, Cooke RG: Vagus nerve stimulation for treatment-resistant depression: a randomized, controlled acute phase trial. Biol Psychiatry 2005; 58:347–354Google Scholar
11. Rush AJ, Sackeim HA, Marangell LB, George MS, Brannan SK, Davis SM, Lavori P, Howland R, Kling MA, Rittberg B, Carpenter L, Ninan P, Moreno F, Schwartz T, Conway C, Burke M, Barry JJ: Effects of 12 months of vagus nerve stimulation in treatment-resistant depression: a naturalistic study. Biol Psychiatry 2005; 58:355–363Google Scholar
12. Rush AJ, George MS, Sackeim HA, Marangell LB, Husain MM, Giller C, Nahas Z, Haines S, Simpson RK Jr, Goodman R: Vagus nerve stimulation (VNS) for treatment-resistant depressions: a multicenter study. Biol Psychiatry 2000; 47:276–286Google Scholar
13. Watson D, Clark LA: Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol 1988; 6:1063–1070Google Scholar
14. Lozano A, Hutchison W, Kiss Z, Tasker R, Davis K, Dostrovsky J: Methods for microelectrode-guided posteroventral pallidotomy. J Neurosurg 1996; 84:194–202Google Scholar
15. Whitty CW, Duffield JE, Tow PM, Cairns H: Anterior cingulectomy in the treatment of mental disease. Lancet 1952; 1:475–481Google Scholar
16. Dougherty DD, Weiss AP, Cosgrove GR, Alpert NM, Cassem EH, Nierenberg AA, Price BH, Mayberg HS, Fischman AJ, Rauch SL: Cerebral metabolic correlates as potential predictors of response to anterior cingulotomy for treatment of major depression. J Neurosurg 2003; 99:1010–1017Google Scholar
17. Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, Jerabek PA: Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry 2000; 48:830–843Google Scholar
18. Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT: Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry 1999; 156:675–682Google Scholar
19. Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, Silva JA, McGinnis S, Glass TG, Martin CC, Fox PT: Cingulate function in depression: a potential predictor of treatment response. Neuroreport 1997; 8:1057–1061Google Scholar
20. Mayberg HS, Starkstein SE, Sadzot B, Preziosi T, Andrezejewski PL, Dannals RF, Wagner HN Jr, Robinson RG: Selective hypometabolism in the inferior frontal lobe in depressed patients with Parkinson’s disease. Ann Neurol 1990; 28:57–64Google Scholar
21. Drevets WC, Price JL, Simpson JR, Todd RD, Reich T, Vannier M, Raichle ME: Subgenual prefrontal cortex abnormalities in mood disorders. Nature 1997; 386:824–827Google Scholar




