Reduced Neural Habituation in the Amygdala and Social Impairments in Autism Spectrum Disorders
Abstract
Objective: Amygdala dysfunction has been proposed as a critical component in social impairment in autism spectrum disorders. This study was designed to investigate whether abnormal habituation characterizes amygdala dysfunction in autism spectrum disorders and whether the rate of amygdala habituation is related to social impairment. Method: Using functional MRI, the authors measured change over time in activation of the amygdala and fusiform gyrus to neutral facial stimuli in adults with autism spectrum disorders and healthy comparison adults. Results: The comparison group evidenced significantly greater amygdala habituation bilaterally than the autism spectrum group. There were no group differences in overall fusiform habituation. For the autism spectrum group, lower levels of habituation of the amygdala to the face stimuli were associated with more severe social impairment. Conclusions: These results suggest amygdala hyperarousal in autism spectrum disorders in response to socially relevant stimuli. Further, sustained amygdala arousal may contribute to the social deficits observed in autism spectrum disorders.
Viewing and identifying faces is a fundamental component of social cognition and one of the most earliest emerging social deficits in autism spectrum disorders (1) . Face processing deficits likely contribute to impairments in eye gaze, joint attention, responses to emotional displays, and face recognition (1) . Behavioral studies in autism spectrum disorders have identified specific face processing abnormalities, including poor memory for faces (see reference 2 , for example), difficulty evaluating trustworthiness (3) , reduced inversion effect (4) , reduced attention to the eye region (5) , and abnormalities in perceiving emotions associated with facial expressions. Because of the fundamental role that face processing plays in guiding social interactions, the neural circuitry involved in face processing has been hypothesized to contribute to social dysfunction in individuals with autism spectrum disorders (1 , 6) .
Face processing abnormalities and their concomitant social dysfunction have been hypothesized to be related to overarousal or hyperexcitability of the amygdala, which triggers an exaggerated sympathetic response (7 , 8) . However, amygdala dysfunction in response to faces in autism spectrum disorders also reportedly includes decreased neural response (9 – 11) and reduced task-related modulation of the amygdala (10 , 12 , 13) . Together, these findings indicate that the relationship between face processing and amygdala arousal remains unclear.
In this study, we tested whether differences in amygdala activation between a group with autism spectrum disorders and a healthy comparison group were related to differences in rates of amygdala habituation to face stimuli. Neural habituation is a process by which the neural response decreases over time in response to repeated stimulation (14) . It is thought to reflect plasticity and learning (15) and can be disrupted by neural lesions (14) . In functional MRI (fMRI) studies of typically developing individuals, face activation in the amygdala (16 – 18) and fusiform gyrus (18) habituates rapidly over repeated stimulus exposure. We hypothesized that individuals with autism would show reduced neural habituation of the amygdala to social stimuli, characterized by sustained activation to social stimuli over time. This hypothesis differs from those posited previously (8) , which consider significantly greater activation in an autism spectrum disorders group relative to a comparison group to reflect an overall heightened neural response to social stimuli (i.e., sensitization). Second, we investigated the relationship of amygdala habituation to social impairment in autism spectrum disorders. Some have theorized that hyperarousal of the amygdala contributes to social avoidance and difficulty with social interaction (7 , 8) . Given the individual differences in level of social dysfunction in patients with autism spectrum disorders, we hypothesized that reduced habituation in autism spectrum disorders would be correlated with increased social dysfunction as assessed by standardized clinical diagnostic measures. We did not predict group differences in habituation in the fusiform face area because previous studies have reported either reduced activation (8 , 19 – 21) or no difference in fusiform face area activation (22 – 25) in autism spectrum disorders groups.
Method
Participants
Twenty-four adults with autism spectrum disorders and 23 healthy comparison subjects completed the fMRI protocol. Comparison subjects were screened for current and past psychiatric disorders, developmental learning disabilities, and contraindications to MRI. Data for five individuals with autism spectrum disorders and three comparison subjects were excluded because of excessive motion during the scans. All participants had a full-scale IQ and verbal IQ ≥80 as measured by the WAIS-III. Overall, data from 19 individuals with autism spectrum disorders and 20 comparison subjects were included in the analyses. Diagnoses were confirmed with the Autism Diagnostic Interview–Revised, the Autism Diagnostic Observation Schedule, and DSM-IV criteria. The two groups did not significantly differ in age or IQ (see Table 1 ). Clinical ratings on the social subscale of the Autism Diagnostic Observation Schedule were used in the fMRI analysis to identify the relationship between social impairment and neurofunctional impairment. The Autism Diagnostic Observation Schedule is a semistructured clinical tool used to evaluate current social and communication skills; higher scores reflect a greater number of symptoms.
This study was approved by the University of Washington Human Subjects Institutional Review Board. Informed written consent was obtained from all study participants.
fMRI Data Acquisition
Structural and functional MRI were performed on a 1.5-T Signa MRI system (General Electric, Waukesha, Wisc.). fMRI series were collected using an echo-planar pulse sequence (repetition time=3000 msec, echo time=50 msec, 21 slices 6 mm thick with 1 mm gap, 64×64 matrix, 114 volumes total per run). A spoiled gradient-recall echo was collected for fMRI registration and anatomical localization (sagittal three-dimensional spoiled gradient-recall acquisition, repetition time=11.1 msec, echo time=2 msec, with voxel dimensions of 0.97×1.1×1.2 mm).
fMRI Task
A block design paradigm was used. Two runs were collected within the same scanning session containing blocks of upright neutral faces (eight 36-second blocks), inverted neutral faces (four 36-second blocks), houses (four 36-second blocks), and fixation (six 18-second blocks). Within each run, 144 seconds of neutral faces and 54 seconds of fixation were presented. There was a 6-minute gap between run 1 and run 2. Each face stimulus was presented for 3 seconds with no interstimulus interval. Participants were instructed to press a button when identical stimuli appeared in succession; 30% of the trials were targets. Fixation blocks appeared at the beginning, middle, and end of the experiment. Face stimuli were photos of 24 adults with a neutral facial expression (an example is provided in the supplemental figure in the data supplement that accompanies the online edition of this article). This study investigated only upright neutral faces versus fixation at run 1 and run 2. Different photographs were used in each run.
fMRI Processing and Statistical Analysis
fMRI data analyses were performed using the FMRIB Software Library, version 3.3 (http://www.fmrib.ox.ac.uk/fsl/). The following preprocessing steps were applied: the first two volumes were discarded; motion correction was conducted using MCFLIRT (Motion Correction using FMRIB’s Linear Image Registration Tool); nonbrain structures were removed using Brain Extraction Tool; data were spatially smoothed using a Gaussian kernel of full width at half maximum 5 mm and temporally smoothed using a high-pass filter of sigma=72 seconds. Motion-related components, identified using Multivariate Exploratory Linear Optimized Decomposition into Independent Components, were filtered from the data prior to statistical analyses. Time-series statistical analyses were carried out using FMRIB’s Improved Linear Model (FILM) with local autocorrelation correction. Condition effects were estimated at each voxel for the contrast “upright face > fixation” for each participant. Individual fMRI data were registered to the high-resolution spoiled gradient-recall acquisition and then warped to the Montreal Neurological Institute (MNI152) standard image using FLIRT (FMRIB’s Linear Image Registration Tool).
Mixed-effects analyses were conducted using FLAME (FMRIB’s Local Analysis of Mixed Effects), a method for modeling and estimating the random-effects component of the measured intersession mixed-effects variance. To investigate group differences in habituation, four analyses were conducted 1) upright face > fixation run 1; 2) upright face > fixation run 2; 3) paired-samples two-group t test upright face > fixation run 1 versus run 2; and 4) paired-samples two-group t test upright face > fixation run 2 versus run 1. To investigate the relationship between habituation to faces and social functioning in autism spectrum disorders, an additional paired t test was conducted comparing upright face > fixation in run 1 versus run 2 with Autism Diagnostic Observation Schedule social subscale score entered as an explanatory variable. Four a priori regions of interest were defined on the MNI152 template and tested separately: right amygdala, left amygdala, right lateral fusiform gyrus, left lateral fusiform gyrus. Statistical corrections for multiple comparisons were conducted using cluster thresholding based on Markov Chain Monte Carlo sampling for each region of interest. All fMRI results were corrected for multiple comparisons using this method.
Results
fMRI Behavioral Performance
Independent-samples t tests were conducted to assess between-group differences in number of errors and reaction time during the upright face blocks. No significant group differences in accuracy were observed. However, the autism spectrum disorders group responded significantly more slowly on average than the comparison group at time 1 (see Table 1 ).
Repeated-measures analyses were conducted to determine whether there were changes in performance across time and whether the amount of change between time 1 and time 2 depended on diagnostic status. The within-subjects factor was time (faces 1, faces 2), and the between-subjects factor was group. No significant change in the number of errors between time 1 and time 2 was found, and the difference in number of errors committed at time 1 compared with time 2 did not depend on which group the participant was in. Overall, participants responded more slowly at time 2 than at time 1 (F=18.839, df=1, 37, p<0.001). However, the group-by-time interaction was not significant, indicating that both groups slowed down over time at approximately the same rate. Thus, overall, there were no group differences in performance changes from time 1 to time 2.
fMRI Results
Faces 1 > fixation
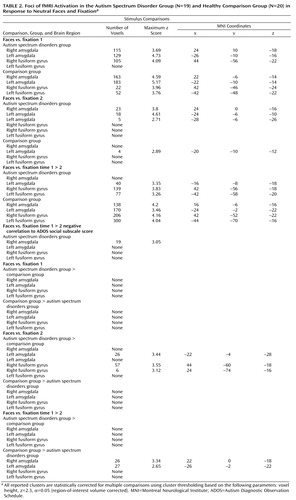
Both groups showed significant activation in the amygdala and fusiform gyrus (p<0.05, two-tailed, corrected). No between-group differences in fusiform or amygdala activation to faces were observed ( Table 2 ).
Faces 2 > fixation
The autism spectrum disorders group showed significant activation in the amygdala bilaterally, and the comparison group showed significant activation in the left amygdala (p<0.05, two-tailed, corrected). Neither group evidenced significant fusiform activation to faces. The autism spectrum disorders group showed significantly greater activation in the left amygdala and the right fusiform gyrus than the comparison group (p<0.05, two-tailed, corrected), suggesting abnormally elevated neural activity related to emotional processing and face identification. The comparison group did not show greater activation than the autism spectrum disorders group in either brain region (see Table 2 ).
Faces 1 > faces 2
Both groups showed significant habituation in the fusiform gyrus bilaterally (p<0.05, two-tailed, corrected). However, significantly reduced amygdala habituation was observed bilaterally in the autism spectrum disorders group (p<0.05, two-tailed, corrected). This result is consistent with our hypothesis that a component of amygdala dysfunction in autism spectrum disorders is a failure of the neural activity to habituate appropriately to repeated stimulation. No between-group differences were observed in the fusiform gyrus. Results are presented in Table 2 and Figures 1 and 2 .

a The comparison group showed significant bilateral habituation in the amygdala and fusiform gyrus at p<0.05, small volume corrected. The autism spectrum disorders group showed significant bilateral habituation in the fusiform gyrus and left-lateralized habituation in the amygdala at p<0.05, small volume corrected. Significantly greater habituation in the comparison group was observed in the amygdala bilaterally at p<0.05, small volume corrected. No significant between-group differences in habituation were observed in the fusiform gyrus. The autism spectrum disorders group did not show greater habituation than comparison subjects in either brain region (not pictured).

a The comparison group’s amygdala activation exceeds the autism spectrum disorders group’s average at time 1 but shows a substantial decrease by time 2. The autism spectrum disorders group is characterized by a shallower rise and smaller drop in activation over the two runs relative to the comparison group. Average z scores were calculated including all voxels in the amygdala region of interest for the contrasts faces 1 > fixation and faces 2 > fixation, by group.
Faces 2 > faces 1
Neither group showed significantly increased activation to faces at time 2 compared with time 1 in the amygdala or fusiform gyrus.
Correlation Analysis
The Autism Diagnostic Observation Schedule social subscale score was significantly correlated with the degree of habituation between run 1 and run 2 in the right amygdala (p<0.05, two-tailed, corrected) in the autism spectrum disorders group ( Figure 3 ). Thus, individuals with the greatest level of current clinical impairment demonstrated the least amount of neural habituation in response to repeated exposure to neutral faces. Consistent with our theory, abnormally sustained amygdala arousal may contribute to more severe social dysfunction in autism spectrum disorders. No relationship between neural habituation and clinical severity was observed in the fusiform gyrus, indicating that this relationship may be specific to the amygdala.

a A significant inverse correlation between habituation in the right amygdala and the Autism Diagnostic Observation Schedule social subscale score was observed. The scatterplot depicts the relationship of habituation to faces and social severity on the Autism Diagnostic Observation Schedule (R 2 =0.3736). The mean z score value for the amygdala was based on the average z score of the cluster showing a significant relationship between habituation (i.e., based on the contrast “face vs. fixation” time 1 > time 2) and Autism Diagnostic Observation Schedule social subscale score (see Table 2). These results demonstrate that the more severely affected an individual is, the less habituation to faces occurs, with the most severely affected individuals displaying increased activation over time (as evidenced by negative z scores for habituation).
Discussion
Our goal in this study was to investigate habituation of amygdala response during face processing in individuals with autism spectrum disorders relative to healthy comparison subjects. Consistent with our hypotheses, the autism spectrum disorders group demonstrated significantly reduced amygdala habituation relative to the comparison group. This finding refines earlier assertions that individuals with autism spectrum disorders experience hyperarousal of the amygdala while looking at faces (8) . While hyperarousal was indeed present in individuals with autism spectrum disorders, the relative magnitude of activation levels in the autism spectrum disorders group never exceeded the maximum level of the comparison group (see Figure 3 ). Instead, individuals with autism spectrum disorders showed a marginal decrease of initial levels of amygdala arousal over time while comparison subjects rapidly habituated to repeated face stimuli, presumably as the stimuli were rendered less salient (17) .
Habituation of the neural response is a powerful indicator of learning, and it is observed across species. This mechanism can be identified in fMRI studies of the association of behavioral improvements with diminished activation following repeated stimuli (18) . Habituation in the amygdala may affect social orienting responses (17) and novelty detection (26) . Because individuals with autism spectrum disorders do not show typical amygdala habituation responses to faces, they may have more difficulty extracting and interpreting important social information from faces and the social environment. Difficulties with social orienting are well established in individuals with autism spectrum disorders and have been hypothesized to be associated with amygdala dysfunction (27) . One possible explanation for difficulties with social orienting observed in autism spectrum disorders may be that amygdala dysfunction results in difficulty discriminating between salient and nonsalient social information. This stimulus overload, resulting from the failure of amygdala neurons to habituate to less important stimuli, may have far-reaching effects in terms of social dysfunction. Thus, we propose that reduced amygdala habituation in individuals with autism spectrum disorders may be involved in the difficulty these individuals exhibit in social cognition. In fact, in this study, participants with the most severe social impairments exhibited the least amount of amygdala habituation to faces (see Figure 3 ).
These results add to a growing body of evidence indicating that amygdala abnormalities are present in autism spectrum disorders and may be linked to the social deficits present in these disorders (28) . Amygdala impairment was identified as a potential etiological mechanism of social dysfunction in autism spectrum disorders because of its known involvement in evaluation of the emotional and/or social value of the stimulus being perceived (28 , 29) . Traditionally, the amygdala’s role in social cognition was thought to be limited to processing threatening stimuli, particularly facial expressions that depict fear or anger. However, several studies have reported that the amygdala is also active in response to emotionally ambiguous facial expressions, such as surprise (30) and neutrality (31 , 32) . Together, this suggests that the amygdala may play a more general role in identifying and processing socially relevant information rather than responding exclusively to discrete categories of emotion (31) . It is possible that when the amygdala is engaged as a social relevance detector, amygdala dysfunction affects socioemotional processing in individuals with autism spectrum disorders. The role of the amygdala in social dysfunction has been proposed previously (see reference 28 for example); our findings extend current theory to suggest that reduced habituation may be a mechanism for social impairment.
Reduced habituation may arise from structural and biochemical abnormalities in the amygdala in autism spectrum disorders. Volumetric studies indicate that amygdala development follows an atypical course in autism spectrum disorders. Abnormal enlargement of the amygdala is found in very young children with these disorders (33 – 35) but not in adolescents and adults, where volume reduction relative to comparison subjects is observed (7 , 20 , 36) . Preliminary evidence suggests that the pattern of amygdala growth may be related to clinical severity. Larger amygdala volumes at ages 3–4 years were predictive of a more severe clinical course from ages 3–6 (35) . In contrast, adolescents and adults with the smallest amygdala volumes exhibited the most severe current deficits in processing facial emotions and the greatest illness severity on childhood nonverbal social behaviors estimated from the Autism Diagnostic Interview–Revised (7) . These studies point toward a theoretical developmental model by which the most severely affected individuals undergo excessive amygdala growth early in childhood and subsequent atrophy in adolescence and adulthood. This pattern has been suggested to be the result of hyperactivity leading to excitotoxic changes in the amygdala (7) . Future longitudinal studies will be important in determining whether this pattern indeed exists in autism spectrum disorders.
In addition to the aforementioned structural studies, Dalton and colleagues (8) provided additional support for the hyperactivity model, as demonstrated by the association between increased amygdala activation during face perception and a higher degree of eye fixation in autism spectrum disorders patients. These results were interpreted to indicate a heightened response to social stimuli in comparison with typically developing individuals. A follow-up study by the same group (7) found a relationship between eye gaze, amygdala volume, and clinical impairment; smaller amygdala volume was associated with slower judgment time for emotional expressions, decreased eye fixation, and more childhood social impairment. The abnormally small amygdala volumes found by late adolescence were again suggested to be consistent with a developmental model of hyperactivity-induced excitotoxic changes eventually leading to atrophy (7) . The results of our study are consistent with this hypothesis in that failure to habituate would result in persistent elevated amygdala arousal to social stimuli.
We evaluated the neural response of the amygdala and fusiform gyrus while viewing neutral faces over time in high-functioning adults with autism spectrum disorders. Abnormal amygdala functional activation in autism spectrum disorders has been reported in response to emotional face processing (9 , 10) , discrimination (8) , and attribution (12) and consists of abnormally increased activation (8) , decreased activation (9 – 11) , and reduced task-related modulation of the amygdala (10 , 12 , 13) . We proposed that differential amygdala responsiveness in relation to matched comparison groups in previous studies may be related to reduced habituation in the autism spectrum disorders group. Indeed, studies that included extended scanning periods (e.g., 818 seconds) reported increased amygdala activation in the autism spectrum disorders group (8) , whereas shorter experiments (with scanning periods ranging from 292 to 498 seconds) reported decreased amygdala activation (9 – 11) . Difficulty evaluating and interpreting facial expressions has been reported in autism spectrum disorders and is thought to be associated with amygdala damage; however, in high-functioning individuals with autism spectrum disorders, impaired performance is limited to higher-level processing, such as the evaluation of trustworthiness (3) . Thus, it is unlikely that abnormal amygdala activation in fMRI studies of high-functioning individuals with these disorders is due to difficulty distinguishing an angry facial expression from a neutral one. Similarly, in this study, abnormal amygdala activation is unlikely to stem from the autism spectrum disorders group misinterpreting the neutral faces as depicting a negative emotion given the high level of cognitive functioning in our participants. Furthermore, amygdala habituation has been shown to be modulated by emotion, such that amygdala response to more emotional faces is characterized by increased initial amygdala response and faster habituation (18) . This is opposite to what was found in our autism spectrum disorders group, rendering such an explanation unlikely.
Finally, no significant difference in habituation of the fusiform gyrus was observed between the autism spectrum disorders group and the comparison group. The autism spectrum disorders group showed significantly decreased activation in the fusiform gyrus bilaterally from time 1 to time 2. The inconsistency in activation between these two brain regions in the autism spectrum disorders group is notable given that the amygdala reportedly modulates the response to faces through anatomical projections (18) . In a previous study, we found reduced connectivity between the fusiform face area and the amygdala in autism spectrum disorders (37) . Thus, it is possible that the differential habituation response observed between the fusiform gyrus and the amygdala in the autism spectrum disorders group is secondary to abnormalities in neural signaling originating from the amygdala (38) .
An important limitation of this study is that because the fMRI signal is an indirect measure of neural activity, amygdala hyperarousal in autism spectrum disorders cannot be entirely ruled out. Furthermore, the findings reported here may reflect changes in the relative ratio of inhibitory and excitatory networks, resulting in a net change in the fMRI signal (39) . An additional limitation is that eye tracking within the scanner was not available; therefore we cannot be certain that the differences reported here were not due to differences in visual attention. One autism study found that both fusiform and amygdala activation were correlated with the time spent fixating on the eyes (8) , indicating that this is an important factor in face processing in autism spectrum disorders. However, our pattern of results would not be expected if a significant confound related to eye gaze were present. First, the amygdala and fusiform gyrus were significantly activated by both groups to a comparable degree during the initial scan. Thus, assuming that Dalton et al. (8) are correct, with limited confidence we can infer that individuals in the autism spectrum disorders group did not display substantially reduced attention to the eyes at time 1. Second, the group difference in habituation was specific to the amygdala. Since visual attention to the eyes was shown to be correlated with both amygdala and fusiform activation, had this confound been present, it would be expected to affect activation in both structures similarly. Third, we reviewed eye-tracking data collected outside the magnet by our group (40) . The visual stimuli included pictures of unfamiliar neutral faces that matched the pictures in this fMRI study. The duration of fixation on the eye region when looking at unfamiliar faces was lower in the autism spectrum disorders group, although the difference did not reach statistical significance. Twenty-three of these participants also had valid fMRI data for the current study. To explore the relationship between looking at the eyes and habituation, we reran our between-group analyses on the subset of our sample with valid eye-tracking data, entering the duration of fixation on the eyes as a nuisance variable. The group differences in amygdala habituation remained; reduced habituation was observed in the right amygdala both with and without statistically controlling for duration of time fixating on the eye region. However, because we cannot definitively determine how individuals with autism spectrum disorders allocated their visual attention, it will be important in future studies to incorporate eye tracking in order to clarify the relationship between eye gaze patterns and habituation effects.
In summary, individuals with autism spectrum disorders show abnormally reduced habituation to neutral faces in the amygdala. Individuals who showed the least amount of neural habituation had the greatest social impairment on a clinical measure of current social functioning. This suggests that difficulties in social cognition in autism spectrum disorders may be associated with the abnormalities in this basic function of learning. Reduced habituation in autism spectrum disorders and related social deficits likely arise from previously reported structural abnormalities in the amygdala and abnormal connections between the amygdala and cortical regions.
1. Dawson G, Webb SJ, McPartland J: Understanding the nature of face processing impairment in autism: insights from behavioral and electrophysiological studies. Dev Neuropsychol 2005; 27:403–424Google Scholar
2. Boucher J, Lewis V, Collis G: Familiar face and voice matching and recognition in children with autism. J Child Psychol Psychiatry 1998; 39:171–181Google Scholar
3. Adolphs R, Sears L, Piven J: Abnormal processing of social information from faces in autism. J Cogn Neurosci 2001; 13:232–240Google Scholar
4. Hobson RP, Ouston J, Lee A: What’s in a face? the case of autism. Br J Psychol 1988; 79:441–453Google Scholar
5. Klin A, Jones W, Schultz R, Volkmar F, Cohen D: Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Arch Gen Psychiatry 2002; 59:809–816Google Scholar
6. Schultz RT, Grelotti DJ, Klin A, Kleinman J, Van der Gaag C, Marois R, Skudlarski P: The role of the fusiform face area in social cognition: implications for the pathobiology of autism. Philos Trans R Soc Lond B Biol Sci 2003; 358:415–427Google Scholar
7. Nacewicz BM, Dalton KM, Johnstone T, Long MT, McAuliff EM, Oakes TR, Alexander AL, Davidson RJ: Amygdala volume and nonverbal social impairment in adolescent and adult males with autism. Arch Gen Psychiatry 2006; 63:1417–1428Google Scholar
8. Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, Alexander AL, Davidson RJ: Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci 2005; 8:519–526Google Scholar
9. Critchley HD, Daly EM, Bullmore ET, Williams SC, Van Amelsvoort T, Robertson DM, Rowe A, Phillips M, McAlonan G, Howlin P, Murphy DG: The functional neuroanatomy of social behaviour: changes in cerebral blood flow when people with autistic disorder process facial expressions. Brain 2000; 123:2203–2212Google Scholar
10. Ashwin C, Baron-Cohen S, Wheelwright S, O’Riordan M, Bullmore ET: Differential activation of the amygdala and the “social brain” during fearful face-processing in Asperger syndrome. Neuropsychologia 2007; 45:2–14Google Scholar
11. Baron-Cohen S, Ring HA, Wheelwright S, Bullmore ET, Brammer MJ, Simmons A, Williams SC: Social intelligence in the normal and autistic brain: an fMRI study. Eur J Neurosci 1999; 11:1891–1898Google Scholar
12. Wang AT, Dapretto M, Hariri AR, Sigman M, Bookheimer SY: Neural correlates of facial affect processing in children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry 2004; 43:481–490Google Scholar
13. Williams JH, Waiter GD, Gilchrist A, Perrett DI, Murray AD, Whiten A: Neural mechanisms of imitation and “mirror neuron” functioning in autistic spectrum disorder. Neuropsychologia 2006; 44:610–621Google Scholar
14. Thompson RF, Spencer WA: Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychol Rev 1966; 73:16–43Google Scholar
15. Prescott SA: Interactions between depression and facilitation within neural networks: updating the dual-process theory of plasticity. Learn Mem 1998; 5:446–466Google Scholar
16. Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, Strauss MM, Hyman SE, Rosen BR: Response and habituation of the human amygdala during visual processing of facial expression. Neuron 1996; 17:875–887Google Scholar
17. Fischer H, Wright CI, Whalen PJ, McInerney SC, Shin LM, Rauch SL: Brain habituation during repeated exposure to fearful and neutral faces: a functional MRI study. Brain Res Bull 2003; 59:387–392Google Scholar
18. Ishai A, Pessoa L, Bikle PC, Ungerleider LG: Repetition suppression of faces is modulated by emotion. Proc Natl Acad Sci USA 2004; 101:9827–9832Google Scholar
19. Schultz RT, Gauthier I, Klin A, Fulbright RK, Anderson AW, Volkmar F, Skudlarski P, Lacadie C, Cohen DJ, Gore JC: Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Arch Gen Psychiatry 2000; 57:331–340Google Scholar
20. Pierce K, Muller RA, Ambrose J, Allen G, Courchesne E: Face processing occurs outside the fusiform “face area” in autism: evidence from functional MRI. Brain 2001; 124:2059–2073Google Scholar
21. Hubl D, Bolte S, Feineis-Matthews S, Lanfermann H, Federspiel A, Strik W, Poustka F, Dierks T: Functional imbalance of visual pathways indicates alternative face processing strategies in autism. Neurology 2003; 61:1232–1237Google Scholar
22. Hadjikhani N, Joseph RM, Snyder J, Chabris CF, Clark J, Steele S, McGrath L, Vangel M, Aharon I, Feczko E, Harris GJ, Tager-Flusberg H: Activation of the fusiform gyrus when individuals with autism spectrum disorder view faces. Neuroimage 2004; 22:1141–1150Google Scholar
23. Dapretto M, Davies MS, Pfeifer JH, Scott AA, Sigman M, Bookheimer SY, Iacoboni M: Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat Neurosci 2006; 9:28–30Google Scholar
24. Bird G, Catmur C, Silani G, Frith C, Frith U: Attention does not modulate neural responses to social stimuli in autism spectrum disorders. Neuroimage 2006; 31:1614–1624Google Scholar
25. Hadjikhani N, Joseph RM, Snyder J, Tager-Flusberg H: Abnormal activation of the social brain during face perception in autism. Hum Brain Mapp 2007; 28:441–449Google Scholar
26. Wilson FA, Rolls ET: The effects of stimulus novelty and familiarity on neuronal activity in the amygdala of monkeys performing recognition memory tasks. Exp Brain Res 1993; 93:367–382Google Scholar
27. Dawson G, Toth K, Abbott R, Osterling J, Munson J, Estes A, Liaw J: Early social attention impairments in autism: social orienting, joint attention, and attention to distress. Dev Psychol 2004; 40:271–283Google Scholar
28. Baron-Cohen S, Ring HA, Bullmore ET, Wheelwright S, Ashwin C, Williams SC: The amygdala theory of autism. Neurosci Biobehav Rev 2000; 24:355–364Google Scholar
29. Adolphs R: The neurobiology of social cognition. Curr Opin Neurobiol 2001; 11:231–239Google Scholar
30. Kim H, Somerville LH, Johnstone T, Alexander AL, Whalen PJ: Inverse amygdala and medial prefrontal cortex responses to surprised faces. Neuroreport 2003; 14:2317–2322Google Scholar
31. Fitzgerald DA, Angstadt M, Jelsone LM, Nathan PJ, Phan KL: Beyond threat: amygdala reactivity across multiple expressions of facial affect. Neuroimage 2006; 30:1441–1448Google Scholar
32. Kleinhans NM, Johnson LC, Mahurin R, Richards T, Stegbauer KC, Greenson J, Dawson G, Aylward E: Increased amygdala activation to neutral faces is associated with better face memory performance. Neuroreport 2007; 18:987–991Google Scholar
33. Schumann CM, Hamstra J, Goodlin-Jones BL, Lotspeich LJ, Kwon H, Buonocore MH, Lammers CR, Reiss AL, Amaral DG: The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. J Neurosci 2004; 24:6392–6401Google Scholar
34. Sparks BF, Friedman SD, Shaw DW, Aylward EH, Echelard D, Artru AA, Maravilla KR, Giedd JN, Munson J, Dawson G, Dager SR: Brain structural abnormalities in young children with autism spectrum disorder. Neurology 2002; 59:184–192Google Scholar
35. Munson J, Dawson G, Abbott R, Faja S, Webb SJ, Friedman SD, Shaw D, Artru A, Dager SR: Amygdalar volume and behavioral development in autism. Arch Gen Psychiatry 2006; 63:686–693Google Scholar
36. Aylward EH, Minshew NJ, Goldstein G, Honeycutt NA, Augustine AM, Yates KO, Barta PE, Pearlson GD: MRI volumes of amygdala and hippocampus in non-mentally retarded autistic adolescents and adults. Neurology 1999; 53:2145–2150Google Scholar
37. Kleinhans NM, Richards T, Sterling L, Stegbauer KC, Mahurin R, Johnson LC, Greenson J, Dawson G, Aylward E: Abnormal functional connectivity in autism spectrum disorders during face processing. Brain 2008; 131:1000–1012Google Scholar
38. Schultz RT: Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. Int J Dev Neurosci 2005; 23:125–141Google Scholar
39. Logothetis NK: What we can do and what we cannot do with fMRI. Nature 2008; 453:869–878Google Scholar
40. Sterling L, Dawson G, Webb S, Murias M, Munson J, Panagiotides H, Aylward E: The role of face familiarity in eye tracking of faces by individuals with autism spectrum disorders. J Autism Dev Disord 2008; 38:1666–1675Google Scholar