Apolipoprotein E Genotype, Cortisol, and Cognitive Function in Community-Dwelling Older Adults
Abstract
Background: Elevated cortisol indicates stress and may be a risk factor for cognitive decline in aging. Genetic factors may influence individual vulnerability to the adverse effects of stress on cognitive function in aging. Method: The authors investigated whether the gene-environment interaction between the genotype for the apolipoprotein E gene ( APOE ) and cortisol predicted cognitive performance in older urban adults. Cross-sectional data were analyzed from a population-based sample of 50–70-year-old men and women. Cognitive performance, salivary cortisol levels, and APOE genotype were assessed in 962 subjects. Scores on 20 standard cognitive tests were combined into seven domain scores (language, processing speed, eye-hand coordination, executive functioning, verbal memory and learning, visual memory and learning, visuoconstruction). Results: In adjusted models, while a higher cortisol level was associated with worse cognitive scores, the slopes of the adverse relations were steeper in persons with at least one APOE -ε4 allele. Effect sizes were large: for example, the effect of having one ε4 allele plus a cortisol area under the curve greater than the 75th percentile was equivalent to a decrease in language score expected from an age increase of 8.0 years (95% confidence interval=1.7–14.4), while having two ε4 alleles and a cortisol area under the curve greater than the 75th percentile was equivalent to an age increase of 33.4 years (95% CI=14.8–52.0). Conclusions: These data suggest that APOE genotype modifies cortisol’s relations with cognitive functioning and that the ε4 allele increases vulnerability of the aging brain to adverse effects of stress.
Chronic psychosocial stress has been implicated as a factor contributing to cognitive aging. Animal studies suggest that repeated exposure to psychosocial hazards such as restraint stress or subordinate hierarchical status can cause neuroanatomical changes (1) , including inhibition of adult neurogenesis in the dentate gyrus (2) and dendritic atrophy in the hippocampus (3) and medial prefrontal cortex (4) . This damage is partially attributable to HPA axis dysregulation characterized by excesses in glucocorticoid production and changes in the diurnal pattern of secretion (5 , 6) . Observational studies in older humans indicate that elevation in cortisol (the principal human glucocorticoid) is associated with smaller hippocampal volume (7) and is a risk factor for greater decline in global cognition, verbal memory, and executive functioning (8 – 10) .
It is reasonable to expect that genetic factors may influence individual vulnerability to the adverse effects of stress, and one candidate of immediate interest is the gene for apolipoprotein E ( APOE ). Three alleles of APOE (e2, e3, ε4) code for three protein isoforms that have substantial involvement in lipid metabolism and neurobiology (11) . The ε4 allele increases risk for late-onset familial and sporadic Alzheimer’s disease in a dose-dependent manner (12 , 13) . The ε4 genotype may influence some domains of cognitive performance in nondemented adults, although effects appear small. A meta-analysis of 38 studies found modest associations of APOE -ε4 with decrements in global cognition, episodic memory, and executive functioning (14) . A large study showed greater longitudinal decline for ε4 carriers in verbal memory and processing speed; however, the differences in decline by genotype were relatively small (15) . Aged healthy ε4 carriers have greater risk of hippocampal atrophy (16) as well as reduced white matter integrity in the hippocampus, medial temporal lobe, and corpus callosum (17) . It is noteworthy that epidemiologic evidence suggests that the ε4 allele also amplifies the effects of multiple risk factors for cognitive dysfunction, including head injury, lead exposure, diabetes, peripheral vascular disease, atherosclerosis, hypercholesterolemia, elevated homocysteine, and low vitamin B 12(15 , 18 – 22) .
We have reported that higher salivary cortisol levels over a study visit were associated with worse cognitive performance in a cross-sectional analysis of data from 967 adults aged 50 to 70 years (23) . We now consider whether APOE genotype modifies the associations of cortisol with cognitive performance in the same study population.
Method
Participant Selection and Recruitment
Sampling and recruitment for the Baltimore Memory Study have been described elsewhere (24) . A total of 1,140 persons were enrolled from the eligible population of 50–70-year-old residents of Baltimore neighborhoods who had lived within the greater Baltimore area for at least the previous 5 years. Study participants completed three visits an average of 14 months apart. The Committee for Human Research of the Johns Hopkins Bloomberg School of Public Health approved the study. Participants provided written, informed consent before entering the study and were paid $50 for completion of each visit.
Data Collection
All data collection occurred on site at the study clinic in Baltimore. Trained research assistants collected data in the following order: cognitive testing, blood pressure, height, weight, spot urine collection, structured interview, and venipuncture. The interview captured self-reported information on demographic characteristics and medical history, including chronic conditions, current and past medications, and alcohol and tobacco use. Participant race/ethnicity was collected through self-report by using the 2000 U.S. Census method. Depressive symptoms were measured by using the Center for Epidemiologic Studies Depression (CES-D) Scale (25) . Recent exposure to stressful events was assessed by using a questionnaire that asked whether each of 25 events had occurred in the previous 7 days.
Saliva Sampling and Cortisol Measurement
Sampling and measurement methods have been previously described (23) . We used the cognitive battery to elicit an HPA axis response during the study visit. Although cognitive performance was assessed at three yearly study visits, cortisol samples were obtained at only one visit, either the second or third visit. Four salivary cortisol samples were collected for each of the 992 participants during the study visit (before, during, and after cognitive testing and at visit completion). The mean duration from the first to fourth cortisol sample was 159 minutes (SD=25). Visits were scheduled throughout the day to accommodate the large sample size: 373 (37.6%) submitted the first saliva sample between 08:00 and 09:45 a.m., 501 (50.5%) submitted the first sample between 09:46 a.m. and 2:30 p.m., and 118 (11.9%) submitted the first sample between 2:31 and 6:30 p.m. Self-reported assessments of subjective distress at the times of each of the saliva collections were obtained by using a visual analogue scale.
Laboratory Methods
Salivary cortisol was assayed by the General Clinical Research Center Core Laboratory at the Johns Hopkins Bayview Medical Center campus (Baltimore) by using a standard radioimmunoassay. A trained phlebotomist obtained a 10-ml blood sample through venipuncture, and it was clotted, centrifuged, and stored at –20°C within 1 hour. The samples were then transferred to and stored at –70°C at the Johns Hopkins Bloomberg School of Public Health. The Malaria Research Institute Gene Array Core Facility at the Bloomberg School of Public Health performed APOE genotyping from frozen whole blood according to methods published elsewhere (21) .
Cognitive Battery and Cognitive Domains
The cognitive battery and creation of cognitive domain scores have been described in detail previously (24 , 26) . Each subject completed 20 standard tests classified in seven cognitive domains: 1) language (Boston Naming Test; letter fluency; category fluency), 2) processing speed (simple reaction time), 3) eye-hand coordination (Purdue Pegboard Test for dominant hand, nondominant hand, and both hands; Trail-Making Test A), 4) executive functioning (difference scores: total Purdue Pegboard Test minus performance with both hands; Stroop test C form minus A form; Trail-Making Test B minus A), 5) verbal memory and learning (Rey Auditory Verbal Learning Test immediate recall, delayed recall, recognition), 6) visual memory (Rey Complex Figure delayed recall; symbol digit), and 7) visuoconstruction (Rey Complex Figure copy). Domain scores were created by averaging the z scores for the individual tests within domains. Tests were standardized for direction so that a negative regression coefficient indicates worse performance. The seven domain scores were the primary study outcomes.
Cortisol Metrics
The creation of the metrics from the four cortisol measurements across the study visit has been described elsewhere (23) . Because of skewing, the four cortisol sample values were natural-log transformed before creation of the metrics. We previously noted associations of three cortisol metrics with cognitive performance (23) and therefore limited the present analysis of cortisol- APOE interaction to the same three metrics: pretest (the first sample value), mean (mean of all four sample values), and area under the curve with respect to zero (AUC). The metrics are hypothesized to represent different aspects of HPA axis activity, with the pretest value indicating the nonchallenge cortisol level and the mean value and AUC providing metrics of overall cortisol dose across the study visit. AUC is a standard pharmacological metric representing total hormonal output over a period of time (27) . Unlike other summary measures such as mean, AUC captures both the cortisol levels at the times of sampling and changes over time. The pretest, mean, and AUC values were highly correlated, with Spearman rank correlation coefficients ranging from 0.77 (pretest and AUC) to 0.90 (mean and AUC).
Statistical Analysis
The objective of the present analysis was to evaluate whether the associations of the three cortisol metrics and seven cognitive domain scores (23) were modified by APOE genotype. The adjusted analyses included a maximum of 962 participants who had complete cortisol, genotyping, and cognitive test data.
Multiple linear regression was used to evaluate the relations of the cortisol metrics, APOE , and cognitive domain scores. For each model specification, separate regressions were conducted for each of the three metrics. Model 1 evaluated main effect associations for cortisol (continuous) and APOE genotype (two indicator terms: one ε4 allele versus none, two ε4 alleles versus none). Model 2 evaluated effect modification by APOE genotype of the cortisol associations by including cross-products of the two >APOE indicator terms with each cortisol metric. The following covariates were included in models 1 and 2, on the basis of a priori knowledge of independent associations with the outcome or if they changed the relation of cortisol with the outcome: age (years), sex, race/ethnicity (African American, African American/mixed race, and other, with whites as the reference group), household wealth (natural-log transformed sum of household income and household assets), educational status (nine levels), study visit (second versus third), testing technician, and time of day of cortisol sampling (linear and squared terms, to adjust for nonlinearity). Model 3 included model 1 and 2 covariates plus self-reported recent stressful events (five levels); history (yes versus no) of stroke, diabetes, cardiovascular disease, hypercholesterolemia, and hypertension; depressive symptoms (CES-D score, continuous); and use of antidepressant or antianxiety medications. Statistical interaction was evaluated through likelihood ratio tests and Wald tests. Final models were evaluated for normality, influential points, homoscedasticity, and model fit by using standard diagnostic procedures. Regression analyses were performed by means of the R statistical package, version 2.2.1 (http://www. r-project.org/).
To interpret the magnitude of the estimates, we examined adjusted mean cognitive performance by APOE genotype and by AUC cortisol level (dichotomized at the 75th percentile, 374.40 nmol·min/liter, to indicate presence or absence of high cortisol). Between-group differences in mean cognitive performance were estimated with adjustment for model 1 and 2 covariates. The effects of having higher cortisol and one or two ε4 alleles were then compared with the estimated effect of an increase in 1 year of age at baseline among the study participants. Cognitive performance differences and 95% confidence intervals (CIs) were calculated by using Stata version 8.0 (Stata Corp., College Station, Tex., 2003).
Results
Description of Study Participants
The characteristics of the study participants have been reported (23) and are now summarized by genotype ( Table 1 ). Genotype frequencies did not differ from Hardy-Weinberg equilibrium (χ 2 =4.39, df=3, p>0.05). Approximately 30% of the sample had at least one ε4 allele, with differences in prevalence by race; 35.0% of nonwhites, predominantly African Americans, had at least one ε4 allele, as compared with 25.8% of whites. The prevalences of hypercholesterolemia and elevated depressive symptom level (CES-D score of 16 or more) were higher in persons with one ε4 allele, although these differences were not detected in persons with two ε4 alleles ( Table 1 ). There were no differences in time of sampling or levels of the cortisol metrics by ε4 genotype.
Cortisol Metrics, APOE Genotype, and Cognitive Function
In the adjusted analysis of APOE main effects with the pretest metric as a covariate, the presence of one ε4 allele (versus none) was not associated with lower cognitive domain scores ( Table 2 , model 1); the results for the mean value and AUC are similar and are not shown. In contrast, the presence of two ε4 alleles was associated with worse performance in the domains of processing speed, executive functioning, and verbal memory and learning.
APOE genotype modified cortisol’s relations with cognitive performance. Inclusion of cortisol- APOE interaction terms significantly improved fit (p<0.05) over nested models in 14 of 21 models. Cortisol- APOE interactions for pretest, mean, and AUC values were observed in the domains of language, eye-hand coordination, verbal memory and learning, and visuoconstruction ( Table 2 , model 2; Table 3 ); results for mean values are similar to those for pretest and AUC and are not shown. Adjusted associations for AUC (from model 2) stratified by ε4 genotype are graphically displayed in Figure 1 . Interaction was most evident for participants with two ε4 alleles (ε4/4 genotype). For the cross-product of cortisol with two ε4 alleles, beta coefficients were negative in all 21 models, with p<0.05 in 13 Wald tests. Additional adjustment for psychosocial and cardiovascular health and medication use in model 3 did not substantially change these patterns of associations (results not shown).
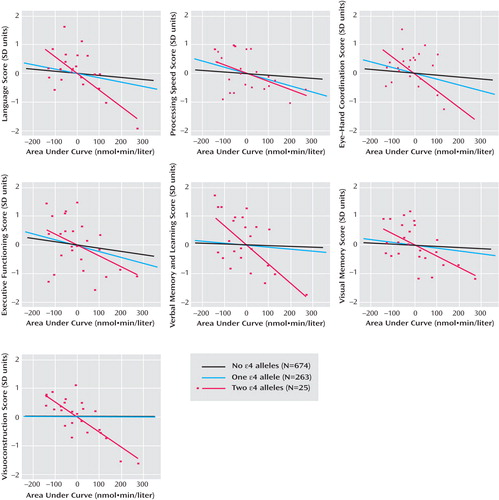
a Associations are adjusted for age, sex, technician, visit, household wealth, education, race/ethnicity, and time of day. Straight lines represent predicted linear fits for all participants (N=962). Points are plotted only for persons with two ε4 alleles (N=25). Cortisol values were natural-log transformed before metrics creation (see Method section of text). To ensure comparability, data for persons with no or one ε4 allele are restricted to the range of values of cortisol metrics for persons with two ε4 alleles.
Evaluation of Magnitude of Effect Modification
Compared with persons with lower AUC cortisol (≤75th percentile) and no ε4 alleles (reference group), persons with higher cortisol (>75th percentile) and no ε4 alleles performed significantly worse in language, processing speed, eye-hand coordination, and executive functioning ( Table 4 ). Persons with one or two ε4 alleles but not higher cortisol did not perform significantly different from the reference group in any domain. However, persons with higher cortisol and one ε4 allele performed significantly worse in language, processing speed, eye-hand coordination, and executive functioning. Persons with higher cortisol and two ε4 alleles performed significantly worse in all domains, with differences ranging from –0.84 SD (visuoconstruction) to –1.71 SD (verbal memory and learning). Additional analyses with the 75th percentile split applied to pretest and mean cortisol and at additional cutoff points (e.g., 70th and 80th percentiles) yielded similar results.
The magnitude of the cortisol- APOE interaction was next compared to the magnitude of the association of a 1-year increase in age at baseline. Possession of higher cortisol and one ε4 allele was equivalent to an increase ranging from 8.0 (95% CI=1.7–14.4) to 12.7 (95% CI=7.5–18.0) years of age at baseline for the domains of language, eye-hand coordination, and executive functioning. Estimates for the group with higher cortisol and two ε4 alleles were considerably larger; for example, decrements in language scores for this group were equivalent to 33.4 years (95% CI=14.8–52.0) of increased age.
Discussion
Previously, we found that higher levels of the pretest, mean, and AUC cortisol metrics were associated with worse cognitive performance in a population of community-dwelling older adults. The present findings from the same study population suggest that APOE -ε4 genotype modifies the relation between cortisol and cognitive function such that the slopes of the adverse relations are steeper in the presence of the ε4 allele.
We observed cortisol- APOE interactions in a broad range of domains, including language, eye-hand coordination, executive functioning, verbal memory and learning, visual memory, and visuoconstruction. While memory-related associations with cortisol have been well documented, there are few prior studies of nonmemory associations. Therefore, we cannot speculate about the significance of domain-specific associations. However, since glucocorticoid receptors are well distributed throughout the brain and APOE genotype influences multiple aspects of brain physiology, we believe that the observed interaction in multiple cognitive domains is biologically plausible. The strongest interactions were observed with the AUC metric, with statistically significant interactions observed in six of seven domains. This may be because AUC, in contrast to pretest and mean values, reflects both nonchallenge and challenge cortisol levels as a more comprehensive indicator of HPA axis activity.
It was interesting that there were more significant associations, and the magnitudes of the associations were larger, for the cortisol- APOE -ε4 interactions than for ε4 alone. There were several associations of APOE genotype with cognitive decrements, especially among persons with two ε4 alleles, and these findings were generally consistent with prior evidence (14 , 15) . However, the data suggest that among our study subjects the independent influence of APOE -ε4 genotype on cognitive function may be less important than the combined roles of the genotype and environmental exposures such as stress (as measured by the cortisol metrics), an example of gene-environment interaction.
Our findings are supported by a recent study showing that persons with high self-reported stress levels and any APOE -ε4 alleles performed worse on several memory tasks (28) . Because the relation of self-reported chronic stress to the underlying biology of the HPA axis is somewhat unclear, the assessment of HPA axis functioning in the present study provides additional insight into possible biological mechanisms, discussed in the following. We also extend these findings through use of a broader cognitive battery and a larger and younger population-based sample, in which cognitive decline may not be as readily evident.
Population stratification is a possibility, given the strong representation of African Americans in our study group and the higher proportion of African American individuals with an ε4 allele. We addressed this concern by conducting stratified analyses separately for whites and African Americans. We analyzed a model with an indicator for the ε4 allele and its cross-product with cortisol along with model 1 covariates, and we compared the results for the two race/ethnicity groups. While the patterns of statistical significance changed because of the decreased sample size in each stratum, the magnitudes and signs of coefficients for cross-product terms were qualitatively similar by race/ethnicity. This suggests that population stratification is unlikely to account for our findings.
Our results have several limitations. First, the subjects were not clinically assessed for dementia. Estimates place the prevalence of dementia at age 60–64 at less than 1% and at age 65–69 at less than 2% (29) . In our relatively young community-dwelling and population-based sample, it is unlikely that the prevalence of dementia would be high enough to influence our results. In addition, subjects were not excluded from the study on the basis of drug or medication use that could have altered cognition. However, our previous analysis showed that adjustment for alcohol, tobacco, recreational drug use, and medication use (antidepressants, anxiety medications, and hormone replacement therapy) did not substantially alter the main effect associations of the cortisol metrics with cognitive performance (23) . Another limitation is the collection of cortisol across an approximately 10-hour time range. However, prior studies suggest that comparable HPA axis responses to psychosocial challenges can be reliably measured in the morning and afternoon (30) . Furthermore, our analysis of cortisol main effects showed no indication that the results varied between participants sampled in the morning and afternoon (23) , and the time of sampling did not vary by genotype. We therefore do not believe that sampling at different times of the day accounts for our results.
Because our analysis was cross-sectional, we cannot be certain about the temporal relations of our measurements of cortisol and cognitive function. However, several lines of evidence allow us to infer that the HPA axis dysregulation we believe the cortisol metrics measure can be placed before the measured cognitive function. Although cortisol was assessed at the same time as cognitive testing, we previously found that 1) adjustment for perceived distress at the time of sampling did not alter the associations of cortisol and cognitive performance and 2) the rate of change in cortisol from the pretest to the second measurement shortly after the peak difficulty in cognitive testing (slope from cortisol samples 1-2) was not associated with cognitive performance (23) . These observations indicate that associations between cortisol and cognitive function likely were not due to acute, short-latency effects but rather longer-term effects of cortisol that preceded the study visit. This is in accordance with longitudinal studies (8 – 10) that provide evidence for the chronic effects of excess glucocorticoids on cognitive decline. Because temporality in the case of a genetic polymorphism is not a concern, we believe that elevated cortisol and APOE genotype-specific effects both temporally precede any decrements in cognitive function in older age.
While the roles of both APOE and cortisol in the pathogenesis of cognitive dysfunction are still not well understood, several plausible biological mechanisms can be proposed for the cortisol- APOE interaction. APOE genotype may modify the physiological consequences of HPA axis activity. For instance, the effects of environmental stressors (31) and administered corticosterone (32) on cognitive performance are conditional on APOE genotype in mice. In addition, APOE genotype may lower the neuronal threshold required for cortisol or neurotoxicants to produce neurodegenerative changes. Experimental stress conditions such as restraint or corticosterone injection produce dendritic atrophy of the hippocampus in rats, which is reversible with either termination of stress-inducing conditions or pharmacological treatment (33 , 34) . Apolipoprotein E has isoform-specific effects on neurite remodeling, and its significant involvement with proliferation, repair, and remyelination can affect recovery from neurotoxic insults (11) . Thus, the ability to recover from potentially reversible detrimental effects of cortisol, especially in the aging brain, may differ by APOE genotype.
It is also possible that cortisol may increase susceptibility of the brain to adverse events, such as the structural and functional changes mediated by apolipoprotein E. Corticosterone has been shown to exacerbate damage to hippocampal cell cultures by glutamate, FeSO 4 , and amyloid beta toxicities (35) . In addition, elevated cortisol reduces hippocampal glucose metabolism in elderly persons (36) . Given that older, nondemented ε4 carriers exhibit cerebral glucose hypometabolism in the parietal, temporal, and posterior cingulate cortices (37) , one possible mechanism for the interaction of APOE and cortisol takes place through coincident energetics disruption and the resulting impairment of neuronal calcium regulation (38) .
Until this study’s findings are replicated, discussion of relevant clinical implications may be premature. Nevertheless, it is intriguing that both glucocorticoids and APOE have been implicated as playing central roles in the disease process of Alzheimer’s disease. Experiments in a mouse model have shown that stress-level glucocorticoid administration increases production of amyloid beta and accumulation of tau pathology (39) , suggesting that the higher cortisol levels often found in patients with Alzheimer’s disease are not merely a consequence of the disease. Additionally, patients’ higher plasma cortisol levels are associated with accelerated progression of dementia symptoms and more rapidly decreasing cognitive performance (40) . It is possible that the HPA axis and APOE genotype may have joint effects in the disease process of Alzheimer’s disease.
In summary, the results from this study indicate that APOE -ε4 genotype may influence vulnerability to the effects of HPA axis dysregulation on cognitive function. Given that the cortisol metrics may capture HPA axis dysregulation and that such dysregulation could result from chronic stress, it is plausible that the results here are evidence of a gene-environment interaction concerning the adverse effects of the environment and its psychosocial hazards for the cognitive function of older persons.
1. Uno H, Tarara R, Else JG, Suleman MA, Sapolsky RM: Hippocampal damage associated with prolonged and fatal stress in primates. J Neurosci 1989; 9:1705–1711Google Scholar
2. Gould E, Tanapat P, McEwen BS, Flugge G, Fuchs E: Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci USA 1998; 95:3168–3171Google Scholar
3. Magarinos AM, Verdugo JM, McEwen BS: Chronic stress alters synaptic terminal structure in hippocampus. Proc Natl Acad Sci USA 1997; 94:14002–14008Google Scholar
4. Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, McEwen BS, Morrison JH: Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex 2006; 16:313–320Google Scholar
5. McEwen BS: The neurobiology of stress: from serendipity to clinical relevance. Brain Res 2000; 886:172–189Google Scholar
6. Sapolsky RM: Glucocorticoids, stress, and their adverse neurological effects: relevance to aging. Exp Gerontol 1999; 34:721–732Google Scholar
7. Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NP, Thakur M, McEwen BS, Hauger RL, Meaney MJ: Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci 1998; 1:69–73Google Scholar
8. Karlamangla AS, Singer BH, Chodosh J, McEwen BS, Seeman TE: Urinary cortisol excretion as a predictor of incident cognitive impairment. Neurobiol Aging 2005; 26(suppl 1):80–84Google Scholar
9. Li G, Cherrier MM, Tsuang DW, Petrie EC, Colasurdo EA, Craft S, Schellenberg GD, Peskind ER, Raskind MA, Wilkinson CW: Salivary cortisol and memory function in human aging. Neurobiol Aging 2006; 27:1705–1714Google Scholar
10. Seeman TE, McEwen BS, Singer BH, Albert MS, Rowe JW: Increase in urinary cortisol excretion and memory declines: MacArthur studies of successful aging. J Clin Endocrinol Metab 1997; 82:2458–2465Google Scholar
11. Huang Y: Apolipoprotein E and Alzheimer disease. Neurology 2006; 66(2 suppl 1):S79–S85Google Scholar
12. Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA: Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 1993; 261:921–923Google Scholar
13. Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-MacLachlan DR, Alberts MJ, et al: Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology 1993; 43:1467–1472Google Scholar
14. Small BJ, Rosnick CB, Fratiglioni L, Backman L: Apolipoprotein E and cognitive performance: a meta-analysis. Psychol Aging 2004; 19:592–600Google Scholar
15. Blair CK, Folsom AR, Knopman DS, Bray MS, Mosley TH, Boerwinkle E: APOE genotype and cognitive decline in a middle-aged cohort. Neurology 2005; 64:268–276Google Scholar
16. Moffat SD, Szekely CA, Zonderman AB, Kabani NJ, Resnick SM: Longitudinal change in hippocampal volume as a function of apolipoprotein E genotype. Neurology 2000; 55:134–136Google Scholar
17. Persson J, Lind J, Larsson A, Ingvar M, Cruts M, Van Broeckhoven C, Adolfsson R, Nilsson LG, Nyberg L: Altered brain white matter integrity in healthy carriers of the APOE epsilon4 allele: a risk for AD? Neurology 2006; 66:1029–1033Google Scholar
18. Teasdale GM, Murray GD, Nicoll JA: The association between APOE epsilon4, age and outcome after head injury: a prospective cohort study. Brain 2005; 128:2556–2561Google Scholar
19. Stewart WF, Schwartz BS, Simon D, Kelsey K, Todd AC: ApoE genotype, past adult lead exposure, and neurobehavioral function. Environ Health Perspect 2002; 110:501–505Google Scholar
20. Bunce D, Kivipelto M, Wahlin A: Apolipoprotein E, B vitamins, and cognitive function in older adults. J Gerontol B Psychol Sci Soc Sci 2005; 60:P41–P48Google Scholar
21. Schafer JH, Glass TA, Bolla KI, Mintz M, Jedlicka AE, Schwartz BS: Homocysteine and cognitive function in a population-based study of older adults. J Am Geriatr Soc 2005; 53:381–388Google Scholar
22. Haan MN, Shemanski L, Jagust WJ, Manolio TA, Kuller L: The role of APOE epsilon4 in modulating effects of other risk factors for cognitive decline in elderly persons. JAMA 1999; 282:40–46Google Scholar
23. Lee BK, Glass TA, McAtee MJ, Wand GS, Bandeen-Roche K, Bolla KI, Schwartz BS: Associations of salivary cortisol with cognitive function in the Baltimore Memory Study. Arch Gen Psychiatry 2007; 64:810–818Google Scholar
24. Schwartz BS, Glass TA, Bolla KI, Stewart WF, Glass G, Rasmussen M, Bressler J, Shi W, Bandeen-Roche K: Disparities in cognitive functioning by race/ethnicity in the Baltimore Memory Study. Environ Health Perspect 2004; 112:314–320Google Scholar
25. Radloff LS: The CES-D Scale: a self-report depression scale for research in the general population. Applied Psychol Measurement 1977; 1:385–401Google Scholar
26. Shih RA, Glass TA, Bandeen-Roche K, Carlson MC, Bolla KI, Todd AC, Schwartz BS: Environmental lead exposure and cognitive function in community-dwelling older adults. Neurology 2006; 67:1556–1562Google Scholar
27. Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH: Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 2003; 28:916–931Google Scholar
28. Peavy GM, Lange KL, Salmon DP, Patterson TL, Goldman S, Gamst AC, Mills PJ, Khandrika S, Galasko D: The effects of prolonged stress and APOE genotype on memory and cortisol in older adults. Biol Psychiatry 2007; 62:472–478Google Scholar
29. Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y, Jorm A, Mathers C, Menezes PR, Rimmer E, Scazufca M: Global prevalence of dementia: a Delphi consensus study. Lancet 2005; 366:2112–2117Google Scholar
30. Maheu FS, Collicutt P, Kornik R, Moszkowski R, Lupien SJ: The perfect time to be stressed: a differential modulation of human memory by stress applied in the morning or in the afternoon. Prog Neuropsychopharmacol Biol Psychiatry 2005; 29:1281–1288Google Scholar
31. Grootendorst J, de Kloet ER, Vossen C, Dalm S, Oitzl MS: Repeated exposure to rats has persistent genotype-dependent effects on learning and locomotor activity of apolipoprotein E knockout and C57Bl/6 mice. Behav Brain Res 2001; 125:249–259Google Scholar
32. Grootendorst J, Kempes MM, Lucassen PJ, Dalm S, de Kloet ER, Oitzl MS: Differential effect of corticosterone on spatial learning abilities in apolipoprotein E knockout and C57BL/6J mice. Brain Res 2002; 953:281–285Google Scholar
33. Conrad CD, LeDoux JE, Magarinos AM, McEwen BS: Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci 1999; 113:902–913Google Scholar
34. Sousa N, Lukoyanov NV, Madeira MD, Almeida OF, Paula-Barbosa MM: Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience 2000; 97:253–266Google Scholar
35. Goodman Y, Bruce AJ, Cheng B, Mattson MP: Estrogens attenuate and corticosterone exacerbates excitotoxicity, oxidative injury, and amyloid beta-peptide toxicity in hippocampal neurons. J Neurochem 1996; 66:1836–1844Google Scholar
36. de Leon MJ, McRae T, Rusinek H, Convit A, De Santi S, Tarshish C, Golomb J, Volkow N, Daisley K, Orentreich N, McEwen B: Cortisol reduces hippocampal glucose metabolism in normal elderly, but not in Alzheimer’s disease. J Clin Endocrinol Metab 1997; 82:3251–3259Google Scholar
37. Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, Thibodeau SN, Osborne D: Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med 1996; 334:752–758Google Scholar
38. Yusim A, Ajilore O, Bliss T, Sapolsky R: Glucocorticoids exacerbate insult-induced declines in metabolism in selectively vulnerable hippocampal cell fields. Brain Res 2000; 870:109–117Google Scholar
39. Green KN, Billings LM, Roozendaal B, McGaugh JL, LaFerla FM: Glucocorticoids increase amyloid-beta and tau pathology in a mouse model of Alzheimer’s disease. J Neurosci 2006; 26:9047–9056Google Scholar
40. Csernansky JG, Dong H, Fagan AM, Wang L, Xiong C, Holtzman DM, Morris JC: Plasma cortisol and progression of dementia in subjects with Alzheimer-type dementia. Am J Psychiatry 2006; 163:2164–2169Google Scholar







