Mortality Risk in Patients With Dementia Treated With Antipsychotics Versus Other Psychiatric Medications
Abstract
Objective: Mortality rates in the year following new antipsychotic medication starts for neuropsychiatric symptoms of dementia were compared with rates after starts of other psychiatric medications. Method: The retrospective, cohort study used national data from the Department of Veterans Affairs (fiscal years 2001–2005) on patients older than 65 years who began outpatient treatment with psychiatric medication following a dementia diagnosis (N=10,615). Twelve-month mortality rates were compared in patients taking antipsychotics and those taking other psychiatric medications. The authors controlled for confounding by using multivariate models and propensity-scoring methods. Secondary analyses included a no-medication group and examination of mortality causes. Results: All groups taking antipsychotics had significantly higher mortality rates (22.6%–29.1%) than patients taking nonantipsychotic medications (14.6%). Adjusted mortality risks for atypicals and for combined atypical and conventional antipsychotics were similar to those for conventional antipsychotics. The mortality risk was significantly lower for nonantipsychotic medications than conventional antipsychotics. Except for anticonvulsants, the adjusted risks for all individual classes of nonantipsychotics were significantly lower than the risk for antipsychotics. Mortality risks did not change over 12 months. The proportions of patients taking antipsychotics who died from cerebrovascular, cardiovascular, or infectious causes were not higher than rates for those taking nonantipsychotic psychiatric medications. Conclusions: Antipsychotic medications taken by patients with dementia were associated with higher mortality rates than were most other medications used for neuropsychiatric symptoms. The association between mortality and antipsychotics is not well understood and may be due to a direct medication effect or the pathophysiology underlying neuropsychiatric symptoms that prompt antipsychotic use.
Neuropsychiatric symptoms are present in more than 80% of persons with dementia (1) , and they are associated with more hospitalizations, nursing home placement, caregiver stress, and depression and with less caregiver employment and income (2 – 7) . Such symptoms may be more critical to institutionalization than cognitive symptoms (7) , accounting for up to one-third of the costs for care of Alzheimer’s dementia (8) . Research examining treatment of neuropsychiatric symptoms of dementia is modest, and no medication is approved by the Food and Drug Administration (FDA) for this indication. Nevertheless, conventional antipsychotics have long been used to treat neuropsychiatric symptoms. Following the introduction of atypical antipsychotics, with lower reported rates of parkinsonism and tardive dyskinesia, there was a significant shift from the use of conventionals to atypicals (9 , 10) . Modest reductions of neuropsychiatric symptoms of dementia had been reported with risperidone and olanzapine (11 – 15) , although results from the recently published Clinical Antipsychotic Trials of Intervention Effectiveness—Alzheimer’s Disease (CATIE-AD) indicate that response rates with olanzapine, risperidone, and quetiapine are not significantly different from the rates with placebo (16) . Additionally, discontinuation rates owing to side effects were significantly higher in subjects taking atypical antipsychotics (16) . Therefore, only a subgroup of subjects may have benefited from antipsychotic medication.
In 2002, randomized, controlled trials of atypical antipsychotics raised concerns regarding cerebrovascular adverse events among patients with dementia (14 , 17 , 18) . Consequently, safety information updates were published in Canada (18) , the United States, and the United Kingdom (19) . A recent independent meta-analysis found an elevated risk of cerebrovascular adverse events associated with atypical antipsychotics, compared to placebo, and a significantly higher risk with risperidone (20) . Two studies have compared the risks of cerebrovascular adverse events for typical and atypical antipsychotics. The first, a retrospective population-based cohort study, found that olanzapine and risperidone use in elderly patients was not associated with a significantly higher risk of stroke compared to conventional antipsychotic use (21) . The second, a reanalysis of olanzapine trial data, found that the incidence of cerebrovascular adverse events was approximately three times as high for olanzapine (1.3%) as for placebo (0.4%), with no significant difference between olanzapine and risperidone or between olanzapine and conventional antipsychotics (22) .
In 2005, the FDA issued a warning (23) that, among elderly patients with dementia, the treatment of behavioral disorders with atypical antipsychotics was associated with a higher mortality rate. Of 17 placebo-controlled trials with olanzapine, aripiprazole, risperidone, or quetiapine in patients with neuropsychiatric symptoms of dementia, 15 showed greater mortality (approximately 1.7-fold). Specific causes of deaths were mostly cardiac related or infections. The FDA noted that a warning for conventional antipsychotics was being considered because the limited available data suggested a similarly higher mortality risk. This concern was confirmed by a meta-analysis by Schneider et al. of data from trials of atypicals that included ad hoc haloperidol analyses showing a relative mortality risk of 2.07 (24) and by a study (25) demonstrating that mortality risks with conventional antipsychotics were higher than with atypicals in elderly patients. A recent reanalysis of olanzapine trial data found no significant differences in mortality between olanzapine and risperidone and between olanzapine and conventionals (22) .
Although nonantipsychotic psychiatric medications (antidepressants, anticonvulsants, anxiolytics, and sedative/hypnotics) are also used for management of neuropsychiatric symptoms of dementia, there is little research support for their efficacy for this indication (26) . The wide range of psychiatric medications could also be associated with higher mortality risks in this often frail population (24 , 27) . Given these concerns, further study comparing the risks of antipsychotics with those of nonantipsychotic psychiatric medications often used as alternatives is warranted. Because psychotropic agents for neuropsychiatric symptoms are frequently used for long periods, it is also important to compare mortality risks during both acute and maintenance treatment.
The purpose of this study was to compare 12-month mortality risks among patients who had recently had prescriptions filled for conventional antipsychotics, atypical antipsychotics, or nonantipsychotic psychiatric medications in outpatient settings following a dementia diagnosis. We controlled for potential differences in patient characteristics among medication groups and performed various sensitivity analyses. We hypothesized that mortality rates for patients taking antipsychotics would be higher than for patients taking nonantipsychotic psychiatric medications.
Method
Study Cohort
The study used data, with identifiers removed, from national Department of Veterans Affairs (VA) registries maintained by the VA Serious Mental Illness Treatment, Research, and Evaluation Center for veterans who received a dementia diagnosis in VA health care settings. The VA Ann Arbor Healthcare System Institutional Review Board approved the study.
Patients were included in the study if they 1) were over age 65, 2) had received a dementia diagnosis in fiscal year 2002 or 2003, 3) had not used psychiatric medications in the 6 months before the dementia diagnosis, and 4) had begun outpatient treatment with psychiatric medication in the 6 months following the dementia diagnosis. This strategy was intended to increase the likelihood that the medication was prescribed for neuropsychiatric symptoms associated with dementia rather than another condition and that dementia was an active condition at the time the new prescription was filled. The final sample included 10,615 patients.
Antipsychotic Medications
The atypical antipsychotic agents included aripiprazole, clozapine, olanzapine, quetiapine, risperidone, and ziprasidone. The conventional agents included chlorpromazine, fluphenazine, mesoridazine, perphenazine, thioridazine, trifluoperazine, haloperidol, loxapine, molindone, pimozide, and thiothixene.
Other Psychiatric Medications
Antidepressants (amitriptyline, clomipramine, doxepin, imipramine, trimipramine, desipramine, nortriptyline, protriptyline, amoxapine, maprotiline, fluoxetine, fluvoxamine, paroxetine, sertraline, citalopram, escitalopram, bupropion, nefazodone, trazodone, venlafaxine, mirtazapine, isocarboxazid, phenylzine, and tranylcypromine), anticonvulsants (carbamazepine, felbamate, lamotrigine, valproic acid, valproate, and divalproex), and anxiolytics/hypnotics (alprazolam, chlordiazepoxide, clonazepam, chlorazepate, diazepam, halazepam, lorazepam, oxazepam, prazepam, buspirone, estazolam, flurazepam, quazepam, temazepam, triazolam, zolpidem, zaleplon, and chloral hydrate) were included as nonantipsychotic psychotropics. Patients taking lithium (which is seldom used for neuropsychiatric symptoms of dementia) or with seizure disorders (for whom anticonvulsant use is less likely to be related to dementia) were excluded.
As cholinesterase inhibitors may affect medication use and their vagotonic effects may increase mortality risks, we controlled for use of these agents (donepezil, rivastigmine, tacrine, and galantamine) in all analyses.
Mortality
Data on vital status were obtained from the 1) VA Patient Treatment File, 2) VA Beneficiary Identification Records Locator System, 3) Social Security Administration Death Master File, and 4) National Death Index (National Center for Health Statistics, Hyattsville, Md.). If there were discrepancies between sources, we used the earliest date of death judged as valid. Dates were considered invalid if there were more than two visits after the date of death (as patients’ families may use one or two visits for bereavement counseling). Data on underlying causes of death were obtained from the National Death Index.
Other Variables
Age and ethnicity were obtained for each patient from national data in the VA Serious Mental Illness Treatment, Research, and Evaluation Center registries. Indicators of comorbid schizophrenia and bipolar disorder were obtained from the VA National Psychosis Registry maintained by the Serious Mental Illness Treatment, Research, and Evaluation Center. We used a modified version of the Charlson comorbidity index (28) to measure medical comorbidity with the original Charlson weights based on 18 medical comorbidities (excluding dementia) in the year prior to new medication start. In the final model, we used indicators for comorbidity and included only those showing statistically significant associations or meaningfully large coefficients.
Delirium frequently occurs during inpatient stays among elderly patients with dementia and is an independent risk factor for mortality (29) , and antipsychotics are preferentially prescribed for delirium. Although the members of our cohort were outpatients when medication began, we included the presence of a delirium diagnosis at the time the prescription was filled as a covariate in our analyses, using an inclusive coding scheme for confusional states based on prior work (30) . We included calendar time at the start of new medication as a covariate to control for potential changes in health care over the study period.
Statistical Analysis
Descriptive statistics were used to characterize demographic and clinical characteristics by the four types of medications prescribed: 1) conventional antipsychotics, 2) atypical antipsychotics, 3) combined conventionals and atypicals (either concurrently or switching from one class to another during the 6 months following dementia diagnosis), and 4) other psychotropic medications. Patients filling prescriptions for both antipsychotics and other psychotropic medications were classified as taking antipsychotics. Chi-square tests and t tests were used to compare the characteristics of the patients who died within 1 year of receiving psychotropic medication and those who were still alive after 1 year.
Mortality during the 12-month follow-up (encompassing acute and maintenance treatment) was calculated by medication type. Each patient was followed for 12 months after a new medication start. The outcome measure was the length of time from the first filled prescription until death or 12 months, whichever was earlier. Across medication types, Kaplan-Meier survival curves were created, and Cox proportional-hazards regression was used to compare survival over the 12 months after treatment initiation. The Cox model was adjusted for the variables shown in Table 1 .
We plotted estimated hazards according to life-table methods with follow-up time broken into monthly intervals to assess whether the risks and risk differences remained similar over time. To test whether mortality risks were parallel over time across medication groups, we included terms for the interaction of time and medication type and tested for their statistical significance. We also examined the interaction of medication type with dichotomized follow-up time (first versus second half of the 12-month period) to determine whether the mortality risks of the medication types were different during the two time periods. Because we may not have had statistical power to detect interaction because of a smaller number of deaths at later follow-up times, we also plotted log-log survival curves by medication type for visual inspection.
As confirmatory analyses, we applied propensity scores to match patients in the different medication groups, and we compared survival across medication types in the matched cohorts. Because there were four medication types, propensity scores were estimated in several ways. First, propensity score was estimated for the probability of initiating any antipsychotic versus nonantipsychotic psychiatric medication. The propensity-matched cohort excluded patients with no matches. Then we repeated similar propensity-matched cohort construction using three subsets of patients: 1) those taking atypical antipsychotics or nonantipsychotic psychiatric medications, 2) those taking conventional antipsychotics or nonantipsychotic psychiatric medications, and 3) those taking both types of antipsychotics or nonantipsychotic psychiatric medications. In each subset, the propensity score was estimated by using a logistic regression model that included all baseline covariates and multiple terms for interactions, including the interaction of fill date and schizophrenia and the interaction of fill date and delirium. To make propensity-score adjusted comparisons of mortality risks, we utilized the proportional-hazards model with the corresponding subset of the propensity-matched cohort, stratified by blocks of propensity scores. The proportional-hazards models were adjusted for the same variables as in the analysis of the unmatched cohort.
A secondary analysis included patients taking no psychiatric medications. These patients, by definition, did not have prescriptions filled for psychotropic drugs and could not be followed from the time of prescription fill to death. Thus, we used a different design, matching every patient taking a psychiatric medication with a randomly chosen patient from the no-medication group (who met our eligibility criteria except having filled a prescription for a psychotropic drug). Patients were matched on the week of diagnosis as well as the time lag between the date of the dementia diagnosis and the fill date. For each no-medication patient, a phantom fill date was created that resulted in the same number of days since dementia diagnosis as the corresponding matching patient in the medication group. The proportional-hazards model adjusted the covariance for matching.
In addition to the main and secondary analyses, several sensitivity analyses were performed in order to 1) use the time since the dementia diagnosis as a proxy for dementia severity and 2) remove the restriction for a “clean period” (without psychotropic medications) prior to the new medication start. We defined “time since dementia diagnosis” as the time between the initial appearance of a registry dementia diagnosis and the time of the patient’s index diagnosis in fiscal year 2002 or 2003. This variable was intended as a proxy for dementia severity, given that dementia gradually worsens over time and that changes can be seen on a yearly basis. Analyses without the restriction of a clean period prior to dementia diagnosis were completed as neuropsychiatric symptoms often precede dementia diagnoses.
Results
Characteristics of the Study Population
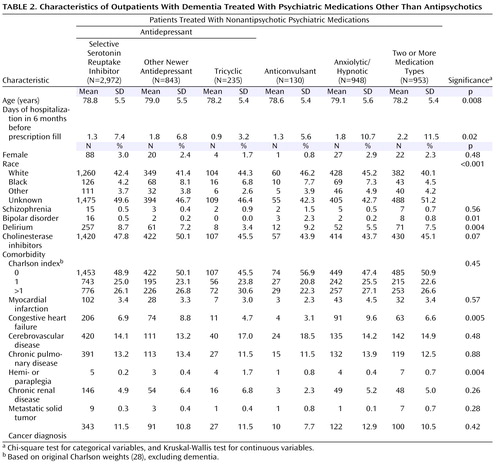
The study population included 10,615 patients over 65 years old with dementia. Table 1 and Table 2 show demographic and clinical characteristics of the entire cohort, the three antipsychotic groups, the group receiving nonantipsychotic psychiatric medications, and the group receiving no psychotropic medication.
The statistically and clinically significant between-group differences included 1) race, with a higher percentage of African Americans (15.6%) receiving conventional antipsychotics versus other psychiatric medications (5.5%), and 2) cholinesterase inhibitor use, which was lower among patients receiving conventional antipsychotics (36.3%) than among those receiving other antipsychotics or nonantipsychotic psychiatric medications (41.2%–50.1%).
Type of Psychiatric Medication and Associated 12-Month Mortality
Overall mortality in the cohort was 18.0%. Twelve-month mortality differed significantly by medication type (χ 2 =130.0, df=3, p<0.001). Mortality among patients taking conventional agents (25.2%), atypicals (22.6%), or both types of antipsychotics (29.1%) was significantly higher than among users of other psychiatric medications (14.6%) ( Table 3 ). The relative risks adjusted for covariates were 0.93 (with a 95% confidence interval [CI] of 0.75–1.16) for patients receiving atypicals, 1.33 (95% CI=0.94–1.86) for those using both types of antipsychotics, and 0.56 (95% CI=0.45–0.70) for patients receiving other psychiatric medications, relative to the group receiving conventional antipsychotics. Twelve-month mortality for individual classes of nonantipsychotic psychiatric medications is shown in Table 3 .
We found no evidence for increasing or decreasing risks over time or differential risks across medication types over time, either by testing for interaction terms or by visual inspection of various graphs. Results from the confirmatory analysis comparing mortality risks across medication types by means of the proportional-hazards regression stratified by the propensity score blocks and adjusting for covariates were consistent with the results of multivariate analyses without propensity scores.
Secondary Analyses and Sensitivity Analyses
When the group taking psychotropic medications other than antipsychotics was broken into individual classes of medications, except for anticonvulsants, the adjusted mortality risks for all other classes of nonantipsychotics were significantly lower than the risk for conventional antipsychotics ( Table 3 ). Patients taking no psychotropic medications had a 12-month mortality rate of 17.8%. This analysis showed a significantly lower risk of death for the no-medication group than for the group taking conventional antipsychotics ( Table 3 ). The adjusted relative risk estimates for the individual medication types were similar to those from the main analysis. The Kaplan-Meier survival curves for the main study cohort and the no-medication group are shown in Figure 1 .

a Kaplan-Meier survival curves.
The analysis using the dementia severity proxy included a smaller group (N=1,555) of patients with consistent VA use in the 5 years prior to the new medication start. Each patient’s time since dementia diagnosis was categorized by the number of years. Comparing patients with 5 years since diagnosis to patients within 1 year of diagnosis (roughly corresponding to later versus earlier dementia), we found a higher rate of current conventional antipsychotic use, 10.8% (N=25) versus 3.5% (N=13), and a lower rate of other psychiatric medication use, 49.8% (N=115) versus 55.7% (N=210), among those with longer times since dementia diagnosis (χ 2 =16.6, df=8, p=0.03). In this subset, the relative risks of the various medication groups from the Cox proportional-hazards model adjusting for year since diagnosis also showed a lower risk associated with nonantipsychotic medication use (relative risk=0.65, 95% CI=0.43–0.99).
The results of the second sensitivity analysis, which included patients without a medication clean period, were similar to those from the main analysis.
Comorbidity Analyses and Causes of Death
We stratified patients by a measure of overall medical comorbidity, the Charlson index (28) . These analyses indicated that conventional antipsychotics and nonantipsychotic psychiatric medications were more likely to be prescribed for patients with greater medical comorbidity (those having higher Charlson scores) than for patients taking atypical antipsychotics. Among the “healthiest” group of patients (Charlson score=0), conventionals and atypicals were associated with higher mortality than other psychotropic medications but were not significantly different from one another, while among patients with a greater medical burden (Charlson score >2), conventionals, but not atypicals, were again associated with higher mortality than other psychotropic medications.
Table 4 presents the proportions of patients taking antipsychotics or other psychotropic medications who died from the 10 leading causes of death among the elderly in the United States (31 , 32) , as well as suicide, other (non-Alzheimer’s) dementia, or other causes. The proportion of patients dying from cerebrovascular, cardiovascular, or important infectious causes (e.g., pneumonia, septicemia) appeared to be no higher among the patients receiving antipsychotics than among those taking nonantipsychotic psychiatric medications. A larger proportion of patients taking antipsychotics appeared to die from dementia-related causes, as compared to those receiving other psychotropic medications, and a larger proportion of patients taking nonantipsychotic medications appeared to die from cancer.
Discussion
In this national sample of 10,615 VA patients diagnosed with dementia and starting treatment with psychiatric medications as outpatients, both conventional and atypical antipsychotics were associated with significantly higher 12-month mortality than most other psychotropic medications. Risks were elevated consistently over the 12-month follow-up. Although it has been suggested that the higher mortality associated with antipsychotics could also be associated with many classes of psychotropic drugs used to treat neuropsychiatric symptoms of dementia (24 , 27) , our results suggest that is not the case. We found similar mortality risks in the group using nonantipsychotic psychotropic medications and in those receiving no psychotropic medications; both of these groups showed lower risks than those taking antipsychotic medications.
In this study of outpatients, conventional antipsychotics conferred an adjusted risk similar to that for atypical antipsychotics. This confirms other recent work (24 , 25) suggesting that conventional antipsychotics are not safer than atypicals and should not be used to replace the latter agents in response to recent FDA warnings.
The causality of greater mortality with antipsychotics is not yet understood. While it is possible that mortality results from the underlying causes of neuropsychiatric symptoms of dementia, as opposed to a direct medication effect, antipsychotic use has been found to be associated with increased cognitive decline independent of dementia severity and type of behavioral disturbance (33) . Our secondary analyses examining medical comorbidity indicate that the observed relationship between antipsychotics and mortality is complex. This relationship may be due, in part, to a direct influence of antipsychotics on mortality risk but may also reflect remaining confounds from medical and dementia severity. Our data on the cause of death do not point to a specific cardiotoxic, vascular, or immunological mechanism but instead show proportionately more deaths due to dementia-related causes among users of antipsychotic medications.
The overall 12-month mortality rates observed for our patients ranged from 10.2% to 29.1%, higher percentages than observed in the CATIE-AD trial (16) , from which patients with “severe or unstable medical illness” were excluded (34) . While patients receiving care at VA hospitals may be sicker (poorer health status, more medical conditions, and higher use of medical resources) than those in general patient populations (35) , the mortality rates in our study were closer to those of Wang et al. (25) (18% and 15% for users of conventionals and atypicals, respectively, in 180 days).
A major limitation of our study, like that of Wang et al. (25) , is the lack of data on dementia severity and type of behavioral disorder. To the extent that such factors determine preferential prescription of antipsychotics for more severe disorders, our analyses must be interpreted with caution. Similarly, the lower mortality rates seen with tricyclic antidepressants may reflect low rates of tricyclic use in patients with more severe dementia due to side effect concerns (as well as use of low-dose tricyclics for nonpsychiatric purposes). Patients receive medications nonrandomly, on the basis of their clinical state and clinician preference. Our propensity-score matching and various sensitivity analyses attempted to address this issue. Analyses using a proxy for severity suggest that patients with longer-standing diagnoses (corresponding to more severe dementia) were more likely to be taking conventional antipsychotics and less likely to be taking other psychiatric medications than patients more recently diagnosed. Additionally, cholinesterase use was lower in patients taking conventional antipsychotics; perhaps this relationship was correlated with dementia severity.
Several other limitations are inherent in the use of registry data for this type of research. Given the VA population, the cohort was primarily male, and the study results may not be generalizable to other clinical populations. We relied on discharge or visit diagnoses to identify comorbid conditions and to adjust for differences in medical burden. Because registry dementia diagnoses are clinical, cases not yet diagnosed with dementia (likelier earlier cases) will not be ascertained. No observational study can completely exclude the possibility of bias due to unmeasured differences in illness burden; nevertheless, we attempted to adjust for this bias in our analyses by controlling for characteristics indicating greater medical burden or frailty, as well as our propensity scoring and sensitivity analyses. Only a randomized trial could completely exclude the possibility of such biases. In terms of medication data, prescription fills can be an imprecise measure of actual drug exposure; patients may not be fully adherent to drug regimens, and medication fills may not reflect day-to-day usage. However, poor adherence with antipsychotics may be less prevalent among older patients (36) . We also could not control for medication doses in the analyses. Finally, the number of patients taking conventional antipsychotics in our sample was much smaller than the number of patients taking atypical agents. Over the study period, conventionals were largely replaced by atypicals. Thus, patients taking conventionals may be a nonrepresentative subsample.
Given the potential risks of mortality with antipsychotics, recruiting the large numbers of elderly patients necessary for clinical trials may be difficult (20) . Large observational studies such as ours are important to address longer-term risks. Since antipsychotic medications may benefit only a minority of patients (16) , new approaches clearly are needed to manage the neuropsychiatric symptoms of dementia. More research is required regarding psychosocial and behavioral interventions, as well as alternative pharmacotherapeutic approaches to these often-troublesome symptoms. No behavioral/psychosocial treatment has been shown to be effective in this population in large-scale randomized, controlled trials (37) . In the meantime, some patients with severe behavioral problems may need immediate pharmacotherapy, particularly the targeted use of antipsychotics for patients with clear-cut psychotic symptoms (38) .
1. Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S: Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the Cardiovascular Health Study. JAMA 2002; 288:1475–1483Google Scholar
2. Coen RF, Swanick GR, O’Boyle CA, Coakley D: Behavioral disturbance and other predictors of carer burden in Alzheimer’s disease. Int J Geriatr Psychiatry 1997; 12:331–336Google Scholar
3. Devanand DP, Marder K, Michaels KS, Sackeim HA, Bell K, Sullivan MA, Cooper TB, Pelton GH, Mayeux R: A randomized, placebo-controlled dose-comparison trial of haloperidol for psychosis and disruptive behaviors in Alzheimer’s disease. Am J Psychiatry 1998; 155:1512–1520Google Scholar
4. Covinsky KE, Eng C, Lui LY, Sands LP, Sehgal AR, Walter LC, Wieland D, Eleazer GP, Yaffe K: Reduced employment in caregivers of frail elders: impact of ethnicity, patient clinical characteristics, and caregiver characteristics. J Gerontol A Biol Sci Med Sci 2001; 56:M707–M713Google Scholar
5. Wancata J, Windhaber J, Krautgartner M, Alexandrowicz R: The consequences of non-cognitive symptoms of dementia in medical hospital departments. Int J Psychiatr Med 2003; 33:257–271Google Scholar
6. Steele C, Rovner B, Chase GA, Folstein M: Psychiatric symptoms and nursing home placement of patients with Alzheimer’s disease. Am J Psychiatry 1990; 147:1049–1051Google Scholar
7. Kales HC, Chen PJ, Blow FC, Welsh DE, Mellow AM: Rates of clinical depression diagnosis, functional impairment and nursing home placement in coexisting dementia and depression. Am J Geriatr Psychiatry 2005; 13:441–449Google Scholar
8. Beeri MS, Werner P, Davidson M, Noy S: The cost of behavioral and psychological symptoms of dementia in community dwelling Alzheimer’s disease patients. Int J Geriatr Psychiatry 2002; 17:403–408Google Scholar
9. Lopez OL, Becker JT, Sweet RA, Klunk W, Kaufer DI, Saxton J, DeKosky ST: Patterns of change in the treatment of psychiatric symptoms in patients with probable Alzheimer’s disease from 1983 to 2000. J Neuropsychiatry Clin Neurosci 2003; 15:67–73Google Scholar
10. Rapoport M, Mamdani M, Shulman KI, Herrmann N, Rochon PA: Antipsychotic use in the elderly: shifting trends and increasing costs. Int J Geriatr Psychiatry 2005; 20:749–753Google Scholar
11. Katz IR, Jeste DV, Mintzer JE, Clyde C, Napolitano J, Brecher M: Comparison of risperidone and placebo for psychosis and behavioral disturbances associated with dementia: a randomized double-blind trial: Risperidone Study Group. J Clin Psychiatry 1999; 60:107–115Google Scholar
12. De Deyn PP, Rabheru K, Rasmussen A, Bocksberger JP, Dautzenberg PL, Eriksson S, Lawlor BA: A randomized trial of risperidone, placebo, and haloperidol for behavioral symptoms of dementia. Neurology 1999; 53:946–955Google Scholar
13. Street JS, Clark WS, Gannon KS, Cummings JL, Bymaster FP, Tamura RN, Mitan SJ, Kadam DL, Sanger TM, Feldman PD, Tollefson GD, Breier A: Olanzapine treatment of psychotic and behavioral symptoms in patients with Alzheimer disease in nursing home care facilities: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry 2000; 57:968–976Google Scholar
14. Brodaty H, Ames D, Snowdon J, Woodward M, Kirwan J, Clarnette R, Lee E, Lyons B, Grossman F: A randomized, placebo-controlled trial of risperidone for the treatment of aggression, agitation, and psychosis of dementia. J Clin Psychiatry 2003; 64:134–143Google Scholar
15. De Deyn PP, Carrasco MM, Deberdt W, Jeandel C, Hay DP, Feldman PD, Young CA, Lehman DL, Breier A: Olanzapine versus placebo in the treatment of psychosis with or without associated behavioral disturbances in patients with Alzheimer’s disease. Int J Geriatr Psychiatry 2004; 19:115–126Google Scholar
16. Schneider LS, Tariot PN, Dagerman KS, Davis SM, Hsiao JK, Ismail MS, Lebowitz BD, Lyketsos CG, Ryan JM, Stroup TS, Sultzer DL, Weintraub D, Lieberman JA: Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med 2006; 355:1525–1538Google Scholar
17. Wooltorton E: Risperidone: increased rate of cerebrovascular events in dementia. CMAJ 2002; 167:1269–1270Google Scholar
18. Therapeutic Products Directorate: Risperdal (risperidone) and Cerebrovascular Adverse Events in Placebo-Controlled Dementia Trials. Ottawa, Health Canada, Therapeutic Products Directorate, Oct 11, 2002 (http://www.hc-sc.gc.ca/dhp-mps/medeff/advisories-avis/prof/2002/risperdal_hpc-cps_e.html)Google Scholar
19. Medicines and Healthcare Products Regulatory Agency: Summary of Clinical Trial Data on CAEs (Cerebrovascular Adverse Events) in Randomised Clinical Trials of Risperidone Conducted in Patients With Dementia. London, Department of Health, Medicines and Healthcare Products Regulatory Agency, March 9, 2004 (http://medicines.mhra.gov.uk)Google Scholar
20. Schneider LS, Dagerman K, Insel PS: Efficacy and adverse effects of atypical antipsychotics for dementia: meta-analysis of randomized, placebo-controlled trials. Am J Geriatr Psychiatry 2006; 14:191–210Google Scholar
21. Herrmann N, Mamdani M, Lanctôt KL: Atypical antipsychotics and risk of cerebrovascular accidents. Am J Psychiatry 2004; 161:1113–1115Google Scholar
22. Kryzhanovskaya LA, Jeste DV, Young CA, Polzer JP, Roddy TE, Jansen JF, Carlson JL, Cavazzoni PA: A review of treatment-emergent adverse events during olanzapine clinical trials in elderly patients with dementia. J Clin Psychiatry 2006; 67:933–945Google Scholar
23. Food and Drug Administration: FDA Public Health Advisory: Deaths With Antipsychotics in Elderly Patients With Behavioral Disturbances. Washington, DC, FDA, April 11, 2005 (http://www.fda.gov/cder/drug/advisory/antipsychotics.htm)Google Scholar
24. Schneider LS, Dagerman KS, Insel P: Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA 2005; 294:1934–1943Google Scholar
25. Wang PS, Schneeweiss S, Avorn J, Fischer MA, Mogun H, Solomon DH, Brookhart MA: Risk of death in elderly users of conventional vs atypical antipsychotic medications. N Engl J Med 2005; 353:2335–2341Google Scholar
26. Sink KM, Holden KF, Yaffe K: Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA 2005; 293:596–608Google Scholar
27. Rabins PV, Lyketsos CG: Antipsychotic drugs in dementia: what should be made of the risks? JAMA 2005; 294:1963–1965Google Scholar
28. Charlson ME, Pompei P, Ales KL, MacKenzie CR: A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–383Google Scholar
29. Adamis D, Treloar A, Martin FC, Macdonald AJ: Recovery and outcome of delirium in elderly medical inpatients. Arch Gerontol Geriatr 2006; 43:289–298Google Scholar
30. Kales HC, Kamholz BA, Visnic SG, Blow FC: Recorded delirium in a national sample of elderly inpatients: potential implications for recognition. J Geriatr Psychiatry Neurol 2003; 16:32–38Google Scholar
31. Gorina Y, Hoyert D, Goulding M: Trends in Causes of Death Among Older Persons in the United States: Aging Trends, number 6. Hyattsville, Md, National Center for Health Statistics, 2006Google Scholar
32. Anderson RN, Minino AM, Hoyert DL, Rosenberg HM: Comparability of Cause of Death Between ICD-9 and ICD-10: Preliminary Estimates: National Vital Statistics Reports, vol 49, number 2. Hyattsville, Md, National Center for Health Statistics, 2001Google Scholar
33. McShane R, Keene J, Gedling K, Fairburn C, Jacoby R, Hope T: Do neuroleptic drugs hasten cognitive decline in dementia? prospective study with necropsy follow up. BMJ 1997; 314:266–270Google Scholar
34. Schneider LS, Tariot PN, Lyketsos CG, Dagerman KS, Davis KL, Davis S, Hsiao JK, Jeste DV, Katz IR, Olin JT, Pollock BG, Rabins PV, Rosenheck RA, Small GW, Lebowitz B, Lieberman JA: National Institute of Mental Health Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE): Alzheimer disease trial methodology. Am J Geriatr Psychiatry 2001; 9:346–360Google Scholar
35. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM: Are patients at Veterans Affairs medical centers sicker? a comparative analysis of health status and resource use. Arch Intern Med 2000; 160:3252–3257Google Scholar
36. Valenstein M, Copeland LA, Blow FC, McCarthy JF, Gillon L, Bingham CR, Stavenger T: Pharmacy data identify poorly adherent patients with schizophrenia at increased risk for admission. Med Care 2002; 40:630–639Google Scholar
37. Cohen-Mansfield J: Nonpharmacological interventions for inappropriate behaviors in dementia. Am J Geriatr Psychiatry 2001; 9:361–381Google Scholar
38. Ropacki SA, Jeste DV: Epidemiology of and risk factors for psychosis of Alzheimer’s disease: a review of 55 studies published from 1990 to 2003. Am J Psychiatry 2005; 162:2022–2030Google Scholar