Effect of COMT Val158Met Polymorphism on the Continuous Performance Test, Identical Pairs Version: Tuning Rather Than Improving Performance
Abstract
OBJECTIVE: It has been suggested that variation in catechol O-methyltransferase (COMT) activity associated with variation in COMT Val158Met genotypes may result in enhanced or reduced cognitive performance, depending on whether the phenotype requires cognitive stability or cognitive flexibility. The authors’ goal was to determine whether, in confirmation of this prediction, performance on a measure of cognitive stability would be associated with Met loading. METHOD: COMT genotyping was investigated in relation to a measure of reaction time variability on the Continuous Performance Test, Identical Pairs Version, in a large and representative sample of 527 young men (mean age=21 years). RESULTS: Met loading was associated with reduced reaction time variability. CONCLUSIONS: Met genotype loading may confer enhanced “tuning” or greater stability in performance, possibly by stabilizing active neural representations in the prefrontal cortex during tasks involving working memory.
A G-to-A transition at codon 158 of the catechol O-methyltransferase (COMT) gene, resulting in a valine (Val)-to-methionine (Met) substitution yielding different Val158Met genotypes, has been associated with differential COMT activity. The enzymatic activity of the Val allele is three to four times higher than that of the Met allele, and this high level of activity is hypothesized to result in poorer performance of frontally mediated cognitive tasks because of lower dopamine levels (1).
Findings relating the COMT genotype with measures of cognition have been inconsistent, however (e.g., references 1–4). In the largest study to date involving a representative sample of 543 young men, no effect of COMT genotype was found on either working memory or attention (5).
Inconsistencies in the findings relating COMT genotype to cognition may be caused by the failure to distinguish between cognitive tasks that differentially depend on tonic and phasic dopamine signaling. The low-activity Met allele may be expected to be of benefit in tasks of cognitive stability requiring tonic dopamine activation but have negative effects on tasks of cognitive flexibility requiring phasic activation (6).
In the current study, we conducted further analyses in a representative general population sample of 543 young men to test for an association between COMT genotype and a cognitive measure more specific for cognitive stability: reaction time variability. Intraindividual variability in cognitive tasks is a measure of “noise” and as such constitutes an important source of information (7). Reaction time variability is considered a measure of dispersion and efficiency of processing information (7), which recently has been hypothesized to be a fundamental mechanism underlying frontal lobe deficits in schizophrenia (8). We used a measure of reaction time variability derived from the Continuous Performance Test, Identical Pairs Version (9), to test the hypothesis that Met carriers, whose synaptic dopamine degrades less rapidly, would have reduced levels of cortical noise.
Method
The Athens Study of Psychosis Proneness and Incidence of Schizophrenia (5, 10) examined 2,243 randomly selected new male Air Force conscripts who were 18–24 years old. The study was approved by the Ethics Committee of the University Mental Health Research Institute, and all subjects provided written informed consent. Six hundred three conscripts were randomly selected for COMT genotyping; results for 543 of these subjects were valid.
The Continuous Performance Test, Identical Pairs Version, requires a subject to respond whenever two identical stimuli (4-digit numbers) appear in a row (target) within a sequence of 300 rapidly flashed trials presented in a 5-minute trial. There are 60 such target pairs in every test condition and an equal number of false alarm trials, in which two successive stimuli are very similar but not identical. A sensitivity index d′ is computed on the basis of the ability to discriminate between target and false alarm. Mean response latencies to target and false alarm are computed, as well as the standard deviation of response latency to target. This latter measure of intraindividual variability of response latencies to target, derived from the Continuous Performance Test, Identical Pairs Version, provides the main index of reaction time variability for this study (5).
DNA was extracted from mouthwash samples as described previously (5). Genotyping was performed by polymerase chain reaction amplification, digestion with the restriction enzyme NlaIII, and 3% agarose electrophoresis, as described elsewhere (5).
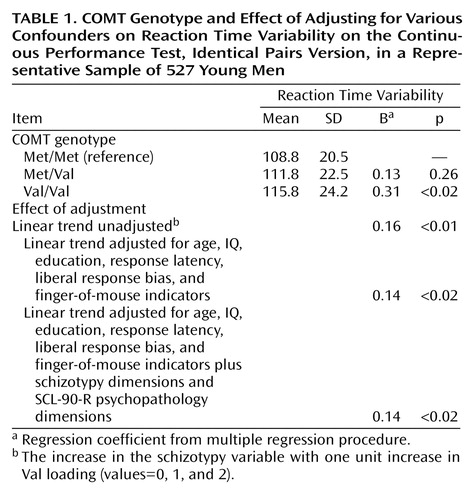
Linear effects of Val allelic loading were tested by using multiple regression in Stata (11) with reaction time variability as the dependent variable. In addition, dummy variables for each genotype group (Met/Met, Met/Val, Val/Val) were created to assess dose-response effects. Effect sizes were expressed as standardized regression coefficients (B) from the multiple regression procedures. We adjusted reaction time variability analyses for mean response latency and for motivational factors, using the three finger-of-mouse indicators and the beta criterion (a measure of liberal response bias). Other a priori confounders included age, IQ assessed with Raven’s Progressive Matrices (12), number of years of schooling (range=6–17 years), the nine psychopathology dimensions of the SCL-90-R, and four dimensions of schizotypy (5).
Results
The mean age of the 543 subjects was 21.0 years (SD=1.9), their mean number of years of schooling was 13.0 years (SD=2.0), and their mean Raven IQ was 46.6 (SD=7.7). Alleles of Val and Met were present at overall frequencies of 56.4% and 43.6%, respectively. The three genotype frequencies were 20.6% for Met/Met (N=112), 46% for Met/Val (N=250), and 33% for Val/Val (N=181), not deviant from that expected for genotypes in Hardy-Weinberg equilibrium.
No large or significant differences were observed between Val allelic loading and age, years of schooling, Raven IQ, and other confounding factors derived from the Continuous Performance Test.
Of the 543 individuals, 527 had valid values for reaction time variability (mean=112.5, SD=22.8, median=111.1), which was normally distributed. Greater level of reaction time variability was weakly correlated with greater response latency (r=0.19, N=527, p<0.001) and moderately correlated with decreased Continuous Performance Test sensitivity index d′ (ability to discriminate between targets and false alarms, r=–0.39, N=527, p<0.001). Val loading was associated with reaction time variability (B=0.16, χ2=6.7, df=1, p<0.01) in a dose-response fashion, and this was independent of confounding factors (Table 1).
Discussion
Cognitive tests used in previous studies of cognition and COMT genotype require both cognitive flexibility and cognitive stability, which, in association studies with the COMT genotype, may cancel each other out statistically given their hypothesized directionally inverse dependence on phasic and tonic dopamine (6). The same applies to the Continuous Performance Test, Identical Pairs Version, which requires both maintenance (stability) and updating (flexibility) of memory representations. The positive association between Val loading and reaction time variability may be explained by the fact that Met genotype loading confers enhanced “tuning” or greater stability in performance, possibly by stabilizing active neural representations in the prefrontal cortex during tasks involving working memory.
 |
Received Oct. 3, 2004; revision received Jan. 18, 2005; accepted Feb. 4, 2005. From the University Mental Health Research Institute and the Department of Psychiatry, National and Kapodistrian University of Athens, Eginition Hospital, Athens; the Department of Psychiatry and Neuropsychology, South Limburg Mental Health Research and Teaching Network, European Graduate School of Neuroscience, Maastricht University, Maastricht, the Netherlands; and the Division of Psychological Medicine, Institute of Psychiatry, De Crespigny Park, London. Address correspondence and reprint requests to Professor Stefanis, Department of Psychiatry, National and Kapodistrian University of Athens Medical School, Eginition Hospital, 74 Vas. Sofias Ave., Athens 11528, Greece; [email protected] (e-mail). Supported by grant EKBAN 97 from the General Secretariat of Research and Technology of the Greek Ministry of Development (Professor Stefanis). Technical support for this project was provided by Intrasoft Co. The authors thank Ioannis Giouzelis, Ioanna Hantoumi, Georgios Kastrinakis, Emanouil Kattoulas, Catherine Paximadis, and Christos Theleritis for their help with data acquisition and preprocessing.
1. Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR: Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA 2001; 98:6917–6922Crossref, Medline, Google Scholar
2. Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS, Goldman D, Weinberger DR: Executive subprocesses in working memory: relationship to catechol-O-methyltransferase Val158Met genotype and schizophrenia. Arch Gen Psychiatry 2003; 60:889–896Crossref, Medline, Google Scholar
3. Bilder RM, Volavka J, Czobor P, Malhotra AK, Kennedy JL, Ni X, Goldman RS, Hoptman MJ, Sheitman B, Lindenmayer JP, Citrome L, McEvoy JP, Kunz M, Chakos M, Cooper TB, Lieberman JA: Neurocognitive correlates of the COMT Val(158)Met polymorphism in chronic schizophrenia. Biol Psychiatry 2002; 52:701–707Crossref, Medline, Google Scholar
4. Rosa A, Peralta V, Cuesta MJ, Zarzuela A, Serrano F, Martínez-Larrea A, Fañanás L: New evidence of association between COMT gene and prefrontal neurocognitive function in healthy individuals from sibling pairs discordant for psychosis. Am J Psychiatry 2004; 161:1110–1112Link, Google Scholar
5. Stefanis NC, van Os J, Avramopoulos D, Smyrnis N, Evdokimidis I, Hantoumi I, Stefanis CN: Variation in catechol-O-methyltransferase val158 met genotype associated with schizotypy but not cognition: a population study in 543 young men. Biol Psychiatry 2004; 56:510–515Crossref, Medline, Google Scholar
6. Nolan KA, Bilder RM, Lachman HM, Volavka J: Catechol O–methyltransferase Val158Met polymorphism in schizophrenia: differential effects of Val and Met alleles on cognitive stability and flexibility. Am J Psychiatry 2004; 161:359–361Link, Google Scholar
7. Stuss DT, Murphy KJ, Binns MA, Alexander MP: Staying on the job: the frontal lobes control individual performance variability. Brain 2003; 126(part 11):2363–2380Google Scholar
8. Winterer G, Coppola R, Goldberg TE, Egan MF, Jones DW, Sanchez CE, Weinberger DR: Prefrontal broadband noise, working memory, and genetic risk for schizophrenia. Am J Psychiatry 2004; 161:490–500Link, Google Scholar
9. Cornblatt BA, Lenzenweger MF, Erlenmeyer-Kimling L: The Continuous Performance Test, Identical Pairs Version, II: contrasting attentional profiles in schizophrenic and depressed patients. Psychiatry Res 1989; 29:65–85Crossref, Medline, Google Scholar
10. Stefanis NC, Smyrnis N, Avramopoulos D, Evdokimidis I, Ntzoufras I, Stefanis CN: Factorial composition of self-rated schizotypal traits among young males undergoing military training. Schizophr Bull 2004; 30:335–350Crossref, Medline, Google Scholar
11. Stata Reference Manual: Release 8.0. College Station, Tex, Stata Corp, 2003Google Scholar
12. Raven JC: Raven’s Progressive Matrices. San Antonio, Tex, Psychological Corp (Harcourt), 1988Google Scholar



