No Association Between Schizophrenia and Polymorphisms in COMT in Two Large Samples
Abstract
OBJECTIVE: A valine/methionine polymorphism in the catechol O-methyltransferase (COMT) gene has been proposed to influence susceptibility to schizophrenia, as has a COMT haplotype in Ashkenazi Jewish and Irish subjects. The authors examined these hypotheses. METHOD: They reviewed data from more than 2,800 individuals, including almost 1,200 with schizophrenia, from case-control and family-based European association samples. RESULTS: The authors found no support for the hypothesis that a valine/methionine polymorphism in the COMT gene influences susceptibility to schizophrenia or the hypothesis that a COMT haplotype influences susceptibility to schizophrenia in Ashkenazi Jewish and Irish subjects. CONCLUSIONS: The data suggest that the valine allele of COMT does not increase susceptibility to schizophrenia in Europeans and that the Ashkenazi or Irish haplotype does not increase susceptibility. Ethnic variation in the linkage disequilibrium structure at COMT means that the haplotype data may not generalize across populations. However, the authors’ examination of the hypothesis that the valine allele confers susceptibility, with a particularly strong effect in Europeans, reveals that no such caveat applies.
Catechol O-methyltransferase (COMT) is a strong positional and functional candidate gene for schizophrenia (1). COMT contains a valine/methionine polymorphism. The valine allele raises COMT enzyme activity, including the form predominant in the brain, and is associated with reduced performance in tests of frontal lobe function (2), but its proposed role in schizophrenia is more controversial. A recent meta-analysis (1) reported no overall support for this role, although it was postulated that a true association might exist, particularly in European populations. The situation has become more complex with a three-marker haplotype yielding evidence for association with schizophrenia in Ashkenazi Jewish and Irish samples (3, 4). Although the associated haplotype carried the valine allele in each study, the evidence for haplotype association was stronger than for valine alone, suggesting that valine may not be the cause of the association. Indeed, in one study (4), a haplotype carrying the valine allele was significantly underrepresented in subjects with schizophrenia. Given the enduring controversy surrounding COMT, we have examined these specific genetic findings at COMT in two samples: a case-control sample (709 subjects with schizophrenia and 710 comparison subjects) and 488 pro-band-parent trios.
Method
Case-control subjects were unrelated, white, and born in the United Kingdom or Ireland. Trios were ascertained through unrelated Bulgarian probands. All subjects defined as cases met DSM-IV criteria for schizophrenia except for 40 Bulgarian probands who met criteria for schizoaffective disorder. Diagnoses were based on direct standardized diagnostic interviews with probands and review of all retrievable case records (see reference 5). Comparison subjects were unrelated blood donors ascertained in the same regions as subjects with schizophrenia. Given the characteristics of U.K. blood donors and the frequency of schizophrenia, it was not necessary to screen the comparison subjects for schizophrenia to retain power (5). Samples have, respectively, 0.99 and 0.84 power to detect the effect of the Ashkenazi (G-G-G) risk haplotype at alpha=0.05 and power of 0.88 and 0.66 to replicate the A-G-A Irish risk haplotype. We have previously reported data for the Val/Met polymorphism in half of the sample (5).
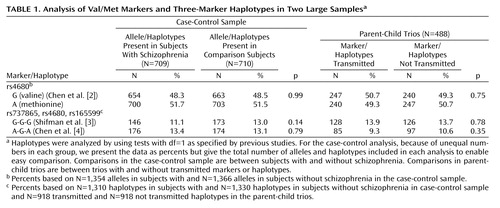
Markers (Table 1) were genotyped by using SNaPshot (ABI, Foster City, Calif.) according to the manufacturer’s instructions with an ABI3100 sequencer. Primers and reaction conditions are provided at the web site of Cardiff University (www.cardiff.ac.uk/medicine/psychological_medicine/pub_data/comt.htm).
Marker associations were tested by chi-square in the case-control sample and by transmission disequilibrium test in the trios (cited in reference 1). Haplotypes were examined by using EHPLUS (6) and the permutation test PMPLUS (7) (case-control) or TDTPHASE (8) (trios).
Results
No marker or haplotype was associated with schizophrenia in any sample or when each was analyzed by gender. For simplicity, we present only the whole sample data for the specific hypotheses (Val/Met, G-G-G haplotype, and A-G-A haplotype) (Table 1). More details are available through our web site (www.cardiff.ac.uk/medicine/psychological_medicine/pub_data/comt.htm). U.K. subjects with schizophrenia were not in Hardy-Weinberg equilibrium for rs737865 in males (p=0.03). U.K. comparison subjects were not in Hardy-Weinberg equilibrium for rs165599 in females (p=0.006). Neither finding was significant after correction for multiple tests in the case-control study (both genders, males and females, subjects with schizophrenia and comparison subjects, three single nucleotide polymorphisms [SNPs]=18 tests). To exclude genotyping error, we resequenced the region and, after detection of several polymorphisms (data not shown), redesigned our assays so none of the primers sat on polymorphic sites. Regenotyping 96 individuals for each marker blindly resulted in 100% concordance between assays.
Discussion
In this large study, we were unable to detect significant association between schizophrenia and the Val/Met COMT locus or haplotypes previously associated with schizophrenia (3, 4). Interpreting the results of haplotype analysis is complex. Given the power, it is unlikely that our findings are attributable to type II error. The modest evidence in the smaller study of Irish subjects (4) may be prone to type I error, but this is unlikely in the large Ashkenazim study because of its strong statistical support. It is always tempting to speculate that discrepancies in case-control analysis result from poor matching, but we and the other researchers (3) have taken considerable care in this respect. Moreover, our trio analyses are robust to stratification, and the distribution of p values we obtained in our own analysis of 361 SNPs at 97 different loci in a case-control study is not different from chance expectations (p=0.3). Another possibility is that the findings reflect differences in the effect size of the risk haplotype, either as a result of differences in the extent of linkage disequilibrium in the region or because the risk variant has itself a larger effect size in the Ashkenazi population. Analysis of the linkage disequilibrium relationships between the markers provides evidence for population linkage disequilibrium differences (9) (www.cardiff.ac.uk/medicine/psychological_medicine/pub_data/comt.htm). Thus, although pairwise estimates of linkage disequilibrium between markers are very similar in U.K. and Bulgarian samples, the linkage disequilibrium between markers rs737865 and rs4608 was considerably higher in the Ashkenazi Jewish sample (p<0.0001 for differences in r2). More surprising, given expected similarities (9), the extent of linkage disequilibrium in the Irish sample is much lower for both of the marker pairs that include the Val/Met polymorphism compared with our U.K. samples (p<0.001 for each pair). To resolve these questions, more detailed high-density SNP analyses of COMT are indicated with the aim of identifying true susceptibility variants if indeed they do exist. However, the evidence concerning the Val/Met locus is now relatively clear. Although vanishingly small effects can never be excluded, data from our own case-control and family study together with the meta-analysis (1) and subsequent studies (3, 4) make it unlikely that the valine allele confers susceptibility to schizophrenia.
 |
Received June 22, 2004; revision received Oct. 6, 2004; accepted Oct. 22, 2004. From the Department of Psychological Medicine, Henry Wellcome Institute for Biomedical Research, Cardiff University. Address correspondence and reprint requests to Professor O’Donovan or Professor Owen, Department of Psychological Medicine, Henry Wellcome Building for Biomedical Research, Cardiff University, Heath Park, Cardiff, CF14 4XN, UK; [email protected] or [email protected] (e-mail). Supported by grants from the Medical Research Council and the Wellcome Trust.
1. Glatt SJ, Faraone SV, Tsuang MT: Association between a functional catechol O–methyltransferase gene polymorphism and schizophrenia: meta-analysis of case-control and family-based studies. Am J Psychiatry 2003; 160:469–476Link, Google Scholar
2. Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, Apud J, Egan MF, Kleinman JE, Weinberger DR: Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet 2004; 75:807–821Crossref, Medline, Google Scholar
3. Shifman S, Bronstein M, Sternfeld M, Pisante-Shalom A, Lev-Lehman E, Weizman A, Reznik I, Spivak B, Grisaru N, Karp L, Schiffer R, Kotler M, Strous RD, Swartz-Vanetik M, Knobler HY, Shinar E, Beckmann JS, Yakir B, Risch N, Zak NB, Darvasi A: A highly significant association between a COMT haplotype and schizophrenia. Am J Hum Genet 2002; 71:1296–1302Crossref, Medline, Google Scholar
4. Chen X, Wang X, O’Neill AF, Walsh D, Kendler KS: Variants in the catechol-o-methyltransferase (COMT) gene are associated with schizophrenia in Irish high-density families. Mol Psychiatry 2004; 9:962–967Crossref, Medline, Google Scholar
5. Norton N, Kirov G, Zammit S, Jones G, Jones S, Owen R, Krawczak M, Williams NM, O’Donovan MC, Owen MJ: Schizophrenia and functional polymorphisms in the MAOA and COMT genes: no evidence for association or epistasis. Am J Med Genet 2002; 114:491–496Crossref, Medline, Google Scholar
6. Xie X, Ott J: Testing linkage disequilibrium between a disease gene and marker loci (abstract). Am J Hum Genet 1993; 53:1107Medline, Google Scholar
7. Zhao JH, Curtis D, Sham PC: Model-free analysis and permutation tests for allelic associations. Hum Hered 2000; 50:133–139Crossref, Medline, Google Scholar
8. Dudbridge F: Pedigree disequilibrium tests for multilocus haplotypes. Genet Epidemiol 2003; 25:115–121Crossref, Medline, Google Scholar
9. Palmatier MA, Pakstis AJ, Speed W, Paschou P, Goldman D, Odunsi A, Okonofua F, Kajuna S, Karoma N, Kungulilo S, Grigorenko E, Zhukova OV, Bonne-Tamir B, Lu RB, Parnas J, Kidd JR, DeMille MM, Kidd KK: COMT haplotypes suggest P2 promoter region relevance for schizophrenia. Mol Psychiatry 2004; 9:859–870Crossref, Medline, Google Scholar



