Metabolic Disconnection Between the Mediodorsal Nucleus of the Thalamus and Cortical Brodmann’s Areas of the Left Hemisphere in Schizophrenia
Abstract
OBJECTIVE: The authors’ goal was to examine interregional correlations of thalamocortical metabolic activity during a verbal learning task in schizophrenia. METHOD: They used [18F]fluorodeoxyglucose positron emission tomography in 41 unmedicated patients with schizophrenia and 59 normal comparison subjects. RESULTS: A metabolic disconnection was observed in patients with schizophrenia in the left hemisphere between the mediodorsal nucleus and widespread frontotemporal cortical regions, and stronger-than-normal intercorrelations were found between the pulvinar and superior temporal, selected parietal, posterior cingulate, and occipital areas. CONCLUSIONS: Deficits in the functional interrelationships between the left frontotemporal cortices and the left mediodorsal nucleus of the thalamus complement inferences from postmortem and magnetic resonance imaging volumetric studies identifying a thalamic diathesis in schizophrenia.
Dysfunctional thalamocortical pathways are thought to be important in the pathogenesis of schizophrenia (1). They may be studied by correlational analysis of metabolic mapping data, a valid and sensitive technique (2, 3) that has been applied in schizophrenia research with variable results. Some (4) but not all (5) earlier analyses of thalamocortical intercorrelations demonstrated differences between normal subjects and patients with schizophrenia. However, those studies used metabolic rates across the whole thalamus, although abnormalities in schizophrenia may be more subtle and may involve particular nuclei, as suggested by our report of selective metabolic decreases in the mediodorsal nucleus (6). In this article we apply the metabolic intercorrelation method and magnetic resonance imaging (MRI) of mediodorsal, centromedian, and pulvinar regions of interest in a large study group of unmedicated patients with schizophrenia to test the hypothesized frontothalamic dysfunction.
Method
Forty-one patients with schizophrenia (32 men, nine women) were recruited from outpatient and emergency departments of Mount Sinai School of Medicine. The patients’ mean age was 36.9 years (SD=15.2, range=18–73); 36 were right-handed. All patients met criteria for schizophrenia (N=37) or schizoaffective disorder (N=4) as determined by semistructured interview with the Comprehensive Assessment of Symptoms and History (7). The patients were neuroleptic naive (N=15) or had been neuroleptic free (N=26) for a median period of 3 weeks (range=12 days–22 years); no patients were receiving depot preparations. Patients’ Brief Psychiatric Rating Scale scores ranged from 25 to 89 (mean=52.4, SD=12.7, median=51).
Fifty-nine normal subjects (45 men, 14 women) were similarly screened to exclude any psychiatric anamnesis. The normal subjects’ mean age was 40.1 years (SD=15.9, range=20–81); 57 were right-handed. Individuals with a positive urine toxicology screen on the positron emission tomography (PET) day and patients with a history of substance abuse, neurological disorders, or head trauma were excluded.
During [18F]fluorodeoxyglucose uptake, participants performed a serial verbal learning task developed for the 32-minute uptake period (6). PET and MRI scans were acquired and coregistered exactly as reported previously (6, 8), as was morphometry of the thalamic nuclei and cortical Brodmann’s areas (8–10). We calculated the product-moment correlation matrices for relative glucose metabolism among the mediodorsal, pulvinar, and centromedian nuclei of the thalamus and each of the 39 cortical Brodmann’s areas identified, with separate analyses for each hemisphere. Some regional volumetric and PET data from this group of subjects have been reported (6, 10).
Results
Intercorrelations between the left mediodorsal nucleus and frontal, temporal (medial and lateral), and selected parietal cortical regions (Figure 1) demonstrated a metabolic disconnection in patients with schizophrenia, with especially strong differences between patients and normal subjects in prefrontal (area 9) and medial temporal (areas 28 and 34) cortices. In the right hemisphere, the only intergroup difference concerned a single positive correlation between the mediodorsal nucleus and occipital area 17 in patients that did not reach statistical significance in normal subjects.
A positive correlation between the left centromedian nucleus and occipital area 17 was significantly lower in patients than in normal subjects. In the right hemisphere, a negative correlation between the centromedian nucleus and area 7a was seen in patients but not in normal subjects.
Unexpectedly, positive intercorrelations between the pulvinar and cortical regions (encountered only in the left hemisphere: posterior cingulate areas 23 and 31, superior temporal area 22, parietal area 39, and occipital area 18) were significantly lower in normal subjects than in patients.
Discussion
A metabolic disconnection between the mediodorsal nucleus of the thalamus and frontotemporal cortex in the left hemisphere, as inferred from diminished correlations between these regions, was observed during a serial verbal learning task in patients with schizophrenia. These results replicate and extend previous findings of weaker positive metabolic intercorrelations between the dorsomedial thalamus and the frontal (4) and limbic (11) cortices during a Continuous Performance Test in patients with schizophrenia compared with normal subjects. They further implicate the mediodorsal nucleus in schizophrenia pathophysiology by indicating that in addition to the metabolic and volumetric impairments in the nucleus (reviewed elsewhere [6, 9, 10]), its connectivity with the frontotemporal cortex in the left hemisphere may likewise be impaired.
Mediodorsal thalamic pathology in schizophrenia is not invariable (12, 13) and may be secondary to the nuclear disconnection from the associative cortical networks (14). Alternatively, neuronal loss in the mediodorsal nucleus in patients may result in its failure to activate associative cortical networks, resulting in lower intercorrelations, but this would not adequately explain the asymmetry of the metabolic disconnections with a backdrop of bilateral volumetric deficits in the nucleus previously reported for this group of subjects (10). Our unilateral findings accord with some earlier studies showing that mediodorsal pathology in patients with schizophrenia was asymmetrically confined to the left hemisphere (4, 14).
Given the putative information-processing role of the mediodorsal nucleus (15), the mediodorsal/cortical dissociations could be related to the left-hemisphere recognition-based memory disturbances in schizophrenia. Indeed, in the serial verbal learning task used in this study, our patients recalled significantly fewer correct words, achieved lower semantic clustering strategy scores, and exhibited more perseveration and intrusion errors than normal subjects (6). Arguably, the verbal memory demands of the task would be expected to activate functional corticothalamic networks preferentially in the left hemisphere, thus selectively emphasizing left hemispheric dysfunction in patients. However, intercorrelations with most of the temporal, some of the frontal, and other cortical areas in the right hemisphere were also detected, with no significant differences between normal subjects and patients with schizophrenia.
In the schizophrenia group, stronger-than-normal correlations were observed between the left pulvinar and left posterior cingulate, superior temporal, lower parietal, and occipital areas thought relevant to higher-order visual information processing. In view of the widespread left mediodorsal/cortical disconnectivities and the role of the pulvinar in selective visual attention and information filtering (16, 17), it is plausible that greater pulvinocortical activity in these patients may represent a stronger reliance on compensatory or alternative strategies within a widely distributed network subservient to across-level visuospatial information processing. Chronic shifting to alternative cognitive strategies in the patient group, away from the mediodorsal/cortical functional networks, might in turn adversely affect mediodorsal nuclear volumes. Studies applying other neuropsychological paradigms may shed further light on the role of thalamocortical disconnectivities in information processing deficits in schizophrenia.
Received Oct. 15, 2004; revisions received Jan. 31 and Feb. 7, 2005; accepted Feb. 22, 2005. From the Department of Psychiatry, Mount Sinai School of Medicine, New York. Address correspondence and reprint requests to Dr. Mitelman, Department of Psychiatry, Neuroscience-PET Laboratory, Box 1505, Mount Sinai Medical Center, One Gustave L. Levy Place, New York, NY 10029; [email protected] (e-mail). Supported by NIMH grants MH-60023, MH-56489, and MH-40071 (Dr. Buchsbaum). The authors thank the Charles Dana Foundation for support of scan acquisition; Rachel Bloom, Jimcy Platholi, and Adam M. Brickman for technical support; Lina Shihabuddin and M. Mehmet Haznedar for clinical recruitment and diagnosis; Cheuk Tang for instrumentation physics consultations; and Igor A. Mitelman for programming support.
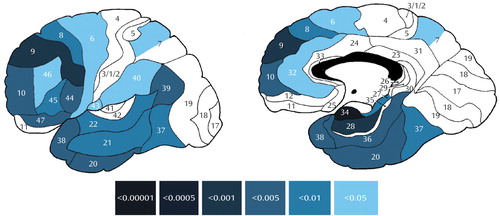
Figure 1. Significant Differences Between Normal Subjects and Patients With Schizophrenia in Correlation Coefficients Between Relative Glucose Metabolic Ratesa of the Left Mediodorsal Nucleus of the Thalamus and Cortical Brodmann’s Areasb
aRelative glucose metabolic rate was defined as region of interest/mean value for all pixels within the brain outline.
bSignificance assessed with Fisher’s z transformation. Color bar illustrates p values for Fisher’s z scores for each cortical Brodmann’s area. All z scores were positive, reflecting positive and significant correlation coefficients in normal subjects, but none of the correlations reached statistical significance in patients with schizophrenia. Correlations where significant differences were found in Fisher’s z transformation comparisons between normal subjects and patients are presented if at least one of the compared correlation coefficients also reached statistical significance.
1. Andreasen NC, Paradiso S, O’Leary DS: “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull 1998; 24:203–218Crossref, Medline, Google Scholar
2. Clark CM, Kessler R, Buchsbaum MS, Margolin RA, Holcomb HH: Correlational methods for determining regional coupling of cerebral glucose metabolism. Biol Psychiatry 1984; 19:663–678Medline, Google Scholar
3. Horwitz B: Simulating functional interactions in the brain: a model for examining correlations between regional cerebral metabolic rates. Int J Biomed Comput 1990; 26:149–170Crossref, Medline, Google Scholar
4. Katz M, Buchsbaum MS, Siegel BV, Wu J, Haier RJ, Bunney WE: Correlational patterns of cerebral glucose metabolism in never-medicated schizophrenics. Neuropsychobiology 1996; 33:1–11Crossref, Medline, Google Scholar
5. Volkow ND, Wolf AP, Brodie JD, Cancro R, Overall JE, Rhoades H, Van Gelder P: Brain interactions in chronic schizophrenics under resting and activation conditions. Schizophr Res 1988; 1:47–53Crossref, Medline, Google Scholar
6. Hazlett EA, Buchsbaum MS, Kemether E, Bloom R, Platholi J, Brickman AM, Shihabuddin L, Tang C, Byne W: Abnormal glucose metabolism in the mediodorsal nucleus of the thalamus in schizophrenia. Am J Psychiatry 2004; 161:305–314Link, Google Scholar
7. Andreasen NC, Flaum M, Arndt S: The Comprehensive Assessment of Symptoms and History (CASH): an instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry 1992; 49:615–623Crossref, Medline, Google Scholar
8. Mitelman SA, Shihabuddin L, Brickman AM, Hazlett EA, Buchsbaum MS: MRI assessment of gray and white matter distribution in Brodmann’s areas of the cortex in patients with schizophrenia with good and poor outcomes. Am J Psychiatry 2003; 160:2154–2168Link, Google Scholar
9. Byne W, Buchsbaum MS, Kemether E, Hazlett EA, Shinwari A, Mitropoulou V, Siever LJ: Magnetic resonance imaging of the thalamic mediodorsal nucleus and pulvinar in schizophrenia and schizotypal personality disorder. Arch Gen Psychiatry 2001; 58:133–140Crossref, Medline, Google Scholar
10. Kemether EM, Buchsbaum MS, Byne W, Hazlett EA, Haznedar M, Brickman AM, Platholi J, Bloom R: Magnetic resonance imaging of mediodorsal, pulvinar, and centromedian nuclei of the thalamus in patients with schizophrenia. Arch Gen Psychiatry 2003; 60:983–991Crossref, Medline, Google Scholar
11. Wu JC, Siegel BV, Haier RJ, Buchsbaum MS: Testing the Swerdlow/Koob model of schizophrenia pathophysiology using positron emission tomography. Behav Brain Sci 1990; 13:169–170Crossref, Google Scholar
12. Cullen TJ, Walker MA, Parkinson N, Craven R, Crow TJ, Esiri MM, Harrison PJ: A postmortem study of the mediodorsal nucleus of the thalamus in schizophrenia. Schizophr Res 2003; 60:157–166Crossref, Medline, Google Scholar
13. Dorph-Petersen KA, Pierri JN, Sun Z, Sampson AR, Lewis DA: Stereological analysis of the mediodorsal thalamic nucleus in schizophrenia. J Comp Neurol 2004; 472:449–462Crossref, Medline, Google Scholar
14. Danos P, Baumann B, Kramer A, Bernstein HG, Stauch R, Krell D, Falkai P, Bogerts B: Volumes of association thalamic nuclei in schizophrenia. Schizophr Res 2003; 60:141–155Crossref, Medline, Google Scholar
15. Van der Werf YD, Jolles J, Witter MP, Uylings HB: Contributions of thalamic nuclei to declarative memory functioning. Cortex 2003; 39:1047–1062Crossref, Medline, Google Scholar
16. LaBerge D, Buchsbaum MS: Positron emission tomographic measurements of pulvinar activity during an attention task. J Neurosci 1990; 10:613–619Crossref, Medline, Google Scholar
17. Shipp S: The functional logic of cortico-pulvinar connections. Philos Trans R Soc Lond B Biol Sci 2003; 358:1605–1624Crossref, Medline, Google Scholar



