Cingulate Cortex Anatomical Abnormalities in Children and Adolescents With Bipolar Disorder
Abstract
OBJECTIVE: In vivo imaging studies have suggested anatomical and functional abnormalities in the anterior cingulate in adults with mood disorders. This anatomical magnetic resonance imaging study examined the cingulate cortex in children and adolescents with bipolar disorder and matched healthy comparison subjects. METHOD: Sixteen patients (mean age=15.5 years, SD=3.4) with DSM-IV bipolar disorder and 21 matched healthy comparison subjects (mean age=16.9 years, SD=3.8) were studied. Three-dimensional gradient echo imaging was performed (TR=25 msec, TE=5 msec, slice thickness=1.5 mm) in a 1.5-T GE Signa magnet. Cingulate volumes were compared by using analysis of covariance, with age and intracranial volume as covariates. RESULTS: The patients with bipolar disorder had significantly smaller mean volumes relative to the healthy subjects in the left anterior cingulate (mean=2.49 cm3 [SD=0.28] versus 3.60 cm3 [SD=0.12], respectively), left posterior cingulate (2.53 cm3 [SD=0.32] versus 2.89 cm3 [SD=0.09]), and right posterior cingulate (2.19 cm3 [SD=0.13] versus 2.28 cm3 [SD=0.08]). No significant between-group difference was found for the right anterior cingulate (2.64 cm3 [SD=0.21] versus 2.71 cm3 [SD=0.10]). CONCLUSIONS: The findings indicate smaller cingulate volumes in children and adolescents with bipolar disorder, suggesting that such abnormalities may be present early in the illness course.
The pathophysiology of bipolar disorder appears to involve abnormalities in brain neuroanatomic circuits that are involved in mood regulation (1). The cingulate gyrus, by having extensive connections with limbic regions, is possibly a key structure in brain circuits that modulate mood. Broca defined the cingulate, hippocampal, and parahippocampal cortices as the limbic lobe (2), which is part of Olds’ “reward system” (3) that borders all sensory and motor areas mediating the appraisal of sensory impressions and movement. The cingulate is contained within a gyrus that lies between the corpus callosum and the cingulate sulcus, which is anterior to the genu of the corpus callosum. The entire cingulate is a heterogenous structure with respect to its cytoarchitecture and functions. It can be divided into anterior domains (Brodmann’s areas 24, 25, 33) and posterior domains (Brodmann’s areas 23, 29, 30) (4). Vogt et al. (4) subdivided the cingulate into four functional areas: affect regulation, response selection, visuospatial processing, and memory access.
The involvement of abnormalities in the cingulate cortices, particularly the anterior cingulate, in the pathophysiology of bipolar disorder is supported by several anatomical magnetic resonance imaging (MRI) studies with different methodological approaches (5). Two previous MRI studies (6, 7) did not find any anatomical abnormalities in the cingulate volumes of patients with bipolar disorder. Drevets et al. (8) reported that a specific part of the anterior cingulate gyrus lying ventral to the genu of the corpus callosum, the subgenual region of Brodmann’s area 24 (also called the subgenual prefrontal cortex), was 40% smaller in subjects with familial mood disorder relative to comparison subjects. This finding was supported by another study (9) in which smaller volumes in the left subgenual region of Brodmann’s area 24 were found in subjects with first episode affective psychosis who had a familial history of mood disorders. Of interest is that smaller left anterior cingulate cortex volumes were observed in drug-free adult patients with bipolar disorder but not in lithium-treated patients (10), which might be the result of lithium’s proposed neuroprotective effect in the brain (11, 12), but the precise mechanism remains elusive. Acute lithium treatment was also associated with a reduced anterior cingulate myo-inositol/creatine ratio in bipolar children (N=11) (13).
Positron emission tomography studies in bipolar subjects during episodes of mania and depression have also demonstrated regional alterations in cerebral perfusion and metabolism in the anterior cingulate cortex (14–17). Ito et al. (14) found decreased cerebral blood flow in the prefrontal cortices, limbic systems, and paralimbic areas of subjects with unipolar and bipolar depression on a pixel-by-pixel basis, using single photon emission computed tomography, and indicated that dysfunction of these regions might be related to the attentional, cognitive, and emotional impairments that are recognized as usual symptoms of depression. The left dorsal anterior cingulate exhibited increased activity in manic patients in two different reports (18, 19). These findings are supported by postmortem studies reporting synaptic abnormalities (20) and decreased neuronal density of the anterior cingulate in patients with bipolar disorder (21, 22).
It is not known whether neuroanatomical abnormalities in the cingulate cortices are already present early in the course of the illness. To address this question, we used MRI techniques to compare the cingulate cortex volumes of children and adolescents diagnosed with bipolar disorder with those of age-matched healthy comparison subjects. To our knowledge, this is the first anatomical MRI study examining the cingulate in bipolar children and adolescents. We hypothesized that children and adolescents with bipolar disorder would have smaller cingulate volumes relative to healthy comparison subjects. On an exploratory basis, we also examined gender and laterality effects.
Method
Subjects
Sixteen children and adolescents with bipolar disorder (eight female and eight male subjects; mean age=15.5 years [SD=3.4, range=10–21]) and 21 healthy subjects (nine female and 12 male subjects; mean age=16.9 years [SD=3.8, range=11–21]) were recruited. Subjects were recruited from the outpatient clinics of the Department of Psychiatry, University of Pittsburgh Medical Center, or through advertisements in the local community. All patients met DSM-IV criteria for bipolar disorder as determined by either the Structured Clinical Interview for DSM-IV (SCID) (23) for subjects ages 18–21 years old or the Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (K-SADS-PL) (24) for those younger than 18. Subjects did not have any history of alcohol/substance abuse, neurological problems, or current medical problems.
The bipolar patient group met DSM-IV criteria for bipolar type I (N=12), bipolar type II (N=3), or bipolar not otherwise specified (N=1). At the time of the study, two subjects were in a depressive episode, and 14 were euthymic. Only two patients were drug-free, the other 14 patients were receiving treatment at the time they participated in the study (Table 1). Eleven patients had a history of previous antipsychotic use, while five patients had no such history. Fifteen of the 16 patients had a family history of mood disorders in a first-degree relative. First-degree relatives were considered mood disorder positive if they ever received a diagnosis from a psychiatrist of unipolar or bipolar disorder, as ascertained by patient and relative reports and available medical records. Patients did not have any axis I comorbid psychiatric disorders other than ADHD (N=5), oppositional defiant disorder (N=1), and conduct disorder (N=1). Symptoms of depression were assessed with the Hamilton Depression Rating Scale.
Healthy subjects (N=21) had no DSM-IV axis I disorders as determined by either the SCID nonpatient version (23) for subjects ages 18–21 years old or the K-SADS-PL (24) for subjects younger than 18. For inclusion, healthy subjects had to be 10–21 years of age with no history of or current psychiatric, neurologic, or substance-related disorder. Exclusion criteria were history of any axis I psychiatric disorder in first-degree relatives, use of any psychoactive medication within 2 weeks of the study, and any current medical problems.
This study protocol was approved by the University of Pittsburgh biomedical institutional review board. All subjects, as well as their parents (if subject was younger than 18), signed consent forms, after having understood all issues pertaining to participation in the study.
MRI Procedure
A 1.5-T GE Signa Imaging System running version Signa 5.4.3 software (General Electric Medical Systems, Milwaukee) was used to acquire the MRI scans. First, a sagittal scout series was obtained to confirm patients’ head position and image quality and to locate a midline sagittal image. Graphic prescription of the coronal and axial images was obtained through a T1-weighted sagittal scout image. Three-dimensional gradient echo imaging (spoiled gradient recalled acquisition) was performed in the coronal plane (TR=25 msec, TE=5 msec, nutation angle=40°, field of view=24 cm, slice thickness=1.5 mm, number of excitations=1, matrix size=256×192) in order to obtain 124 images covering the entire brain. A double echo-spin sequence was used to obtain a T2 and proton density image in the axial plane to screen for any possible neuroradiological abnormalities.
All anatomical measurements were conducted on a Dell PC workstation (Dell Computers, Austin, Tex.) using the semiautomated software Scion Image Beta-4.0.2 for Windows (Scion Corporation, Inc., Frederick, Md.). A trained evaluator, blind to group assignment and to subjects’ identity, obtained volumetric measurements of the cingulate cortex, following the tracing method used by Noga et al. (25). The interrater reliability (intraclass correlation coefficient [ICC]), established by two independent evaluators tracing 10 training scans, was high (intracranial volume: r=0.94; left anterior cingulate: r=0.97; left posterior cingulate: r=0.96; right anterior cingulate: r=0.95; right posterior cingulate: r=0.91). The volumes of these brain structures were expressed in cm3.
Anatomical Landmarks: Cingulate Cortex Measurements
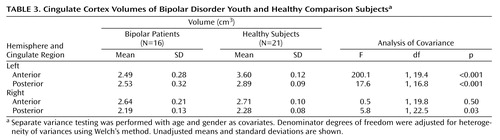
The cingulate was divided into four sections, anterior and posterior, right and left. We measured each section separately. All tracings of the cingulate were done in the coronal view. The first slice traced was two slices anterior to the final slice where the genu was visible. Tracing was continued until the anterior commissure was apparent, and this marked the posterior limit of the anterior cingulate. The subsequent slice marked the anterior border of the posterior cingulate. The final slice measured was the one where the cerebral aqueduct appeared within the pons (Figure 1). Cingulate cortex tracing was done for gray matter only.
Statistical Analysis
Data were analyzed by using factorial analysis of covariance (ANCOVA), with factors corresponding to diagnosis (two levels representing bipolar disorder and healthy groups) and gender (two levels representing male and female subjects) and with age and intracranial volume as continuous covariates. Normality and homogeneity of variances assumptions were assessed by using residuals analysis. To explore the possible effects of comorbidity, we repeated the ANCOVA after excluding the five subjects with comorbid ADHD. Group variances were inversely proportional to means and were not stabilized by transformations attempted. Therefore, separate variance testing was performed. Testing of pairwise comparisons was performed by using separate variance post hoc F tests with Bonferroni correction. Asymmetry of cingulate cortex volumes within left and right hemispheres was analyzed by adding a repeated measures factor to the model having two levels (corresponding to left and right hemispheres), with age, gender, and intracranial volume as covariates. All null hypotheses were rejected at the 0.05 level of significance using two-sided testing. Data are reported as unadjusted means plus or minus one standard deviation.
All analyses were conducted using SYSTAT (SPSS Science, Chicago) software, version 8.
Results
The patients with bipolar disorder did not differ significantly from the healthy subjects in terms of age, gender, race, or education (Table 2).
The bipolar group had significantly smaller mean gray matter volumes relative to the healthy comparison group for all regions studied except the right anterior cingulate (Table 3). Analysis without the five ADHD subjects showed the same results, with significantly smaller mean cingulate volumes in the bipolar compared with the healthy group for left anterior cingulate (F=223.4, df=1, 27, p<0.001), left posterior cingulate (F=40.8, df=1, 27, p<0.001), and right posterior cingulate (F=7.1, df=1, 27, p<0.02), but not the right anterior cingulate (F=0.03, df=1, 27, p<0.88). The main effect for subject gender was not statistically significant for any region. The diagnosis-by-gender interaction was significant only in the case of the left posterior region (F=5.6, df=1, 31, p<0.03). Pairwise testing revealed that healthy male subjects (mean=2.90, SD=0.09) and healthy female subjects (mean=2.87, SD=0.08) had larger posterior cingulate cortices than female bipolar disorder patients (mean=2.36, SD=0.32) (F=17.5, df=1, 7.8, p<0.02).
Both anterior and posterior cingulate cortices exhibited hemispheric asymmetry, which varied as a function of diagnostic group and gender. For the posterior cingulate cortex, significantly larger volumes in the left relative to the right hemisphere were seen in both the bipolar disorder patients (F=39.5, df=1, 31, p<0.001) and healthy comparison subjects (F=155.7, df=1, 31, p<0.001) (Table 3, Figure 2). A significant group-by-hemisphere interaction demonstrated that the interhemispheric asymmetry was larger in the healthy comparison group (F=12.6, df=1, 31, p<0.001). Similar findings were observed with respect to the anterior cingulate cortex (Table 3, Figure 3). Healthy subjects had significantly larger anterior cingulate cortex volumes in the left compared to the right hemisphere (F=357.5, df=1, 31, p<0.001). In contrast, patients with bipolar disorder had larger right hemisphere anterior cingulate volumes (F=8.3, df=1, 31, p<0.02). A significant group-by-hemisphere interaction (F=235.0, df=1, 33, p<0.001) demonstrated a larger interhemispheric asymmetry in the healthy comparison group.
Interhemispheric asymmetry of the posterior cingulate cortex also varied as a function of subject gender. While significantly larger left relative to right posterior cingulate volumes were seen in both male (F=105.7, df=1, 31, p<0.001) and female (F=41.9, df=1, 31, p<0.001) subjects, the interhemispheric difference was significantly larger in the case of male subjects (mean=0.57, SD=0.17) than female subjects (mean=0.41, SD=0.32), as demonstrated by a significant gender-by-hemisphere interaction (F=4.3, df=1, 31, p<0.05).
Discussion
We observed significantly smaller left anterior, left posterior, and right posterior cingulate cortices in children and adolescents with bipolar disorder relative to healthy comparison subjects. Right anterior cingulate volumes were not significantly different between the bipolar and healthy groups. These findings are in agreement with a previous study conducted in adult patients with bipolar disorder (10) that used a similar method to ours (slice thickness, MRI procedures), but they are not in line with two earlier structural MRI studies that did not find significant differences in cingulate cortex volume between adult patients with bipolar disorder and healthy subjects (6, 7). One of these negative studies examined adult first-episode mania patients (N=17) and used 6-mm thick coronal slices (6). In the other study (7), the cingulate was not divided into two regions of interests but instead measured as a single anterior cingulate volume.
Although postmortem studies have reported decreased cell density or size in the anterior cingulate in bipolar disorder, there is no general agreement on cell type or cortical layer affected. Bouras et al. (22) reported significant decreases in neural densities and laminar thickness in layers III, V, and VI in the subgenual part of Brodmann’s area 24 in adult patients with bipolar disorder, an area partially encompassed by our anterior cingulate tracing. Ongur et al. (26) also reported reduced number of glial cells in this area in familial mood disorders. Benes and colleagues (21) found a significant decrease in the density of nonpyramidal neurons of the subgenual region of Brodmann’s area 24 only in layer II, postulating that either an early disturbance to the neural migration into layer II, or an excitotoxic injury during late adolescence could explain this finding. Our findings of smaller left anterior, left posterior, and right posterior cingulate cortices in children and adolescents with bipolar disorder are in line with these results.
Another interesting finding of our study is that patients with bipolar disorder showed less interhemispheric asymmetry in both the anterior and posterior cingulate cortices relative to healthy comparison subjects. Reversals, reductions, or absence of normal cerebral asymmetries have been reported in patients suffering from schizophrenia, involving several structures such as occipital and frontal lobes (27) and the cerebral ventricles (28). Yucel et al. (29) reported a lack of leftward paracingulate sulcus asymmetry among right-handed men with schizophrenia relative to healthy comparison subjects. This finding was replicated in another study where a lack of paracingulate sulcus asymmetry was noted among male patients with early-onset schizophrenia, and this was due to both the less frequent leftward asymmetry and the more frequent rightward asymmetry of paracingulate sulcus patterns (30). The presence of a prominent paracingulate sulcus could indicate a marked local connectivity within the paralimbic cortex (Brodmann’s areas 32, 6, 8 and 9), and a reduction in the paracingulate sulcus could be the consequence of weaker local connectivity in these areas. The reduction in normal hemispheric asymmetries may mark a neurodevelopmental risk factor for major mental illnesses and support the psychotic “continuum” hypothesis (31).
In support of this psychotic “continuum” that would include schizophrenia, schizoaffective disorder, and both unipolar and bipolar affective psychoses, the cingulate cortex is shown to play an important role in the pathophysiology of schizophrenia (20, 21). Three anatomical MRI studies examining schizophrenia patients have shown bilateral (32), left (25), and right-sided (33) reduction in anterior cingulate volumes, while three others did not show significant abnormalities (34–36).
In interpreting these abnormalities, some particular limitations should be considered. Our study group size was relatively small and mostly comprised patients who were being treated with mood stabilizers (lithium or valproate), antidepressants, and antipsychotics. Thus, we were not able to conclusively ascertain whether smaller cingulate volumes might be related to illness duration, severity, or medication effects. Sassi et al. (10) suggested that lithium treatment might influence gray matter volumes in patients with bipolar disorder, either by preventing a disease-related atrophy or by reversing some preexisting abnormalities. However, we were not able to do further analysis of the effects of lithium in our present study, since very few patients were untreated or receiving lithium monotherapy. Nonetheless, in our prior study with adult patients with bipolar disorder (10), our group demonstrated that cingulate gray matter abnormalities were present in untreated bipolar individuals, suggesting that such changes are not due to medication effects. Another limitation of our study is the presence of comorbid psychiatric conditions among our bipolar subjects, particularly attention deficit hyperactivity disorder (ADHD). These conditions limit our ability to draw specific conclusions concerning the association of the observed cingulate abnormalities and bipolar disorder. However, exclusion of subjects with comorbid ADHD did not materially alter the results. With regard to gender differences, the results reported here relied on small samples and should be considered tentative.
It is clear from a wealth of neuropsychological, neuroanatomical, and functional imaging data that abnormalities in the anterior cingulate play a role in pathophysiology of mood disorders (37). This is also consistent with the increasingly sophisticated models of psychopathology that place the cingulate at the interface of emotion, cognition, drive, and motor control (38–40). Abnormalities of the anterior cingulate were also reported in obsessive-compulsive disorder (41), autism (42), and posttraumatic stress disorder (43).
In conclusion, we found decreased cingulate volumes in children and adolescents with bipolar disorder. Because such changes are already present early in illness course, they could possibly be related to abnormalities in the neurodevelopment of structures that play a key role in the pathophysiology of this illness. Alternatively, they may be more characteristic of the early-onset, mostly familial bipolar group included in this study, which probably constitutes a more biologically loaded subgroup. Longitudinal studies with larger numbers of patients are needed to understand the time course of cingulate abnormalities in bipolar disorder, their relationship with illness symptoms and medication response, and the specificity of such findings for the mechanisms potentially involved in bipolar mood disorders.
 |
 |
 |
Presented in part at the 58th annual meeting of the Society of Biological Psychiatry, San Francisco, May 15–17, 2003. Received Sept. 30, 2003; revision received Oct. 21, 2004; accepted Oct. 29, 2004. From the Department of Psychiatry (Division of Mood and Anxiety Disorders) and the Department of Radiology, The University of Texas Health Science Center at San Antonio; the South Texas Veterans Health Care System, Audie L. Murphy Division, San Antonio; the Department of Psychiatry, Dokuz Eylul University School of Medicine, Izmir, Turkey; the Department of Psychiatry, Western Psychiatric Institute and Clinic, University of Pittsburgh Medical Center, Pittsburgh; the Department of Psychiatry, Institute of Psychiatry, University of Sao Paulo School of Medicine, Sao Paulo, Brazil; and the Department of Pathology and Experimental and Clinical Medicine, Section of Psychiatry, University of Udine, Udine, Italy. Address correspondence and reprint requests to Dr. Soares, Department of Psychiatry, Division of Mood and Anxiety Disorders, The University of Texas Health Science Center at San Antonio, 7703 Floyd Curl Dr., San Antonio, TX 78229; [email protected] (e-mail). Supported in part by NIMH grants MH-01736, MH-55123, MH-30915, and MH-59929 and by the National Alliance for Research on Schizophrenia and Depression. Dr. Sassi was supported by a scholarship from the CAPES Foundation (Brazil).
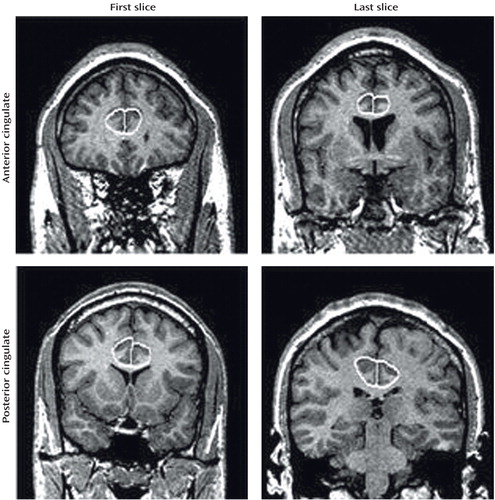
Figure 1. Anatomical Landmarks for the Cingulate Tracinga
aLeft and right sides, traced separately, are shown together here for illustration purposes only.
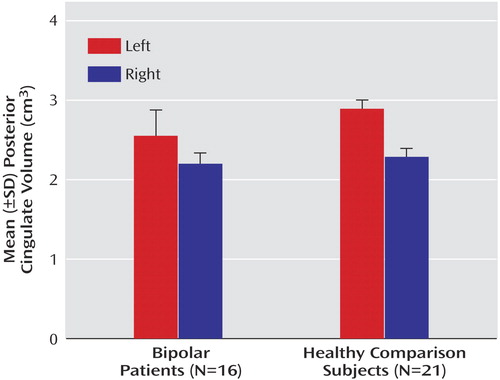
Figure 2. Posterior Cingulate Volume in Bipolar Disorder Youth and Healthy Comparison Subjects
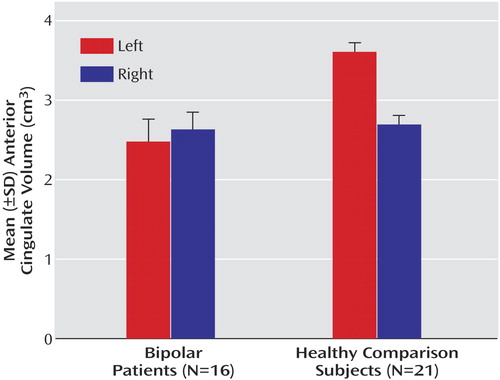
Figure 3. Anterior Cingulate Volume in Bipolar Disorder Youth and Healthy Comparison Subjects
1. Soares JC, Mann JJ: The functional neuroanatomy of mood disorders. J Psychiatr Res 1997; 31:393–432Crossref, Medline, Google Scholar
2. Vogt BA, Vogt LJ, Nimchinsky EA, Hof PR: Primate cingulate cortex chemoarchitecture and its disruption in Alzheimer’s disease, in Handbook of Chemical Neuroanatomy: The Primate Nervous System. Edited by Bloom FE, Bjorklund A, Hokfelt T. New York, Elsevier, 1997, pp 453–488Google Scholar
3. Olds J: A preliminary mapping of electrical reinforcing effects in the rat brain. J Comp Physiol Psychol 1956; 59:281–283Crossref, Google Scholar
4. Vogt BA, Nimchinsky EA, Vogt LJ, Hof PR: Human cingulate cortex: surface features, flat maps, and cytoarchitecture. J Comp Neurol 1995; 359:490–506Crossref, Medline, Google Scholar
5. Soares JC, Mann JJ: The anatomy of mood disorders—review of structural neuroimaging studies. Biol Psychiatry 1997; 41:86–106Crossref, Medline, Google Scholar
6. Strakowski SM, Wilson DR, Tohen M, Woods BT, Douglass AW, Stoll AL: Structural brain abnormalities in first-episode mania. Biol Psychiatry 1993; 33:602–609Crossref, Medline, Google Scholar
7. Lopez-Larson MP, DelBello MP, Zimmerman ME, Schwiers ML, Strakowski SM: Regional prefrontal gray and white matter abnormalities in bipolar disorder. Biol Psychiatry 2002; 52:93–100Crossref, Medline, Google Scholar
8. Drevets WC, Price JL, Simpson JR Jr, Todd RD, Reich T, Vannier M, Raichle ME: Subgenual prefrontal cortex abnormalities in mood disorders. Nature 1997; 386:824–827Crossref, Medline, Google Scholar
9. Hirayasu Y, Shenton ME, Salisbury DF, Kwon JS, Wible CG, Fischer IA, Yurgelun-Todd D, Zarate C, Kikinis R, Jolesz FA, McCarley RW: Subgenual cingulate cortex volume in first-episode psychosis. Am J Psychiatry 1999; 156:1091–1093Abstract, Google Scholar
10. Sassi RB, Brambilla P, Nicoletti M, Malinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC: Lithium influences the volume of the cingulate cortex in bipolar mood disorder patients (abstract). Biol Psychiatry 2002; 51(April suppl)Google Scholar
11. Lenox RH, Hahn C-G: Overview of the mechanism of action of lithium in the brain: fifty-year update. J Clin Psychiatry 2000; 61(suppl 9):5–15Google Scholar
12. Manji HK, Moore GJ, Chen G: Clinical and preclinical evidence for the neurotrophic effects of mood stabilizers: implications for the pathophysiology and treatment of manic-depressive illness. Biol Psychiatry 2000; 48:740–754Crossref, Medline, Google Scholar
13. Davanzo P, Thomas MA, Yue K, Oshiro T, Belin T, Strober M, McCracken J: Decreased anterior cingulate myo-inositol/creatine spectroscopy resonance with lithium treatment in children with bipolar disorder. Neuropsychopharmacology 2001; 24:359–369Crossref, Medline, Google Scholar
14. Ito H, Kawashima R, Awata S, Ono S, Sato K, Goto R, Koyama M, Sato M, Fukuda H: Hypoperfusion in the limbic system and prefrontal cortex in depression: SPECT with anatomic standardization technique. J Nucl Med 1996; 37:410–414Medline, Google Scholar
15. Vasile RG, Sachs G, Anderson JL, Lafer B, Matthews E, Hill T: Changes in regional cerebral blood flow following light treatment for seasonal affective disorder: responders versus nonresponders. Biol Psychiatry 1997; 42:1000–1005Crossref, Medline, Google Scholar
16. George MS, Ketter TA, Post RM: SPECT and PET imaging in mood disorders. J Clin Psychiatry 1993; 54(suppl l):6–13Google Scholar
17. Kennedy SH, Javanmard M, Vaccarino FJ: A review of functional neuroimaging in mood disorders: positron emission tomography and depression. Can J Psychiatry 1997; 42:467–475Crossref, Medline, Google Scholar
18. Blumberg HP, Stern E, Martinez D, Ricketts S, de Asis J, White T, Epstein J, McBride PA, Eidelberg D, Kocsis JH, Silbersweig DA: Increased anterior cingulate and caudate activity in bipolar mania. Biol Psychiatry 2000; 48:1045–1052Crossref, Medline, Google Scholar
19. Rubinsztein JS, Fletcher PC, Rogers RD, Ho LW, Aigbirhio FI, Paykel ES, Robbins TW, Sahakian BJ: Decision-making in mania: a PET study. Brain 2001; 124(part 12):2550–2563Google Scholar
20. Eastwood SL, Harrison PJ: Synaptic pathology in the anterior cingulate cortex in schizophrenia and mood disorders: a review and a Western blot study of synaptophysin, GAP-43 and the complexins. Brain Res Bull 2001; 55:569–578Crossref, Medline, Google Scholar
21. Benes FM, Vincent SL, Todtenkopf M: The density of pyramidal and nonpyramidal neurons in the anterior cingulate cortex of schizophrenic and bipolar subjects. Biol Psychiatry 2001; 50:395–406Crossref, Medline, Google Scholar
22. Bouras C, Kovari E, Hof PR, Riederer BM, Giannakopoulos P: Anterior cingulate cortex pathology in schizophrenia and bipolar disorder. Acta Neuropathol (Berl) 2001; 102:373–379Medline, Google Scholar
23. Spitzer RL, Williams JBW, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV (SCID). Washington, DC, American Psychiatric Press, 1994Google Scholar
24. Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N: Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 1997; 36:980–988Crossref, Medline, Google Scholar
25. Noga JT, Aylward E, Barta PE, Pearlson GD: Cingulate gyrus in schizophrenic patients and normal volunteers. Psychiatry Res 1995; 61:201–208Crossref, Medline, Google Scholar
26. Ongur D, Drevets WC, Price JL: Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci USA 1998; 95:13290–13295Crossref, Medline, Google Scholar
27. Bilder RM, Wu H, Bogerts B, Degreef G, Ashtari M, Alvir JM, Snyder PJ, Lieberman JA: Absence of regional hemispheric asymmetry in first-episode schizophrenia. Am J Psychiatry 1994; 151:1437–1447Link, Google Scholar
28. Crow TJ, Ball J, Bloom SR: Schizophrenia as an anomaly of development of cerebral asymmetry: a postmortem study and a proposal concerning the genetic basis of the disease. Arch Gen Psychiatry 1989; 46:1145–1150Crossref, Medline, Google Scholar
29. Yucel M, Stuart GW, Maruff P: Paracingulate morphologic difference in males with established schizophrenia: a magnetic resonance imaging morphometric study. Biol Psychiatry 2002; 52:15–23Crossref, Medline, Google Scholar
30. Le Provost JB, Bartres-Faz D, Paillere-Martinot ML, Artiges E, Pappata S, Recasens C, Perez-Gomez M, Bernardo M, Baeza I, Bayle F, Martinot JL: Paracingulate sulcus morphology in men with early-onset schizophrenia. Br J Psychiatry 2003; 182:228–232Crossref, Medline, Google Scholar
31. Bilder RM, Wu H, Bogerts B, Ashtari M, Robinson D, Woerner M, Lieberman JA, Degreef G: Cerebral volume asymmetries in schizophrenia and mood disorders: a quantitative magnetic resonance imaging study. Int J Psychophysiol 1999; 34:197–205Crossref, Medline, Google Scholar
32. Goldstein JM, Goodman JM, Seidman LJ, Kennedy DN, Makris N, Lee H, Tourville J, Caviness VS Jr, Faraone SV, Tsuang MT: Cortical abnormalities in schizophrenia identified by structural magnetic resonance imaging. Arch Gen Psychiatry 1999; 56:537–547Crossref, Medline, Google Scholar
33. Ohnuma T, Kimura M, Takahashi T, Iwamoto N, Arai H: A magnetic resonance imaging study in first-episode disorganized-type patients with schizophrenia. Psychiatry Clin Neurosci 1997; 51:9–15Crossref, Medline, Google Scholar
34. Szeszko PR, Bilder RM, Lencz T, Pollack S, Alvir JM, Ashtari M: Investigation of frontal lobe subregions in first-episode schizophrenia. Psychiatry Res 1999; 90:1–15Crossref, Medline, Google Scholar
35. Uematsu M, Kaiya H: Midsagittal cortical pathomorphology of schizophrenia: a magnetic resonance imaging study. Psychiatry Res 1989; 30:11–20Crossref, Medline, Google Scholar
36. Young AH, Blackwood DHR, Roxborough H, McQueen JK, Martin MJ, Kean D: A magnetic resonance imaging study of schizophrenia: brain structure and clinical symptoms. Br J Psychiatry 1991; 158:158–164Crossref, Medline, Google Scholar
37. Ebert D, Ebmeier KP: The role of the cingulate gyrus in depression: from functional anatomy to neurochemistry. Biol Psychiatry 1996; 39:1044–1050Crossref, Medline, Google Scholar
38. Devinsky O, Morrell MJ, Vogt BA: Contributions of anterior cingulate cortex to behaviour. Brain 1995; 118(part 1):279–306Google Scholar
39. Carter CS, Botvinick MM, Cohen JD: The contribution of the anterior cingulate cortex to executive processes in cognition. Rev Neurosci 1999; 10:49–57Crossref, Medline, Google Scholar
40. Paus T: Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci 2001; 2:417–424Crossref, Medline, Google Scholar
41. Adler CM, McDonough-Ryan P, Sax KW, Holland SK, Arndt S, Strakowski SM: fMRI of neuronal activation with symptom provocation in unmedicated patients with obsessive compulsive disorder. J Psychiatr Res 2000; 34:317–324Crossref, Medline, Google Scholar
42. Haznedar MM, Buchsbaum MS, Wei T-C, Hof PR, Cartwright C, Bienstock CA, Hollander E: Limbic circuitry in patients with autism spectrum disorders studied with positron emission tomography and magnetic resonance imaging. Am J Psychiatry 2000; 157:1994–2001Link, Google Scholar
43. Pitman RK, Shin LM, Rauch SL: Investigating the pathogenesis of posttraumatic stress disorder with neuroimaging. J Clin Psychiatry 2001; 62(suppl 17):47–54Google Scholar



