Higher Levels of Basal Serial CSF Cortisol in Combat Veterans With Posttraumatic Stress Disorder
Abstract
OBJECTIVE: Results of basal peripheral cortisol measures in posttraumatic stress disorder (PTSD) have been variable. The authors’ goal was to measure CSF cortisol concentrations, which more accurately reflect brain glucocorticoid exposure, in subjects with or without PTSD. METHOD: CSF was withdrawn from a subarachnoid catheter and plasma from a venous catheter, both indwelling, over a 6-hour interval to determine hourly plasma ACTH and cortisol concentrations and hourly CSF cortisol levels in eight well-characterized combat veterans with PTSD and eight matched healthy volunteers. RESULTS: Mean CSF cortisol concentrations were significantly higher in the subjects with PTSD (3.18 ng/ml, SD=0.33) than in the normal volunteers (2.33 ng/ml, SD=0.50), largely due to higher CSF cortisol concentration nadirs. No group differences were observed in either plasma ACTH or peripheral (plasma or urinary free) cortisol. CSF corticotropin-releasing hormone and CSF cortisol concentrations were positively and significantly correlated. CONCLUSIONS: Despite normal peripheral cortisol indexes in the veterans with PTSD, their CNS exposure to cortisol was greater than that of normal comparison subjects.
Circadian studies have found low peripheral basal cortisol concentrations in posttraumatic stress disorder (PTSD), whereas results of single time-point plasma and urinary free cortisol studies have been variable (1, 2). Given the elevation in CSF corticotropin-releasing hormone (CRH) in PTSD, even normal circulating cortisol levels could be considered low (3). Cortisol enters the brain readily from plasma, but levels in the CSF cannot be inferred directly from peripheral measures (4–7). CSF cortisol concentrations, however, have never been examined in PTSD. We used serial CSF sampling to extend the study of cortisol in PTSD into the CNS.
Method
Patients and Normal Volunteers
The study was approved by the Institutional Review Board of the University of Cincinnati Medical Center and the Research Committee of the Cincinnati Veterans Affairs Medical Center. Written informed consent was obtained from all participating subjects.
We studied 16 medication-free subjects—eight healthy men and eight men with combat-related PTSD, interviewed with the Structured Clinical Interview for DSM-III-R. We excluded current or past psychiatric disorder or current substance abuse (except tobacco) in healthy volunteers and a history of these disorders in their first-degree relatives. The mean ages of the healthy volunteers and PTSD subjects were 41.3 years (SD=8.7) and 41.0 years (SD=10.4), respectively, and the mean body mass indexes of the volunteers and patients were 25.4 kg/m2 (SD=4.0) and 29.3 kg/m2 (SD=4.2), respectively. All PTSD subjects had been exposed to severe combat trauma, and all were free of current substance abuse (except tobacco) (data on file). The patients’ mean Clinician Administered PTSD Scale and Hamilton Depression Rating Scale scores were 81.9 (SD=23.2) and 12.6 (SD=4.3), respectively.
Subjects were admitted to the Clinical Research Unit the day before the CSF withdrawal procedure, according to a standard protocol (3, 8). A 20-gauge catheter was placed in the lumbar subarachnoid space at 8:00 a.m. the morning of the withdrawal, and continuous CSF withdrawal into iced test tubes was begun approximately 3 hours after catheter placement (3, 8). Blood was withdrawn at intervals from a heparin lock.
CSF cortisol, plasma cortisol, and ACTH were assayed by radioimmunoassay (ICN Pharmaceuticals, Costa Mesa, Calif.). Hourly CSF cortisol samples were assayed, in duplicate, as described elsewhere (9). Respective intraassay and interassay coefficients of variation were 7.2% and 2.5% for cortisol and 11.0% and 6.0% for ACTH. Previous assays of urinary free cortisol and CSF CRH were reported (3).
Repeated-measures analyses were conducted according to a linear mixed model where subjects, nested within subgroups, were treated as random effects while all other covariates (time, age, body mass index) were treated as fixed effects. Pearson correlation coefficients were used in all correlation analyses, and t tests were performed on means; p values less than 0.05 were considered significant.
Results
CSF cortisol concentrations were significantly higher in the patients with PTSD (mean=3.18 ng/ml, SD=0.33) than in the normal volunteers (mean=2.33 ng/ml, SD=0.50) (Figure 1). Using repeated-measures analysis, we found a main effect for group (F=9.81, df=1, 12, p<0.01) but no significant group-by-time interaction. The coefficient of variation (standard deviation/mean) calculated for each study subject across the six time points was significantly lower for the patients with PTSD than for the healthy volunteers (F=15.37, df=1, 11, p<0.003). Additionally, a comparison of zenith versus nadir CSF cortisol concentrations over the collection period revealed a significant group (PTSD versus healthy) difference in CSF minimum cortisol levels (t=5.74, df=14, p<0.0001) but not in maximum cortisol levels (t=1.06, df=14, n.s.) (Figure 1).
Mean plasma ACTH concentrations for the patients with PTSD and the normal volunteers were 108.49 pg/ml (SD=63.01) and 81.90 ng/ml (SD=17.96), respectively; mean plasma cortisol concentrations for the patients and volunteers were 78.26 pg/ml (SD=66.44) and 67.02 ng/ml (SD=27.12), respectively. No group, time, or interaction effects for either plasma ACTH (F=1.41, df=1, n.s.) or cortisol (F=0.94, df=1, n.s.) were found, nor were the group mean 24-hour urinary free cortisol excretions significantly different (χ2=0.013, df=1, n.s.).
There was a trend for a correlation between CSF and plasma cortisol concentrations for all experimental subjects taken together (r=0.50, N=15, p<0.10). (Information on cortisol concentrations was missing for one volunteer in the correlation analysis.) The mean CSF-to-plasma cortisol ratios were 4.45% (SD=1.47%) (all subjects), 4.46% (SD=1.19%) (patients with PTSD), and 4.44% (SD=1.84%) (healthy volunteers), with no significant group differences. However, the CSF-to-urinary free cortisol ratio tended to be higher in the PTSD group (mean=3.94%, SD=2.38%, for all participants; mean=4.61%, SD=3.02%, for patients with PTSD; and mean=3.36%, SD=1.65%, for volunteers) (F=3.99, df=1, 11, p<0.08).
Neither plasma cortisol nor ACTH mean concentrations showed any significant relation to CSF CRH levels. However, mean CSF cortisol concentrations were significantly correlated with CSF CRH for all subjects (r=0.624, N=16, p<0.05) and showed similar, but nonsignificant, correlations within the PTSD group (r=0.47, N=8, n.s.) and the volunteer group (r=0.54, N=8, n.s.) (Figure 1).
Discussion
Higher basal CSF cortisol concentrations were observed in veterans with chronic PTSD than in healthy subjects, despite normal plasma cortisol and 24-hour urinary free cortisol concentrations. The higher CSF cortisol concentrations were uniformly attributable to higher CSF cortisol nadirs in the PTSD subjects over the 6-hour collection period; CSF cortisol zeniths were similar across study groups. Accordingly, intraindividual variability in the CSF cortisol concentration was diminished in the men with chronic PTSD relative to healthy volunteers.
The significant positive correlation observed between mean CSF CRH and CSF cortisol levels may reflect a bidirectional regulation of glucocorticoids and CRH.
Although the ratio of CSF to plasma cortisol was nearly identical in both groups, the ratio of CSF cortisol to urinary free cortisol was higher in the PTSD group than the healthy group. Cortisol, unbound in urine, is mostly free (<10% bound) in CSF and largely protein-bound (>90%) in plasma (4, 10). Studies show that the blood-CSF steroid interchange is complex; CSF glucocorticoid levels respond to increases in peripheral cortisol, but other, as yet poorly understood, factors independently modulate CNS cortisol levels (5, 7, 11, 12). Multidrug-resistant P-glycoprotein regulates CNS cortisol access (6). Preclinical studies demonstrating the CNS presence of precursors and enzymes for cortisol synthesis raise the possibility of de novo brain synthesis in humans (13). Therefore, the elevated ratio of CSF to urinary free cortisol excretion in the PTSD group could be attributable to 1) an increase in unbound cortisol available for transport or differences in rate of transport into the CNS, 2) a decrease in CSF cortisol clearance or metabolism, 3) increased de novo cortisol synthesis in the CNS, or 4) diminished tissue uptake of CSF cortisol by the brain.
Received Oct. 30, 2002; revisions received March 2 and May 11, 2004; accepted June 28, 2004. From the Psychiatry Service, San Diego VA Healthcare System; the Department of Psychiatry, University of California, San Diego; the Psychiatry Service, Cincinnati VA Medical Center; the Department of Psychiatry, University of Cincinnati College of Medicine; and the Department of Mathematics, University of Cincinnati. Address correspondence to Dr. Baker, Department of Psychiatry, UCSD, 9500 Gilman Drive (0603V), La Jolla, CA 92092-0603; [email protected] (e-mail). Supported in part by VA Central Office research funds (Drs. Geracioti and Baker). The authors thank Charles W. Wilkinson, Ph.D. (Seattle VA Medical Center), for technical assistance.
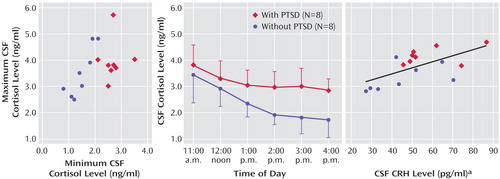
Figure 1. Maximum CSF Concentrations, Serial CSF Cortisol Concentrations, and Correlation Between Mean CSF Corticotropin-Releasing Hormone (CRH) and Mean CSF Cortisol Concentrations in Patients With PTSD and Normal Volunteersa
aWithdrawal of serial concentrations began after 15 hours of fasting and 3 hours after subarachnoid catheter placement; CSF was continuously withdrawn and aliquoted at 1-hour intervals from 11:20 a.m. to 4:20 p.m. In the plot of serial concentrations, each point represents the mean.
1. Yehuda R, Teicher MH, Trestman RL, Levengood RA, Siever LJ: Cortisol regulation in posttraumatic stress disorder and major depression: a chronobiological analysis. Biol Psychiatry 1996; 40:79–88Crossref, Medline, Google Scholar
2. Bremner JD, Vythilingam M, Anderson G, Vermetten E, McGlashan T, Heninger G, Rasmusson A, Southwick SM, Charney DS: Assessment of the hypothalamic-pituitary-adrenal axis over a 24-hour diurnal period and in response to neuroendocrine challenges in women with and without childhood sexual abuse and posttraumatic stress disorder. Biol Psychiatry 2003; 54:710–718Crossref, Medline, Google Scholar
3. Baker DG, West SA, Nicholson WE, Ekhator NN, Kasckow JW, Hill KK, Bruce AB, Orth DN, Geracioti TD Jr: Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. Am J Psychiatry 1999; 156:585–588; correction, 156:986Abstract, Google Scholar
4. Pardridge WM: Transport of protein-bound hormones into tissues in vivo. Endocr Rev 1981; 2:103–123Crossref, Medline, Google Scholar
5. Martensz ND, Herbert J, Stacey PM: Factors regulating levels of cortisol in cerebrospinal fluid of monkeys during acute and chronic hypercortisolemia. Neuroendocrinology 1983; 36:39–48Crossref, Medline, Google Scholar
6. Karssen AM, Meijer OC, van der Sandt IC, Lucassen PJ, de Lange EC, de Boer AG, de Kloet ER: Multidrug resistance P-glycoprotein hampers the access of cortisol but not of corticosterone to mouse and human brain. Endocrinology 2001; 142:2686–2694Crossref, Medline, Google Scholar
7. Herbert J, Keverne EB, Yodyingyuad U: Modulation by social status of the relationship between cerebrospinal fluid and serum cortisol levels in male talapoin monkeys. Neuroendocrinology 1986; 42:436–442Crossref, Medline, Google Scholar
8. Geracioti TD Jr, Orth DN, Ekhator NN, Blumenkopf B, Loosen PT: Serial cerebrospinal fluid corticotropin-releasing hormone concentrations in healthy and depressed humans. J Clin Endocrinol Metab 1992; 74:1325–1330Medline, Google Scholar
9. Peskind ER, Wilkinson CW, Petrie EC, Schellenberg GD, Raskind MA: Increased CSF cortisol in AD is a function of APOE genotype. Neurology 2001; 56:1094–1098Crossref, Medline, Google Scholar
10. Predine J, Brailly S, Delaporte P, Milgrom E: Protein binding of cortisol in human cerebrospinal fluid. J Clin Endocrinol Metab 1984; 58:6–11Crossref, Medline, Google Scholar
11. Murphy BE, Cosgrove JB, McIlquham MC, Pattee CJ: Adrenal corticoid levels in human cerebrospinal fluid. Can Med Assoc J 1967; 97:13–17Medline, Google Scholar
12. Walker MD, Henkin RI, Harlan AB, Casper AGT: Distribution of tritiated cortisol blood, brain, CSF and other tissues of the eviscerated cat. Endocrinology 1971; 88:224–232Crossref, Medline, Google Scholar
13. MacKenzie SM, Clark CJ, Fraser R, Gomez-Sanchez CE, Connell JM, Davies E: Expression of 11beta-hydroxylase and aldosterone synthase genes in the rat brain. J Mol Endocrinol 2000; 24:321–328Crossref, Medline, Google Scholar



