Neuropsychological Impairment and Its Neurological Correlates in Adult Offspring With Heightened Risk for Schizophrenia and Affective Psychosis
Abstract
OBJECTIVE: Schizophrenia is generally considered to be a neurodevelopmental disorder reflected in findings of neuropsychological impairments and neurological abnormality in patients and their relatives. The authors investigated whether neuropsychological impairments are related to neurological abnormality and whether such deficits also characterize risk for affective psychosis. METHOD: In a longitudinal study with a 93% rate of effective follow-up, the authors investigated neuropsychological impairment and its relation to neurological abnormality at a mean age of 22.3 years in 74 offspring of mothers with a history of psychotic disorders (38 offspring with heightened risk for schizophrenia and 36 with risk for affective psychosis) and 88 normal-risk offspring born to mothers with no history of psychosis. RESULTS: Offspring with genetically heightened risk for schizophrenia showed significantly impaired verbal memory, selective attention, and grammatical reasoning, compared with normal-risk offspring. Having impaired verbal memory, attention, and grammatical reasoning functions identified a significantly larger subgroup (16%) among offspring with heightened risk for schizophrenia than among offspring with heightened risk for affective psychosis (0%) and among normal-risk offspring (3%). Multiple neuropsychological functions were significantly related to neurological abnormality in offspring with heightened risk for schizophrenia and in normal-risk offspring but not among offspring with heightened risk for affective psychosis. The extension of schizophrenia and affective psychosis risk groups to include additional offspring of mothers with psychosis-spectrum disorders yielded results similar to those for the core risk groups. CONCLUSIONS: The neurocognitive dysfunction attending heightened risk for schizophrenia is likely based on genetically mediated neurodevelopmental factors, with schizophrenia and affective psychosis belonging to different biological spheres.
Schizophrenia is generally considered to be a disorder with neurodevelopmental roots reflected in findings of brain abnormalities, neurological abnormality, and neuropsychological impairments in both patients and their relatives (1–15). Patients with schizophrenia have a broad range of neuropsychological impairments in abstraction, executive functions, working and long-term memory, sustained and selective attention, and motor and perceptual-motor functions (e.g., references 6–8). Children and other relatives of patients with schizophrenia show an almost identical but less severe pattern of neuropsychological impairments (e.g., references 9–14). Especially, deviations in verbal memory and attention are associated with genetic risk for later development of schizophrenia-spectrum disorders (14–16). It is unclear whether these neuropsychological impairments are concentrated in a subgroup similar to the neurologically “high-scoring” subgroup (25%–50%) found among high-risk offspring of parents with schizophrenia (12, 17–19).
Some family, twin, and linkage studies suggest that schizophrenia and affective psychosis are at least to some degree genetically linked (20–22), and the relatives of patients with schizophrenia and affective psychosis have increased risk for both affective disorder and schizophrenia (23, 24). Neurological abnormalities and neuropsychological impairments are qualitatively similar in the two patient groups but quantitatively more severe in schizophrenia patients, and the offspring of schizophrenia patients have more neurological abnormalities than the offspring of mothers with affective psychosis (7, 8, 17, 25, 26). The neurodevelopmental hypothesis is not usually applied to the development of affective psychosis, and further study should be done to determine whether neuropsychological impairments are less strongly associated with risk for affective psychosis than with risk for schizophrenia, show a different pattern, characterize an especially poor-scoring subgroup, and are related to neurological abnormalities in a specific group of offspring at genetic risk for psychosis.
In this study we investigated 1) whether neuropsychological performance differs across groups of young adult offspring with heightened risk for schizophrenia or affective psychosis, relative to comparison offspring at normal risk, 2) whether verbal memory and attention are particularly impaired in offspring with heightened risk for schizophrenia, 3) whether impairment in verbal memory and attention is especially prominent in a subgroup of offspring with heightened risk for schizophrenia, 4) whether the pattern of neuropsychological impairments is the same for “core” versus extended “spectrum” groups of offspring with heightened risk for schizophrenia or affective psychosis, and 5) whether neuropsychological impairments correlate positively with neurological abnormalities in the different risk and comparison groups.
Method
Subjects
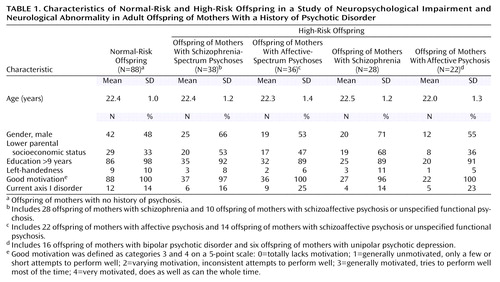
The subjects came from the adult follow-up of the prospective Swedish High-Risk Project; follow-up for this study occurred when the subjects were a mean age of 22.3 years (27). Heightened offspring risk for psychopathology was defined on the basis of a history of psychosis in the (index) mother, diagnosed by senior project diagnosticians (L. Kaij, M.D.; A. Malmquist-Larsson, M.D.) per all known psychiatric records for the woman and, where relevant, her biological relatives at entrance to the project. Normal offspring risk was defined on the basis of an absence of a psychosis history in both the (comparison) mother and biological father, determined through medical records.
Both the index and the comparison mothers were selected from among pregnant women registered at local prenatal clinics in a large geographical area in southwest Sweden during 1973–1977. Comparison mothers were chosen to have the same prenatal clinic, age, parity, social class, and formal marital status in pregnancy as the index mothers at sample selection.
Beginning in 1996, the 178 offspring (80 high-risk, 98 normal-risk) potentially participating in the project were traced through the Swedish population registers and invited to participate in the young adult follow-up. The young adults were contacted over a 4-year period, in order to standardize age at the follow-up examination. In total, 166 (93.3%) of the 178 offspring were followed up (27). Of these, 162 subjects (i.e., 74 high-risk offspring and 88 normal-risk offspring) had data on both neuropsychological function and neurological abnormalities. Four other subjects included in the general follow-up could not be assessed for neuropsychological and neurological abnormalities because of poor current mental health (one high-risk subject and one normal-risk subject), a problematic examination situation (one normal-risk subject), or recent death (one normal-risk subject).
The total high-risk group with neuropsychological and neurological abnormality data consisted of 28 offspring of mothers with schizophrenia (schizophrenia core group), 22 offspring of mothers with affective psychosis (affective core group, with 16 offspring of mothers with bipolar disorder and six offspring of mothers with unipolar depression), 15 offspring of mothers with schizoaffective psychosis, and nine offspring of mothers with unspecified functional psychosis, all defined by Research Diagnostic Criteria (28).
Because of the small subgroup sizes, the 24 offspring in the schizoaffective and unspecified psychosis risk groups were individually reassigned to “mainly schizophrenic” (N=10) or “mainly affective” (N=14) maternal disorder groups, based on mother’s psychiatric history (17, 27). This reclassification was used to establish extended groups of 38 offspring of mothers with schizophrenia-spectrum psychosis and 36 offspring of mothers with affective-spectrum psychosis. The extension of the core schizophrenia and affective risk groups to include additional subjects with maternal psychosis-spectrum disorders had previously shown little effect on the rate and pattern of mental disturbance or neurological abnormality in the respective risk groups in our study (17, 28), indicating that the factors influencing mental health and neurological abnormality are equally present in the additional psychosis-spectrum subjects as in the “core” risk groups. The schizophrenia and affective spectra were selected as the primary diagnostic groupings for the present data analyses (with the core schizophrenia and affective diagnostic groups studied secondarily). The reason for this choice was that the spectrum approach increased group size and statistical power and tended to generally promote similarity in offspring background characteristics across the risk and comparison groups (Table 1).
After complete description of the study to the subjects, written informed consent was obtained.
Neuropsychological Investigation
The subjects were followed up during a full day of assessment at their local general practitioners’ offices, which provided a standardized and neutral environment in each subject’s own geographical area. The standardized procedure during the morning session included the neuropsychological testing and the neurological examination.
The selection of the neuropsychological tests and their particular scores for these analyses was based on 1) the goal of exploring a wide range of neuropsychological and cognitive functions (rather than representing a complete neuropsychological investigation), 2) previous evidence of impaired performance among schizophrenia patients and their relatives (6–14), and 3) avoidance of redundant scores (i.e., different scores showing a Spearman correlation (rs) >0.50 in the total current study group) for the same neuropsychological test (such redundancy concerned different scores for the Wisconsin Card Sorting Test, for the selective attention test, and for the grammatical reasoning test).
The following tests/scores were selected for use in the current analyses: 1) the word-pair test (number of word pairs that the subject could recall immediately and after a 1-hour delay) (29); 2) Trail Making Test A and B (number of seconds to completion) (30); 3) digit span test (number of correctly reproduced digits) both forward and backward (31); 4) verbal fluency test (number of words reported) (32); 5) Wisconsin Card Sorting Test (percent errors and percent preservative responses) (33); 6) block design test (points for correct design and speed to completion) (31); 7) finger-tapping test, alternating right/left hand, implemented on a microcomputer (number of taps per second) (34); 8) reaction time test (simple visual reaction time, mean reaction time in milliseconds) (34); 9) selective attention test (correct hit or rejection of the letter K among other letters (34), scored as compound hits, correct hits, and errors); and 10) grammatical reasoning test (34) assessing the speed and accuracy of evaluating logical statements (scored as level of logical difficulty, correct hits, correct rejections of the statements, and errors).
One physician (E.W.S.), who was trained by an experienced psychologist, examined all subjects on both the neuropsychological and the neurological examinations. During data collection and analysis, the examiner was blind to the subject’s high-risk versus normal-risk status and all previous project data. A high degree of motivation was observed in all subject groups during testing (Table 1).
Neurological Examination
The neurological examination was based on a comprehensive, standardized assessment scale (5, 17), previously used by us to study adult schizophrenia patients and their siblings (5). The neurological abnormality scale comprises investigation of motor coordination, muscle power, muscle tone, sensory functions, reflexes, and cognitive functions. The total neurological abnormality score was the sum of the scores on 44 items (potentially ranging from 0 to 124).
The interrater reliability for this neurological assessment was determined by testing agreement on the total scores with an experienced physician (5, 17) (B. Ismail, M.D.) for 20 subjects (10 patients with psychosis and 10 hospital personnel). The interrater coefficient (intraclass correlation) was 0.97 (F=65.44, df=9, 10, p<0.001).
Statistical Methods
Because of the score distributions, nonparametric tests were used for primary comparison of the groups and investigation of correlation between neuropsychological impairments and neurological abnormalities. The Kruskal-Wallis test was used to compare schizophrenia-spectrum psychosis, affective-spectrum psychosis, and normal-risk offspring groups on quantitative scores for the neuropsychological tests (Table 2). When Kruskal-Wallis analysis showed a significant or nearly significant across-group difference, secondary comparisons of pairs among these three groups were done by using Mann-Whitney tests.
Based on our previous research (17, 27), a score beyond the 90th percentile in the “poor-scoring” direction on the neuropsychological tests for the normal-risk subjects was operationally defined as “poor scoring” for all subjects and was used for classification of performance with respect to confounders and subgroups.
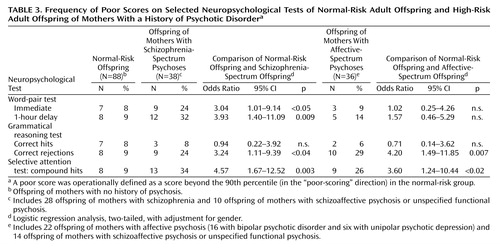
Five neuropsychological test scores (Table 3) were selected for investigating the effect of possible confounders on the significant across-group differences. The five neuropsychological tests that were chosen showed not only a significant across-group difference on the Kruskal-Wallis test but also the possibility of estimating an appropriate 90th percentile cutoff level. (The latter criterion was not fulfilled for the grammatical reasoning test’s level of logical difficulty score because of the score distribution.)
Three subject characteristics (gender, parental socioeconomic status, and current axis I disorder) were identified as potential confounders on the basis of the across-group differences in offspring characteristics shown in Table 1. The method suggested by Greenland (35) was used to define confounders for multivariate logistic regression analysis. Starting with the univariate model, variables were entered into bivariate and multivariate models if they changed the effect estimate by 10% or more (which was found for gender) and were excluded from the model if the effect estimates changed less than 5% (which was found for parental socioeconomic status and current axis I disorder). Logistic regression analysis adjusted for gender, with odds ratios and 95% confidence intervals (CIs), was thereafter used for analysis of the rate with which high-risk subjects scored above the 90th percentile cutoff level for the five selected neuropsychological test scores.
Based on previous findings and the current results, the same tests of verbal memory, attention, and grammatical reasoning functions (Table 3) were used further to investigate the relative size of a “poor-scoring” subgroup among high-risk (compared with normal-risk) subjects. The relative frequency of subjects showing “poor-scoring” on one, two, or all three of these functions was compared across the study groups by using Fisher’s exact test, with odds ratios and 95% CIs.
Spearman’s rank-order correlations were used to examine the relation between neuropsychological and neurological abnormalities within the schizophrenia-spectrum psychosis, affective-spectrum psychosis, and normal-risk offspring groups, separately.
Statistical significance was defined as p≤0.05, two-tailed, with 0.10≥p>0.05 denoting results that approached significance.
The neuropsychological performance for the core schizophrenia-risk and affective-risk groups was evaluated visually rather than submitted to formal statistical analysis.
Results
Neuropsychological Performance
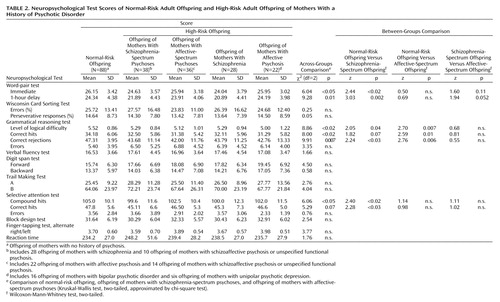
Significant differences or results approaching significance were found across schizophrenia-spectrum psychosis, affective-spectrum psychosis, and normal-risk offspring groups for the word-pair test—immediate recall and 1-hour delayed recall, grammatical reasoning test—level of logical difficulty, correct hits, and correct rejections, and selective attention test—compound hits and correct hits (Table 2 and Table 3). Secondary comparisons among these groups showed that the schizophrenia-spectrum offspring performed significantly more poorly than normal-risk offspring on the word-pair test—immediate recall and 1-hour delayed recall, grammatical reasoning test—level of logical difficulty and correct rejections, and selective attention test—compound hits and correct hits. The results for the grammatical reasoning test—correct hits approached significance. Affective-spectrum offspring performed significantly more poorly than normal-risk offspring on the grammatical reasoning test—level of logical difficulty, correct hits, and correct rejections. Comparison of the two risk groups indicated that schizophrenia-spectrum offspring had poorer performance than affective-spectrum offspring on the word-pair test—immediate recall and 1-hour delayed recall, but the difference only approached significance.
The patterns of neuropsychological function in the core schizophrenia offspring and affective psychosis offspring groups were very similar to those for the extended schizophrenia-spectrum psychosis and affective-spectrum psychosis groups.
The group differences remained after adjustment for gender. Significantly more schizophrenia-spectrum offspring than normal-risk offspring scored above the normal-risk 90th percentile cutoff level for word-pair—immediate recall and 1-hour delayed recall, grammatical reasoning test—correct rejections, and selective attention test—compound hits (Table 3). With adjustment for gender, significantly more affective-spectrum than normal-risk offspring scored above the 90th percentile cutoff for grammatical reasoning test correct rejections and for selective attention test compound hits.
Neuropsychological Poor-Scoring Subgroup
A neuropsychological poor-scoring subgroup was operationally defined by the proportion of individuals within each study group who scored above the 90th percentile cutoff on the verbal memory (word-pair test), attention (selective attention test), and grammatical reasoning scores shown in Table 3. Summarizing across these neuropsychological functions, significantly more schizophrenia-spectrum offspring (than normal-risk offspring) scored above this cutoff on one or more of these neuropsychological functions (Step A), on two or more of the functions (Step B), and on all three functions (Step C) (Table 4).
In contrast, significantly more affective-spectrum offspring (than normal-risk offspring) scored above the 90th percentile cutoff only on one or more of these functions (Step A) but not on two or more or on all three functions (Steps B and C).
Directly comparing the two risk groups, schizophrenia-spectrum offspring more frequently scored above the 90th percentile than affective-spectrum offspring on all three functions (Step C) (p<0.03, Fisher’s exact test; odds ratio=14.6, 95% CI=0.79–269.5) but not on two or more functions (Step B) (p=0.25, Fisher’s exact test; odds ratio=2.21, 95% CI=0.67–7.27) or one or more functions (Step A) (p=1.00, Fisher’s exact test; odds ratio=1.00, 95% CI=0.40–2.49).
Furthermore, the schizophrenia-spectrum offspring showed increasing differences (odds ratios) from normal-risk offspring when progressing from Step A to Step C. In contrast, affective-spectrum offspring did not differ from normal-risk offspring at Step B and Step C, and the odds ratios for those comparisons decreased moving from Step A to Step C.
Neuropsychological Function and Neurological Abnormality
In the schizophrenia-spectrum group, the total neurological abnormality score at a mean age of 22.3 years was significantly correlated with scores for word-pair test—immediate recall and 1-hour delayed recall, grammatical reasoning test—correct rejections, verbal fluency, digit span—forward and backward, block design test, and finger-tapping test (Table 5). In the normal-risk group, the total neurological abnormality score was significantly correlated with scores for the word-pair test—immediate recall and 1-hour delayed recall; Wisconsin Card Sorting Test—percent errors and percent perseverative responses; grammatical reasoning test—level of logical difficulty, correct hits, and correct rejections; verbal fluency; digit span—forward and backward; selective attention test—compound hits; block design test; finger-tapping test; andreaction time. All significant correlations represented a positive relationship between neuropsychological impairments and neurological abnormalities.
In contrast, no significant correlation was found between the total neurological abnormality score and any neuropsychological test results in the affective-spectrum group. However, correlations approaching significance were found between the total neurological abnormality score and scores for 1) the word-pair test—immediate recall and 1-hour delayed recall and 2) Trail Making Test A.
Discussion
In this prospective, longitudinal study, young adult offspring with genetically heightened risk for schizophrenia showed significantly impaired verbal memory, selective attention, and grammatical reasoning, compared with normal-risk offspring. Having impairment on all three functions identified a significantly larger subgroup among offspring of mothers with schizophrenia-spectrum psychosis (16%) than among offspring of mothers with affective-spectrum psychosis (0%) and normal-risk offspring of mothers with no history of psychosis (3%). Several neuropsychological test scores were significantly related to neurological abnormalities at the same age in schizophrenia-spectrum offspring and in normal-risk offspring, but not among affective-spectrum offspring. In total, these findings suggest that the neurodysfunctional correlates of risk for developing schizophrenia differ from those for developing affective psychosis.
We confirmed previous findings of verbal memory impairments in patients with schizophrenia, their children, and other relatives (6–14, 16), and also showed that verbal memory tends to discriminate offspring of mothers with schizophrenia-spectrum psychosis from offspring of mothers with affective-spectrum psychosis. In addition, verbal memory was significantly correlated with neurological abnormalities among schizophrenia-spectrum offspring. These findings are in line with the previous suggestion that verbal memory dysfunction may be a specific trait marker for liability for schizophrenia (14–16).
The grammatical reasoning test was the only neuropsychological test on which both high-risk groups scored significantly more poorly than normal-risk offspring. The grammatical reasoning test is an especially demanding and complex cognitive test that assesses sensorimotor function, visual scanning and perception, and semantic and logical processing. Both high-risk groups had fewer correct hits and achieved a lower level of difficulty, but did not have more errors, than normal-risk offspring. These findings suggest that high-risk offspring process the information in a logically correct manner but at a slower rate than normal-risk offspring. As this test involves integration of multiple neuropsychological functions as well as brain regions, the slower processing may indicate difficulty in integration of these functions or regions (2). Similarly, the schizophrenia-spectrum offspring did not make more errors but achieved fewer correct hits on the selective attention test, compared with the normal-risk offspring. This result may again indicate slower information processing rather than a specific attentional dysfunction.
The identification of a substantial subgroup (16%–50%, depending on the criterion used) (Table 4) of individuals with impairments in verbal memory, attention, and/or grammatical reasoning among the schizophrenia-spectrum offspring corresponds to the occurrence of a neurologically “high-scoring” subgroup (25%–50%) of schizophrenia offspring observed in our own and other studies of high-risk offspring in infancy, childhood, adolescence, and adulthood (12, 17–19). These repeated findings support the hypothesis of a genetically mediated disturbance in the neurodevelopmental maturation in a subgroup of individuals at presumed risk for schizophrenia.
Multiple neuropsychological test scores were significantly related to neurological abnormalities at the same age among schizophrenia-spectrum offspring and among normal-risk offspring, while no significant relation was seen among affective-spectrum offspring. The absence of such a relation among these latter offspring cannot be explained by lack of neurological abnormalities, as normal-risk offspring and affective-spectrum offspring had a similar mean level of neurological abnormalities (17). In total, these findings suggest that the neurocognitive dysfunction attending increased risk for schizophrenia is based on neurological/biological/cerebral factors (rather than, for example, deviations in motivation or personality), that schizophrenia is likely a genetically mediated neurodevelopmental disorder, and that schizophrenia and affective psychosis belong to different biological spheres.
The extension of core schizophrenia and affective psychosis risk groups to include offspring of mothers with additional psychosis-spectrum disorders had little effect on the rate and pattern of neuropsychological function in the respective risk groups. This result is in line with previous findings (17, 27) and seems to indicate that the factors influencing neuropsychological function are equally present in the psychosis-spectrum offspring and in the core group offspring.
The strengths of the study were the prospective design, the high rate of follow-up of adult offspring, the existence of different high-risk groups, the narrow age range at examination, the control of possible confounders, the use of an extensive standardized examination routine conducted by one investigator uninformed with regard to the subject’s study group, and access to other project data.
The limitations of this study include the small numbers of subjects in specific high-risk groups (yielding low statistical power and limiting the possibility for evaluating negative findings), the lack of screening of comparison subjects’ parents for any indicators of cognitive performance, and the lack of independent assessment of the neurological and neuropsychological judgments by different examiners.
In summary, 1) impairments in verbal memory, selective attention, and complex executive function; 2) a substantial poor-scoring subgroup; and 3) significant correlations between neuropsychological functions and neurological abnormalities were found among offspring with increased genetic risk for developing schizophrenia but not among offspring at risk for affective psychosis. This finding supports the position that schizophrenia is a genetically mediated neurodevelopmental disorder whose etiology at least partly differs from that of affective psychosis.
 |
 |
 |
 |
 |
Received March 12, 2004; revision received May 5, 2004; accepted May 13, 2004. From the Department of Psychiatric Epidemiology, University Hospital, Lund University. Address correspondence and reprint request to Dr. McNeil, Psychiatric Epidemiology, Barngatan 2, University Hospital, S-221 85 Lund, Sweden; [email protected] (e-mail). Supported by a grant from the Stanley Medical Research Institute, NIMH grant MH-18857, grant 3793 from the Swedish Medical Research Council, and the Medical Faculty of Lund University, Lund, Sweden.
1. Weinberger DR: Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry 1987; 44:660–669Crossref, Medline, Google Scholar
2. Andreasen NC, Paradiso S, O’Leary DS: “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull 1998; 24:203–218Crossref, Medline, Google Scholar
3. Heinrichs DW, Buchanan RW: Significance and meaning of neurological signs in schizophrenia. Am J Psychiatry 1988; 145:11–18Link, Google Scholar
4. Woods BT, Kinney DK, Yurgelun-Todd D: Neurologic abnormalities in schizophrenic patients and their families, I: comparison of schizophrenic, bipolar, and substance abuse patients and normal controls. Arch Gen Psychiatry 1986; 43:657–663Crossref, Medline, Google Scholar
5. Ismail B, Cantor-Graae E, McNeil TF: Neurological abnormalities in schizophrenic patients and their siblings. Am J Psychiatry 1998; 155:84–89Link, Google Scholar
6. Saykin AJ, Gur RC, Gur RE, Mozley PD, Mozley LH, Resnick SM, Kester DB, Stafiniak P: Neuropsychological function in schizophrenia: selective impairment in memory and learning. Arch Gen Psychiatry 1991; 48:618–624Crossref, Medline, Google Scholar
7. Seidman LJ, Kremen WS, Koren D, Faraone SV, Goldstein JM, Tsuang MT: A comparative profile analysis of neuropsychological functioning in patients with schizophrenia and bipolar psychoses. Schizophr Res 2002; 53:31–44Crossref, Medline, Google Scholar
8. Goldberg TE, Gold JM, Greenberg R, Griffin S, Schulz SC, Pickar D, Kleinman JE, Weinberger DR: Contrasts between patients with affective disorders and patients with schizophrenia on a neuropsychological test battery. Am J Psychiatry 1993; 150:1355–1362Link, Google Scholar
9. Kremen WS, Seidman LJ, Pepple JR, Lyons MJ, Tsuang MT, Faraone SV: Neuropsychological risk indicators for schizophrenia: a review of family studies. Schizophr Bull 1994; 20:103–119Crossref, Medline, Google Scholar
10. Faraone SV, Seidman LJ, Kremen WS, Pepple JR, Lyons MJ, Tsuang MT: Neuropsychological functioning among the nonpsychotic relatives of schizophrenic patients: a diagnostic efficiency analysis. J Abnorm Psychol 1995; 104:286–304Crossref, Medline, Google Scholar
11. Cannon TD, Zorrilla LE, Shtasel D, Gur RE, Gur RC, Marco EJ, Moberg P, Price RA: Neuropsychological functioning in siblings discordant for schizophrenia and healthy volunteers. Arch Gen Psychiatry 1994; 51:651–661Crossref, Medline, Google Scholar
12. Asarnow JR: Children at risk for schizophrenia: converging lines of evidence. Schizophr Bull 1988; 14:613–631Crossref, Medline, Google Scholar
13. Cosway R, Byrne M, Clafferty R, Hodges A, Grant E, Abukmeil SS, Lawrie SM, Miller P, Johnstone EC: Neuropsychological change in young people at high risk for schizophrenia: results from the first two neuropsychological assessments of the Edinburgh High Risk Study. Psychol Med 2000; 30:1111–1121Crossref, Medline, Google Scholar
14. Toomey R, Faraone SV, Seidman LJ, Kremen WS, Pepple JR, Tsuang MT: Association of neuropsychological vulnerability markers in relatives of schizophrenic patients. Schizophr Res 1998; 31:89–98Crossref, Medline, Google Scholar
15. Erlenmeyer-Kimling L: Neurobehavioral deficits in offspring of schizophrenic parents: liability indicators and predictors of illness. Am J Med Genet 2000; 97:65–71Crossref, Medline, Google Scholar
16. Erlenmeyer-Kimling L, Rock D, Roberts SA, Janal M, Kestenbaum C, Cornblatt B, Adamo UH, Gottesman II: Attention, memory, and motor skills as childhood predictors of schizophrenia-related psychoses: the New York High-Risk Project. Am J Psychiatry 2000; 157:1416–1422Link, Google Scholar
17. Schubert EW, McNeil TF: Prospective study of neurological abnormalities in offspring of women with psychosis: birth to adulthood. Am J Psychiatry 2004; 161:1030–1037Link, Google Scholar
18. Marcus J, Hans SL, Lewow E, Wilkinson L, Burack CM: Neurological findings in high-risk children: childhood assessment and 5-year followup. Schizophr Bull 1985; 11:85–100Crossref, Medline, Google Scholar
19. McNeil TF, Harty B, Blennow G, Cantor-Graae E: Neuromotor deviation in offspring of psychotic mothers: a selective developmental deficiency in two groups of children at heightened psychiatric risk? J Psychiatr Res 1993; 27:39–54Crossref, Medline, Google Scholar
20. Cardno AG, Rijsdijk FV, Sham PC, Murray RM, McGuffin P: A twin study of genetic relationships between psychotic symptoms. Am J Psychiatry 2002; 159:539–545Link, Google Scholar
21. Tsuang MT, Taylor L, Faraone SV: An overview of the genetics of psychotic mood disorders. J Psychiatr Res 2004; 38:3–15Crossref, Medline, Google Scholar
22. Berrettini WH: Are schizophrenic and bipolar disorders related? a review of family and molecular studies. Biol Psychiatry 2000; 48:531–538Crossref, Medline, Google Scholar
23. Rosenthal D: Genetic Theory and Abnormal Behavior. New York, McGraw-Hill, 1970, pp 162–168Google Scholar
24. Maier W, Hallmayer J, Minges J, Lichterman D: Morbid risks in relatives of affective, schizoaffective, and schizophrenic patients, in Affective and Schizoaffective Disorders. Edited by Marneros A, Tsuang MT. Berlin, Springer-Verlag, 1990, pp 201–207Google Scholar
25. Bearden CE, Hoffman KM, Cannon TD: The neuropsychology and neuroanatomy of bipolar affective disorder: a critical review. Bipolar Disord 2001; 3:106–153Crossref, Medline, Google Scholar
26. Quraishi S, Frangou S: Neuropsychology of bipolar disorder: a review. J Affect Disord 2002; 72:209–226Crossref, Medline, Google Scholar
27. Schubert EW, McNeil TF: Prospective study of adult mental disturbance in offspring of women with psychosis. Arch Gen Psychiatry 2003; 60:473–480Crossref, Medline, Google Scholar
28. Spitzer RL, Endicott J, Robins E: Research Diagnostic Criteria (RDC) for a Selected Group of Functional Disorders, 3rd ed. New York, New York State Psychiatric Institute, Biometrics Research, 1978Google Scholar
29. Cronholm B, Molander L: Cronholm-Molanders minnesprov (Cronholm-Molander’s Memory Test). Stockholm, Psykologiförlaget, 1959Google Scholar
30. Reitan RM: Trail Making Test: Manual for Administration, Scoring, and Interpretation. Bloomington, Indiana University Medical Center, Department of Neurology, Section of Neuropsychology, 1956Google Scholar
31. Wechsler D: Wechsler Adult Intelligence Scale—Revised (WAIS-R) Manual. San Antonio, Tex, Psychological Corp, 1981Google Scholar
32. Benton AL, Hamsher KD, Varney NR, Spreen O: Contributions to Neuropsychological Assessment. New York, Oxford University Press, 1983Google Scholar
33. Heaton RK: The Wisconsin Card Sorting Test Manual. Odessa, Fla, Psychological Assessment Resources, 1981Google Scholar
34. Levander S: An Automated Psychological Test Battery, IBM-PC version (APT-PC): Research Reports From the Department of Psychiatry and Behavioural Medicine, University of Trondheim, Vol 11, No 65. Trondheim, Norway, University of Trondheim, 1988Google Scholar
35. Greenland S: Modeling and variable selection in epidemiologic analysis. Am J Public Health 1989; 79:340–349Crossref, Medline, Google Scholar



