Prospective Study of Neurological Abnormalities in Offspring of Women With Psychosis: Birth to Adulthood
Abstract
OBJECTIVE: The authors prospectively investigated neurological abnormalities in 75 young adult offspring of mothers with psychotic disorders and 91 offspring of comparison mothers with no psychosis history. They also studied the stability of these abnormalities from birth to adulthood. METHOD: Neurological abnormalities were previously studied in infancy and at 6 years of age. In this study, they were blindly assessed with a comprehensive neurological assessment scale at a mean age of 22.4 years in a 93.3% effective follow-up of the sample. RESULTS: In relation to the comparison subjects (N=88) and offspring of mothers with affective psychosis (N=22), the adult offspring of mothers with schizophrenia (N=28) had significantly more neurological abnormalities. More soft signs, primitive reflexes, involuntary movements, and cranial nerve abnormalities characterized a subgroup (32%) among these offspring. The offspring of mothers with affective psychosis were not different from comparison subjects. The extension of schizophrenia and affective psychosis risk groups to include additional maternal “spectrum cases” (N=10 and N=14, respectively) generally yielded similar results. Neurological abnormalities at 22 years were significantly associated with neurological abnormalities at age 6, but not in infancy, among the total high-risk group, offspring of mothers with schizophrenia, and comparison offspring. CONCLUSIONS: High levels of neurological abnormalities are found in a substantial proportion of offspring of mothers with schizophrenia but not offspring of mothers with affective psychosis. This suggests that familial risk for schizophrenia is associated with neurodevelopmental disturbance that is manifest throughout life and belongs to a different biological continuum from that of affective psychosis.
Schizophrenia is generally considered to be a brain disease resulting from disturbed neurodevelopment (1). One manifestation of this maldevelopment may be the neurological abnormalities, identified on clinical neurological examination, that are so frequently observed among both patients with schizophrenia and their nonpsychotic relatives (2–6). Neurological abnormalities could represent an important early biological marker for schizophrenia risk (7), as a higher rate of neurological abnormalities early in life has been found among individuals who later develop schizophrenia (8–11), and the individual’s level of neurological abnormalities correlates positively between patients and siblings in the same family (6). However, for neurological abnormalities to function optimally as a biological marker for schizophrenia risk, we need to know the specific form of neurological abnormality related to schizophrenia risk, its distribution among high-risk and normal-risk individuals, its personal stability over different ages, and its specificity for risk for schizophrenia versus other psychoses. Current knowledge is incomplete concerning all of these topics.
Both schizophrenia patients and their relatives have thus far shown considerable diversity in form and frequency of neurological abnormalities, although the abnormalities have primarily been in the functional domains representing sensory integration, motor coordination, and sequencing of complex motor acts (2–6). This diversity is perhaps partly due to methodological differences across studies (2). Our study using a comprehensive method showed that schizophrenia patients are more differentially characterized by neurological abnormalities representing “hard signs,” while siblings of schizophrenia patients are more characterized by “soft signs” (6).
Studies of the high-risk offspring of parents with schizophrenia at different ages have detected a subgroup with an especially high frequency of neurological abnormalities and with a higher risk for later development of schizophrenia-related psychopathology (11–17). The size of the subgroup (25%–50%) has varied across studies, possibly because of differences in methods and definitions.
The extent to which high-risk individuals have stable schizophrenia-related neurological abnormalities over time also remains unclear. In the Swedish High-Risk Study, we found no personal stability of neurological abnormalities between the neonatal period and 6 years of age (13, 14). Similarly, the Jerusalem Infant Development Study (10–12) showed only modest stability between infancy and adolescence (14–21 years of age) but strong stability between school age (8–13 years) and adolescence.
It is also unclear whether neurological abnormalities related to schizophrenia are associated primarily with risk for schizophrenia or even affective psychosis. Patients with affective disorders and their relatives generally show more neurological abnormalities than healthy comparison subjects but lower rates of neurological abnormalities than patients with schizophrenia and their relatives (13–16), but results vary across both high-risk and prepatient groups (15, 17–19). This question is related to unresolved issues concerning whether schizophrenia and affective psychosis belong to the same biological continuum and what the definitional limits for each of these disorders are somatically (20).
The type, frequency, personal stability, and specificity of neurological abnormalities for offspring of women with different psychotic disorders were investigated in the Swedish High-Risk Study, which is a prospective, longitudinal investigation of high-risk offspring of women with a history of psychosis and women without such a history (21). The project was begun in 1973 during the mothers’ pregnancies, and the first adult follow-up of this sample was completed when the offspring were about 22 years of age (22). In the current study we investigated 1) whether young adult offspring of mothers with a history of schizophrenia or affective psychosis, as compared with normal-risk comparison offspring, have significantly higher levels of neurological abnormalities in total, in particular functional neurological domains, and in hard versus soft signs, 2) whether such neurological abnormalities are especially prominent among a distinct subgroup, and 3) whether the level of neurological abnormalities is personally stable from birth to adulthood. We also investigated 4) whether extending the groups of mothers with “core” schizophrenia and affective psychosis to include those with broader, “spectrum-type” psychoses affects the offspring’s pattern of neurological abnormalities in adulthood.
Method
Subjects
The current sample was taken from the first adult follow-up (93% effective) of this longitudinal study at a mean age of 22.37 years (SD=1.12) (22). Heightened offspring risk for psychopathology was defined as a history of psychosis in the (index) mother, diagnosed by senior project diagnosticians on the basis of all known psychiatric records for the woman and, where relevant, her biological relatives. Normal offspring risk was defined as the absence of a psychosis history in both the (comparison) mother and biological father, determined through medical records. Further, the index and comparison mothers were systematically interviewed (by T.F.M.) during pregnancy concerning psychiatric symptoms (23).
Both the index and comparison mothers were selected from women registered at the local prenatal clinics in a large geographical area in southwest Sweden during 1973–1977. Comparison subjects were chosen to have the same prenatal clinic, maternal age (±1 year in 75% of the cases, ±2 years in the remaining cases), parity (0 versus 1 versus ≥2), social class (upper, middle, lower), and formal marital status during pregnancy as the index subjects. The offspring and their environment were studied extensively from before birth until 2 years of age (24), with follow-up and investigation at 6 years of age (25).
Beginning in 1996, the 178 offspring (80 with high risk, 98 with normal risk) potentially participating in the project were traced through the Swedish population registers and invited to participate in the young adult follow-up. The sample was contacted over a 4-year period, in order to standardize the age at this follow-up examination. Contact was made first by letter, which was followed by a telephone call. In total, 166 (93.3%) of the 178 offspring were followed up (Table 1) (22). The 162 subjects with data on neurological abnormalities comprised 74 high-risk offspring and 88 normal-risk offspring of comparison mothers. Four other subjects included in the general follow-up could not be assessed neurologically because of poor current mental health (one high-risk, one normal-risk offspring), problematic examination situation (one normal-risk offspring), or recent death (one normal-risk offspring).
The final group of high-risk subjects with data on neurological abnormalities consisted of 28 offspring of mothers with schizophrenia, 22 offspring of mothers with affective psychosis (16 mothers with bipolar disorder and six with unipolar depression), 15 offspring of mothers with schizoaffective psychosis, and nine offspring of mothers with unspecified functional psychosis, all defined by Research Diagnostic Criteria (26).
Because of the small subgroup sizes, the offspring of mothers with schizoaffective psychosis and offspring of mothers with unspecified functional psychosis were not analyzed separately. Instead they were individually reassigned to “mainly schizophrenic” (N=10) or “mainly affective” (N=14) maternal disorder groups, on the basis of the mother’s psychiatric history (22). This reclassification was used to investigate the effect of extending the groups of mothers with schizophrenia and affective psychosis to include additional “spectrum” cases.
After complete description of the study to the subjects, written informed consent was obtained.
Neurological Examination in Adulthood
The subjects were followed up during a full day of assessment at their local general practitioners’ offices. The standardized procedure during the morning session included a neurological examination (22) based on a comprehensive, standardized assessment scale (6) previously used by us to study adult schizophrenia patients and their siblings. The scale consists of 44 items (26 items permitted bilateral assessment) and comprises 21 items from Woods et al. (4), all 19 items from an instrument of Rossi et al. (5), two items (right-left confusion and finger-thumb opposition) from Quitkin et al. (3), and two items (finger-nose test, crossing body midline) from a study by us (14). Neurological soft signs were operationally defined as the items used by Rossi et al. (5), while the remaining items were defined as hard signs (6). The neurological assessment scale includes the following functional domains and items:
Motor functions
Motor functions were represented by the following subdomains: 1) motor coordination (hard signs: finger-nose test, finger-thumb opposition, crossing body midline, Romberg test, gait deviation, whole body clumsiness, dysarthria; soft signs: dysdiadochokinesia, complex motor acts, tapping rhythm), 2) involuntary movements (hard signs: choreiform movements, athetotic movements, intention tremor, postural tremor, resting tremor), 3) mirror movements (hard signs: mirror movements on finger-thumb opposition and on diadochokinesia), 4) deviant muscle power (hard sign: unilateral weakness), 5) deviant muscle tone (hard signs: unilateral cogwheel rigidity, spastic rigidity, hypotonia, pes cavus), and 6) cranial nerve deviations (hard sign: unilateral facial weakness; soft signs: ocular vergence, gaze nystagmus, oral apraxia). (Muscle power and muscle tone were included in the total deviation score but not in the statistical analysis of specific subdomains, because of the low frequency of abnormality in the total sample.)
Sensory functions
Sensory functions were represented by one hard sign, unilateral sensory loss, and four soft signs: astereognosis, graphesthesia, blunt/sharp discrimination, and simultaneous bilateral extinction.
Reflexes
Reflexes were represented by one hard sign, unilateral reflex hyperactivity, and the following soft signs: snout reflex, suck reflex, blink reflex, palmomental test, grasp reflex, and Babinski reflex.
Cognitive functions
Cognitive functions were represented by two hard signs, right-left confusion regarding self and regarding examiner, and four soft signs: imaginary acts, the two-object test, motor perseveration, and gaze impersistence.
Scoring
The total score for neurological abnormalities was the sum of the scores on all 44 items (potentially ranging from 0 to 124), while the score for each domain or subdomain was the sum of the scores on the respective items. On the basis of previous research (14, 22), a score on the total scale that was above the 90th percentile for the normal-risk offspring (i.e., a score higher than 7) was operationally defined as “high” and was used to investigate the possible existence and relative size of a high-scoring subgroup among the high-risk subjects.
One physician (E.W.S.) examined all subjects. During data collection and analysis the examiner was blind to the subject’s high-risk versus normal-risk offspring status and all previous project data. The interrater reliability for the neurological assessment was determined by testing agreement with an experienced physician (6) on the total scores of 20 subjects (10 patients with psychosis and 10 hospital personnel). The interrater coefficient (intraclass correlation) was 0.97 (F=65.44, df=9, 10, p<0.001).
Neurological Examinations Earlier in Life
Neonatal examination
Standardized neurological examinations were blindly performed by one assessor at the delivery hospitals on the third to fourth day after birth (13). The examination was based primarily on the methods of Hagberg (27) and of Prechtl and Beintema (28, 29) and included evaluation of muscle tone, motor movements, simple and complex reflexes, activity level, and sensitivity to stimulation. A summary score composed of deviation points compiled across 19 different items represented the subject’s degree of total neonatal neurological abnormalities. As in our previous study (13), a subject was classified as having a high score if the total neurological abnormality score was greater than 5.
Six-year examination
In the follow-up investigation at 6 years of age, neuromotor assessment was blindly conducted in the subjects’ homes by a different examiner. The neuromotor assessment was primarily based on Touwen and Prechtl’s neurological examination of children (30) and included 26 test items (14). A subject with a total neurological abnormality score higher than 7 was classified as high scoring at that age. The total scores in infancy and at 6 years of age were used for comparison with the total score in the current follow-up at 22 years of age.
Statistical Methods
Kruskal-Wallis one-way analysis of variance was used to compare the offspring of mothers with schizophrenia, offspring of mothers with affective psychosis, and normal-risk offspring groups on the quantitative scores for total neurological abnormalities and each neurological domain and subdomain. When Kruskal-Wallis analysis showed a significant across-group difference or a strong trend, secondary comparisons of pairs among these three groups were done by Wilcoxon Mann-Whitney tests. The Fisher exact test, with odds ratio and 95% confidence interval (CI), was used for analysis of category data regarding rates of high-scoring subjects and confounder effects. Spearman rank correlations were used to study the stability of the quantitative neurological abnormality scores over time. Statistical significance was defined as p≤0.05 two-tailed, with 0.10≥p>0.05 denoting nonsignificant trends.
The effect of adding additional “mainly schizophrenic” or “mainly affective” cases to the core groups of mothers with schizophrenia and affective psychosis, to form extended offspring groups with “schizophrenia risk” and “affective psychosis risk” spectra, was evaluated visually but not submitted to formal statistical analysis.
Results
Neurological Abnormalities in Young Adulthood
Significant differences were found across the offspring of mothers with schizophrenia, offspring of mothers with affective psychosis, and normal-risk offspring for total neurological abnormality score, soft signs, reflexes, and motor functions, with nonsignificant trends for hard signs, motor coordination, and cranial nerves (Table 2). Secondary analyses among these groups showed that the offspring of mothers with schizophrenia had significantly higher scores than the normal-risk offspring on total neurological abnormalities, hard signs, soft signs, reflexes, motor functions, motor coordination, and cranial nerves. The offspring of mothers with schizophrenia also had significantly higher scores than the offspring of mothers with affective psychosis on total neurological abnormalities, soft signs, and reflexes, with a nonsignificant difference in cranial nerves. The offspring of mothers with affective psychosis showed no significant difference from the normal-risk offspring on any neurological abnormality, but they had a trend toward lower scores for deviant reflexes. Furthermore, among the offspring of mothers with affective psychosis, no difference was seen on any scale between the offspring of mothers with bipolar versus unipolar affective psychosis. Receiver operating characteristic curves (not shown) demonstrated that the offspring of mothers with schizophrenia were more readily discriminated from the offspring of mothers with affective psychosis and the normal-risk offspring by soft signs than by hard signs.
Possible Confounders for Group Differences
Schizophrenia spectrum disorders (schizophrenia, schizoaffective psychosis, and cluster A personality disorders) were found in three offspring of mothers with schizophrenia, one offspring of mothers with affective psychosis, and two normal-risk offspring by the time of this follow-up (22). With these six subjects removed, the offspring of mothers with schizophrenia still scored significantly higher on total neurological abnormalities than the normal-risk offspring (z=3.53, p<0.0001) and the offspring of mothers with affective psychosis (z=2.33, p=0.02). There was a somewhat higher rate of male offspring among the offspring of mothers with schizophrenia than among the offspring of mothers with affective psychosis and the normal-risk offspring (Table 1) (χ2=5.04, df=2, p=0.08), but no significant relationship was found between gender and a high score for neurological abnormalities (above the 90th percentile) among the offspring of mothers with schizophrenia (Fisher exact test, p=0.22; odds ratio=4.67, 95% CI=0.48–45.60). Offspring of mothers with schizophrenia have a high rate of substance use disorders (22), but in this study substance use disorders were not significantly related to high scores for neurological abnormalities in the offspring of mothers with schizophrenia (Fisher exact test, p=1.00; odds ratio=0.93, 95% CI=0.14–6.37). At the time of assessment, no subject in any group was receiving neuroleptic medication, and only two subjects were taking selective serotonin reuptake inhibitors (one offspring of mothers with schizophrenia, one offspring of mothers with schizoaffective psychosis).
Subgroup With High Scores on Neurological Abnormalities
The distributions of total neurological abnormality scores for the three offspring groups demonstrate the existence of two subgroups among the offspring of mothers with schizophrenia (Figure 1). Operationally defined, these offspring consisted of one subgroup (68%) with a total score of 7 or less (representing the normal-risk offspring’s 90th percentile cutoff level) and another substantial subgroup (32%) scoring above this level. The offspring of mothers with affective psychosis and the normal-risk offspring did not show such distributions. The proportion of individuals scoring above this cutoff level was significantly greater in the offspring of mothers with schizophrenia than in the normal-risk offspring (Fisher exact test, p=0.005; odds ratio=4.74, 95% CI=1.61–13.89) or in the offspring of mothers with affective psychosis (Fisher exact test, p=0.003; odds ratio=21.92, 95% CI=1.19–401.82). Compared with the normal-risk offspring and the offspring of mothers with affective psychosis (Table 3), this high-scoring subgroup among the offspring of mothers with schizophrenia contained notably more subjects (odds ratio >7) with soft signs, deviant reflexes (especially primitive reflexes), involuntary movements, and cranial nerve deviations. All nine subjects in the high-scoring subgroup had some kind of disturbance in the eye region (glabellar reflex, N=8; gaze nystagmus, N=2; gaze impersistence, N=1).
Stability of Neurological Abnormalities From Infancy to Young Adulthood
The total score for neurological abnormalities at 22 years was significantly positively correlated with the total score at 6 years of age for the total high-risk group (rs=0.38, N=60, p=0.003), the offspring of mothers with schizophrenia (rs=0.46, N=20, p=0.05), and the normal-risk offspring (rs=0.26, N=85, p=0.02) but not for the offspring of mothers with affective psychosis (rs=0.07, N=20, p=0.76). In contrast, the total score at 22 years was not significantly correlated with the score in infancy for any group (rs values ranged from –0.21 to 0.11 across the four groups; the numbers of subjects were 47, 16, 17, and 65 for the total high-risk group, offspring of mothers with schizophrenia, offspring of mothers with affective psychosis, and normal-risk offspring, respectively).
Spectra of Schizophrenia Risk and Affective Psychosis Risk
The addition of mothers with “mainly schizophrenic” and those with “mainly affective” disorders to the original core schizophrenia and affective psychosis groups, to form extended spectra of schizophrenia risk and affective psychosis risk, generally resulted in only minor changes in mean neurological abnormality scores among the offspring (Table 2), with slightly smaller differences between each spectrum group and the normal-risk offspring. Both the pattern of neurological abnormalities and the individual stability over time remained very similar to those for the original offspring of mothers with schizophrenia and offspring of mothers with affective psychosis. The size and characteristics of the neurological abnormalities among the high-scoring subgroup in the schizophrenia risk spectrum offspring (29%) were also similar to those for the high-scoring subgroup among the offspring of mothers with schizophrenia (32%).
Discussion
In this prospective, longitudinal study, personal stability of the level of neurological abnormalities was found in both high-risk and comparison offspring between middle childhood and adulthood but not between infancy and adulthood. This finding is congruent with results in the Jerusalem Infant Development Study (12). At all three ages in the current study, the offspring of women with schizophrenia demonstrated high levels of neurological abnormalities, while the offspring of women with affective psychosis were not different from, or had even lower levels of neurological abnormalities than, comparison subjects (13, 14). This strongly suggests that schizophrenia and affective psychosis belong to different biological continua and, further, that this neurodevelopmental disturbance is already manifest and detectable in childhood. The extension of the core schizophrenia and affective psychosis risk groups to include additional offspring of mothers with spectrum disorders had little effect on the rate and pattern of neurological abnormalities in the respective risk groups, indicating that the factors influencing rates of neurological abnormalities are as present in the additional offspring in the risk spectra as in the core offspring.
In young adulthood, the offspring of mothers with schizophrenia had a wide range of neurological abnormalities, consisting of hard signs, soft signs, deviant reflexes, and disturbances in motor functions, motor coordination, and cranial nerves. The total score for neurological abnormalities of these offspring (mean=6.86) was approximately halfway between the scores of schizophrenia patients (mean=10.13) (6) and normal-risk offspring (mean=3.99). In congruence with findings for siblings (6), the offspring of mothers with schizophrenia were best discriminated from normal-risk offspring by soft signs, rather than hard signs. Higher levels of neurological abnormalities (total score higher than 90th percentile of normal-risk offspring) were present in a substantial proportion (32%) of offspring of mothers with schizophrenia, and this subgroup had high rates of soft signs, primitive reflexes, involuntary movements, and cranial nerve deviations. In total, 18% of the offspring of mothers with schizophrenia had high scores for neurological abnormalities at all three ages, compared with none of the offspring of mothers with affective psychosis or normal-risk offspring. The fact that a substantial proportion of the offspring of mothers with schizophrenia scored in the same range as most normal-risk offspring (Figure 1 and Table 3) may reflect the absence of the presumed number and type of genes or environmental influences that lie behind the high rate of neurological abnormalities in families with schizophrenia.
The existence of similar diverse neurological abnormalities in the offspring of mothers with schizophrenia and in schizophrenia patients is congruent with the findings, emerging from neuroimaging studies, of subtle abnormalities in a widespread area of the brain in schizophrenia patients (31). Such findings speak for a similar etiological, probably genetic, basis for the disturbed neurodevelopmental process found at large in these families.
The high-scoring subgroup of offspring of mothers with schizophrenia had high rates of both neurological disturbances in the eye region (reminiscent of findings in the siblings of schizophrenia patients [6] and high-risk offspring developing psychotic symptoms [31]) and involuntary movements (however, these differences were not significant in the group analyses). These observations are consistent with previous findings in preschizophrenia individuals as early as 2 years of age and in neuroleptic-naive schizophrenia patients and patients’ siblings in childhood and adulthood (7, 8, 14, 32).
The strengths of the study were the prospective design with previous neurological assessments; the high adult follow-up rate; the narrow age range at follow-up; the existence of different high-risk groups; the use of an extensive standardized examination routine with high interassessor reliability, previously tested with schizophrenia patients; and examination by one investigator who was blind with regard to the subject’s study group and previous project data.
The major limitation of this study lies in the small sample sizes of the specific high-risk groups. Given the observed means and variance for total neurological abnormalities, the study had only moderate power (62%) to identify a significant difference (p<0.05) between offspring of mothers with affective psychosis and normal-risk offspring that was of the same magnitude as the difference between offspring of mothers with schizophrenia and normal-risk offspring. The offspring of mothers with affective psychosis nevertheless had somewhat lower neurological abnormality scores than the normal-risk offspring and significantly lower scores than the offspring of mothers with schizophrenia.
In summary, a substantial subgroup of young adult offspring at risk for schizophrenia evidence a notable degree of diverse neurological abnormalities (in line with the neurodevelopmental hypothesis of schizophrenia), which is not observed in offspring at risk for affective psychoses. Although other etiological factors cannot be excluded, the results support the role of genetic factors in neurological abnormalities related to parental schizophrenia. The question of whether the presence of neurological abnormalities in general or a specific type is associated with mental disturbance in adult high-risk offspring will be the focus of our future work.
 |
 |
 |
Presented in part at the XIth Biennial Winter Workshop on Schizophrenia, Davos, Switzerland, Feb. 24 to March 1, 2002. Received Jan. 13, 2003; revision received Sept. 9, 2003; accepted Sept. 19, 2003. From the Department of Psychiatric Epidemiology, University Hospital, Lund University. Address reprint requests to Dr. McNeil, Department of Psychiatric Epidemiology, Barngatan 2, University Hospital, S-221 85 Lund, Sweden; [email protected] (e-mail). Supported by grants from the Stanley Medical Research Institute, NIMH (grant MH-18857), and the Swedish Medical Research Council (grant 3793).
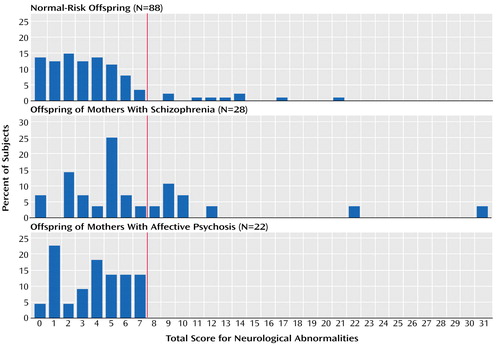
Figure 1. Distribution of Total Scores for Neurological Abnormalities in Young Adult Offspring of Mothers With a History of Schizophrenia or Affective Psychosis and in Normal-Risk Offspringa
aNeurological abnormalities were assessed with a 44-item comprehensive, standardized scale (6). The red line is the 90th percentile for the normal-risk offspring.
1. Weinberger D: Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry 1987; 44:660–669Crossref, Medline, Google Scholar
2. Heinrichs DW, Buchanan RW: Significance and meaning of neurological signs in schizophrenia. Am J Psychiatry 1988; 145:11–18Link, Google Scholar
3. Quitkin F, Rifkin A, Klein DF: Neurological soft signs in schizophrenia and character disorders. Arch Gen Psychiatry 1976; 33:845–853Crossref, Medline, Google Scholar
4. Woods BT, Kinney DK, Yurgelun-Todd D: Neurological abnormalities in schizophrenic patients and their families. Arch Gen Psychiatry 1986; 43:657–663Crossref, Medline, Google Scholar
5. Rossi A, De Cataldo S, Di Michele V, Manna V, Cessoli S, Stratta P, Casacchia M: Neurological soft signs in schizophrenia. Br J Psychiatry 1990; 157:735–739Crossref, Medline, Google Scholar
6. Ismail B, Cantor-Graae E, McNeil TF: Neurological abnormalities in schizophrenic patients and their siblings. Am J Psychiatry 1998; 155:84–89Link, Google Scholar
7. Walker EF, Savoie T, Davis D: Neuromotor precursors of schizophrenia. Schizophr Bull 1994; 20:441–451Crossref, Medline, Google Scholar
8. Erlenmeyer-Kimling L: Neurobehavioral deficits in offspring of schizophrenic parents: liability indicators and predictors of illness. Am J Med Genet 2000; 97:65–71Crossref, Medline, Google Scholar
9. Fish B: Infant predictors of the longitudinal course of schizophrenic development. Schizophr Bull 1987; 13:395–409Crossref, Medline, Google Scholar
10. Hans SL, Marcus J, Nuechterlein KH, Asarnow RF, Styr B, Auerbach JG: Neurobehavioral deficits at adolescence in children at risk for schizophrenia: the Jerusalem Infant Development Study. Arch Gen Psychiatry 1999; 56:741–748Crossref, Medline, Google Scholar
11. Asarnow JR: Children at risk for schizophrenia: converging lines of evidence. Schizophr Bull 1988; 14:613–631Crossref, Medline, Google Scholar
12. Marcus J, Hans SL, Lewow E, Wilkinson L, Burack CM: Neurological findings in high-risk children: childhood assessment and 5-year follow up. Schizophr Bull 1985; 11:85–100Crossref, Medline, Google Scholar
13. Blennow G, McNeil TF: Neurological deviations in newborns at psychiatric high risk. Acta Psychiatr Scand 1991; 84:179–184Crossref, Medline, Google Scholar
14. McNeil TF, Harty B, Blennow G, Cantor-Graae E: Neuromotor deviation in offspring of psychotic mothers: a selective developmental deficiency in two groups of children at heightened psychiatric risk? J Psychiatr Res 1993; 72:39–54Crossref, Google Scholar
15. Dworkin RH, Cornblatt B, Friedman R, Kaplansky LM, Lewis JA, Rinaldi A, Shilliday C, Erlenmeyer-Kimling N: Childhood precursors of affective versus social deficits in adolescents at risk for schizophrenia. Schizophr Bull 1993; 19:563–577Crossref, Medline, Google Scholar
16. Seidman LJ: Schizophrenia and brain dysfunction: an integration of recent neurodiagnostic findings. Psychol Bull 1983; 94:195–238Crossref, Medline, Google Scholar
17. Erlenmeyer-Kimling L, Cornblatt B: The New York High-Risk Project: a follow-up report. Schizophr Bull 1987; 13:451–461Crossref, Medline, Google Scholar
18. Shaffer D, Stokman CS, O’Conner PA, Shafer S, Barmark JE, Hess S, Spalten D, Schonfeld IS: Early soft neurological signs and later psychopathology, in Life-Span Research on the Prediction of Psychopathology. Edited by Erlenmeyer-Kimling L, Miller NE. Hillsdale, NJ, Lawrence Erlbaum Associates, 1986, pp 31–48Google Scholar
19. Cannon M, Caspi A, Moffitt TE, Harrington H, Taylor A, Murray RM, Poulton R: Evidence for early-childhood, pan-developmental impairment specific to schizophreniform disorder. Arch Gen Psychiatry 2002; 59:449–456Crossref, Medline, Google Scholar
20. Cardno AG, Rijsdijk FV, Sham PC, Murray RM, McGuffin P: A twin study of genetic relationships between psychotic symptoms. Am J Psychiatry 2002; 159:539–545Link, Google Scholar
21. McNeil TF, Kaij L, Malmqvist-Larsson A, Näslund B, Persson-Blennow I, McNeil N, Blennow G: Offspring of women with nonorganic psychoses: development of a longitudinal study of children at high risk. Acta Psychiatr Scand 1983; 68:234–250Crossref, Medline, Google Scholar
22. Schubert EW, McNeil TF: Prospective study of adult mental disturbance in offspring of women with psychosis. Arch Gen Psychiatry 2003; 60:473–480Crossref, Medline, Google Scholar
23. McNeil TF, Kaij L, Malmqvist-Larsson A: Women with nonorganic psychosis: mental disturbance during pregnancy. Acta Psychiatr Scand 1984; 70:127–139Crossref, Medline, Google Scholar
24. McNeil TF, Kaij L: Offspring of women with nonorganic psychoses, in Children at Risk for Schizophrenia: A Longitudinal Perspective. Edited by Watt NF, Anthony EJ, Wynne LC, Rolf JE. New York, Cambridge University Press, 1984, pp 465–481Google Scholar
25. McNeil TF, Kaij L: Swedish High-Risk Study: sample characteristics at age 6. Schizophr Bull 1987; 13:373–381Crossref, Medline, Google Scholar
26. Spitzer RL, Endicott J, Robins E: Research Diagnostic Criteria (RDC) for a Selected Group of Functional Disorders, 3rd ed. New York, New York State Psychiatric Institute, Biometrics Research, 1978Google Scholar
27. Hagberg B: Neurologisk diagnostik i nyföddhetsperioden (Neurological diagnostics in the neonatal period). Läkartidningen (Swedish Med Bull) 1964; 32:2232–2238Google Scholar
28. Prechtl HFR, Beintema D: The Neurological Examination of the Fullterm Newborn Infant: Little Club Clinics in Developmental Medicine 12. London, Heinemann, 1964Google Scholar
29. Beintema DA: A Neurological Study of Newborn Infants: Clinics in Developmental Medicine 28. London, Heinemann, 1968Google Scholar
30. Touwen BCL, Prechtl HFR: The Neurological Examination of the Child With Minor Nervous Dysfunction. London, Heinemann, 1970Google Scholar
31. Buckley PF, Larkin C, Friedman L, Lewin JS, Waddington JL: Neurodevelopmental pathobiology of schizophrenia: correlates of magnetic resonance spectroscopy and functional MRI, in The Neurodevelopmental Basis of Schizophrenia. Edited by Waddington JL, Buckley PF. Austin, Tex, RG Landes, 1996, pp 107–117Google Scholar
32. Lawrie SM, Byrne M, Miller P, Hodges A, Clafferty RA, Owens DG, Johnstone EC: Neurodevelopmental indices and the development of psychotic symptoms in subjects at high risk of schizophrenia. Br J Psychiatry 2001; 178:524–530Crossref, Medline, Google Scholar



