Neuropsychological Differences Between Late-Onset and Recurrent Geriatric Major Depression
Abstract
OBJECTIVE: Executive dysfunction, possibly related to vascular pathology, has been well documented in patients with a first episode of major depressive disorder in later life (late-onset geriatric major depression). However, it is unclear whether the neuropsychological presentation differs in patients with a lifetime history of major depressive disorder (recurrent geriatric major depressive disorder). The purpose of this study was to explore differences in neuropsychological function, symptoms, and cardiovascular comorbidity between patients with late-onset and recurrent geriatric major depression. METHOD: The study used a two-by-two factorial design in which one factor was current major depressive disorder (present versus absent) and the second factor was lifetime history of depression (present versus absent). Neuropsychological measures of executive functioning and episodic memory, as well as psychopathological symptoms and comorbid medical illness, were examined in a total of 116 older adults. RESULTS: Patients with late-onset major depressive disorder showed specific deficits in attention and executive function, whereas patients with recurrent major depressive disorder exhibited deficits in episodic memory. The rates of anhedonia and comorbid cardiovascular illness were higher in patients with late-onset geriatric major depressive disorder. CONCLUSIONS: In contrast to recurrent geriatric major depressive disorder, late-onset major depressive disorder is characterized by specific deficits in tasks of attention and executive function, consistent with increased anhedonia and cardiovascular comorbidity. These findings, if confirmed, suggest that recurrent and late-onset geriatric major depressive disorder may represent distinct phenomenological entities. Such phenomenological differences as a function of lifetime history of major depression can guide research in the neurophysiology, prevention, and treatment of geriatric major depressive disorder.
Major depressive disorder is a highly prevalent disease in old age (1–3). Between 1% and 4% of elderly persons experience major depression, and the prevalence increases to between 6% and 32% in older nursing home residents (4). At the same time, the onset of major depression is variable across the life course. For the majority of persons, the age at onset is in the late 20s, but first episodes are also common after age 40 (5, 6). Epidemiologically, about 40% of cases of major depression in old age represent recurrent depressive episodes, whereas about 30% reflect late-onset depression, while in some cases the distinction between recurrent and late-onset depression cannot be determined reliably (7).
There is evidence to suggest that cerebrovascular disease, especially ischemic small-vessel disease, may be a factor in the pathogenesis of late-onset geriatric major depression. Imaging studies of late-onset geriatric major depressive disorder consistently showed signal hyperintensities in deep white matter (8–10) that may go along with structural brain changes in the frontal lobes (11). These findings have led to the hypothesis that among older persons with major depression, there is a subgroup of individuals with what has been termed “vascular depression” (12–14).
At the same time, there is evidence to suggest that recurrent, early-onset major depressive disorder is associated with significant volume loss in the hippocampus (15, 16). Sheline et al. (15) reported an association of the length of untreated depressive episodes with reductions in hippocampal volume in recurrent geriatric major depressive disorder. Bell-McGinty and colleagues (16) found an inverse correlation between bilateral hippocampal-entorhinal volume and years since onset of depression. These findings have recently been linked to models of decreased hippocampal neurogenesis in major depressive disorder, suggesting that recurrent depressive episodes may lead to persistent neuronal alterations on a molecular level in hippocampal cells (17).
Such findings of structural differences between late-onset and recurrent geriatric major depressive disorder inform clinical hypotheses on differences in the neuropsychological and symptom presentation of geriatric major depression. Vascular depression has been associated with a loss of motivation or interest and the presence of cognitive decline, especially in attention and executive functioning, consistent with frontal lobe dysfunction (12, 18).
On the other hand, preliminary evidence suggests that impaired short-term memory functioning may be associated with reduced temporal lobe volume in patients with geriatric depression (19) and in middle-aged adults (20). Other reports suggest that depressed patients may have significant deficits primarily in episodic memory, suggesting a more selective dysfunction in mesial temporal lobe function during episodes of depression (21). Memory dysfunction has been shown to be persistent in older depressed patients, even after response to antidepressant treatment (22). Taken together, these findings suggest that recurrent episodes of major depressive disorder may go along with some degree of persistent dysfunction in the mesial temporal lobe, possibly associated with impairment in episodic memory functions.
Based on these considerations, we hypothesized that the neuropsychological profiles of patients with recurrent versus late-onset geriatric major depressive disorder would differ in the degree of dysfunction in tasks of attention and executive function (consistent with fronto-subcortical circuit dysfunction) versus dysfunction in tasks of episodic memory (consistent with temporal lobe dysfunction). Specifically, we hypothesized that patients with late-onset geriatric major depressive disorder would exhibit specific deficits in attention and executive functioning, whereas patients with recurrent geriatric major depressive disorder would exhibit specific deficits in episodic memory functioning. Furthermore, we hypothesized, consistent with a vascular depression model of late-onset major depressive disorder, that patients with late-onset geriatric major depressive disorder would present with a higher degree with vascular comorbidity.
Method
Subjects
The study builds on the neuropsychological portion of a prospective, longitudinal study of cognition in old age, the Clinical and Biological Studies of Early Alzheimer’s Disease project, at the Department of Psychiatry, Mount Sinai School of Medicine. For the present analyses, we used baseline neuropsychological data from 299 older nursing home residents from the Jewish Home Nursing Home, Bronx, New York (mean age=83.64 years, SD=4.39). These data represent all nondemented participants who completed baseline neuropsychological and psychiatric assessments. The diagnosis of dementia was made in a research consensus conference according to DSM-III-R or DSM-IV criteria. Ethical approval was obtained from the institutional review boards of the Department of Veterans Affairs, Bronx, N.Y., and the Mount Sinai School of Medicine, New York. Written informed consent to participate in the study was obtained from each participant or, if the participant lacked capacity, from a caregiver.
Patients With Geriatric Major Depressive Disorder
The neuropsychological battery was administered, along with a standardized questionnaire assessing psychiatric history and current symptoms, and comprised assessment with the Geriatric Depression Scale (23). Trained research assistants completed a standardized questionnaire assessing the presence or absence of DSM-III-R or DSM-IV symptoms of major depressive disorder. The questionnaire was a modified version of the mood disorders module from the Structured Clinical Interview for DSM-IV Axis I Disorders (24). The presence or absence of a lifetime history of major depressive disorder was extracted from medical information, including charts and information obtained from the treating physician. Both the diagnosis of current major depressive disorder and the diagnosis of a lifetime history of major depressive disorder were reviewed and verified by a research physician with 2 years of specialty training in geriatric psychiatry.
Of the 299 participants, 40 (10.03%) met the criteria for major depressive disorder. Using psychiatric symptom and history data, we defined recurrent geriatric major depressive disorder as present in patients who had at least one episode of major depressive disorder according to psychiatric history, and late-onset geriatric major depressive disorder as present in patients who did not have a history of major depressive disorder. With this classification, 19 patients were defined as having late-onset geriatric major depressive disorder and 21 as having recurrent geriatric major depressive disorder. The external validity of the diagnosis of major depressive disorder was assessed in comparison to scores on the Geriatric Depression Scale by using a cutoff of 11 points. With a standard Geriatric Depression Scale cutoff score of 11 or greater, the diagnosis of major depressive disorder had the following classification accuracy indices (25, 26): hit rate=0.96, sensitivity=0.95, specificity=0.90, false positive rate=0.05, and false negative rate=0.10.
Nondepressed Older Adults
In the remaining 259 nondepressed participants, 123 had a history of lifetime depressive disorder and 111 did not (data were missing for 15 participants). We used twofold oversampling in a randomized matching procedure to avoid inflation of type I error, which has been shown to be significant when sample sizes are unequal by a factor of 5 or more (27). Hence, we randomly selected 42 participants without a lifetime diagnosis of depression and 38 with a lifetime diagnosis of major depressive disorder, matched for age and gender. Two nondepressed older adults without and one nondepressed older adult with a lifetime diagnosis of major depressive disorder had incomplete data and were excluded from the sample. The random sample did not differ from the overall group in age (t=0.28, df=257, p=0.78), gender (χ2=1.40, df=1, p=0.24), and general cognitive status as measured by the Mini-Mental State Examination (MMSE) (28) (t=1.02, df=257, p=0.31).
Assessments
Medical diagnoses were extracted from medical information, including charts and information obtained from the treating physician, by using a standardized checklist assessing the presence or absence of cardiovascular and cerebrovascular diagnoses. Specifically, during the chart review process, checklists were used to assess the documented presence of cardiovascular and cerebrovascular diagnoses.
Specific symptoms of depression were derived from the questionnaire assessing the presence or absence of DSM-III-R or DSM-IV symptoms of major depressive disorder, as either reported by subjective account or observed by clinicians. Specifically, the symptom “mood” represents “depressed mood most of the day, nearly every day.” “Anhedonia” represents “diminished interest or pleasure in activities of the day.” “Neurovegetative symptoms” represent any one of a group of phenomena, including psychomotor agitation or retardation and changes in sleep, weight, and appetite.
The neuropsychological battery was administered by trained research assistants. Scores on all tasks administered were assessed by two independent raters. The MMSE (28) was administered as a global measure of cognitive functioning. The neuropsychological battery consisted of eight tests assessing attention and executive control functions and episodic memory.
Digit symbol
The digit symbol substitution test from the Wechsler Adult Intelligence Scale (29) is a test of perceptual-motor speed and complex attention. The number of squares filled in correctly was determined as the measure of performance.
Trail making
Trail Making Test Part A and Part B (30, 31) were used as measures of visuomotor attention and executive function, respectively. The time to completion in seconds was used as the performance measure.
Fluency
Verbal Fluency for Animals (32) was tested for 60 seconds. The number of animals named, subtracting repetitions, was used as the performance measure.
Episodic memory
Participants were presented with a list of 10 items derived from the Consortium to Establish a Registry for Alzheimer’s Disease (33) battery. The list was presented three times, with the order of item presentation varied. The number of words recalled over three trials was used as a performance measure for overall recall, and the number of words recalled on the third trial was used as a performance measure for learning within episodic memory.
Recognition
Participants were given a list with 20 items, which consisted of the 10 items learned in the episodic memory tasks and 10 distracter items, and were asked to state whether or not the item was part of the original list. The number of items classified correctly was used as the performance measure (34).
Delayed recall
Participants were asked to recall items from the episodic memory tasks after a 10-minute delay. The number of words recalled was used as a performance measure for delayed recall (35).
Data Analysis
Raw scores were used for all analyses. Descriptive analyses were performed using a two-by-two analysis of variance (ANOVA), with two grouping factors (presence versus absence of current major depressive disorder; presence versus absence of lifetime history of depression). Principal-components analysis (with an eigenvalue cutoff set to 1.00) was applied to these variables to determine the structure of cognitive performance, yielding performance indicators for specific cognitive domains.
Analysis of cognitive performance used a two-by-two-by-two mixed-model ANOVA, treating the two groups (presence versus absence of current major depressive disorder; presence versus absence of lifetime history of depression) as between-subjects factors and the two cognitive domains (attention/executive function versus episodic memory) as within-subjects factors. Post hoc tests were performed by using t tests. Chi-square tests were used for analysis of depressive symptoms and medical comorbidity. All statistical tests were two-tailed, and a probability level of <0.05 was considered significant.
Results
Demographic and Clinical Characteristics
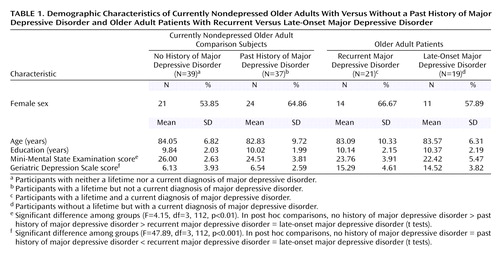
The overall study group comprised 46 men (39.66%) and 70 women (60.34%), with a mean age of 83.41 years (SD=8.37, range=60–97) and a mean education level of 10.04 years (SD=2.05, range=6–14). There were no statistically significant differences between the four study groups in age (F=0.14, df=3, 115, p=0.93), education (F=0.29, df=3, 115, p=0.83), and gender (χ2=1.40, df=3, p=0.71). The baseline characteristics of the four groups are summarized in Table 1.
Overall cognitive status was assessed with the MMSE. Cognitive status was worse in the currently depressed older adults (patients with late-onset and recurrent major depressive disorder), compared to the nondepressed older adults (F=8.43, df=1, 112, p<0.01). Further testing revealed that in the nondepressed group, cognitive performance was significantly worse in the participants who had a lifetime diagnosis of depression (t=1.99, df=74, p<0.05), whereas overall cognitive status was comparable between the older adults with recurrent and late-onset major depressive disorder (t=0.89, df=38, p=0.38).
Depression severity was measured with the Geriatric Depression Scale. Geriatric Depression Scale scores were higher in patients with current major depressive disorder, compared to the nondepressed participants (F=141.93, df=1, 112, p<0.001), but Geriatric Depression Scale scores did not differ between the two currently depressed groups (t=0.56, df=38, p=0.58) nor between the two currently nondepressed groups (t=0.54, df=74, p=0.59).
Differences in Neuropsychology
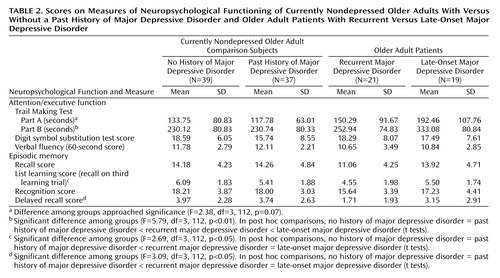
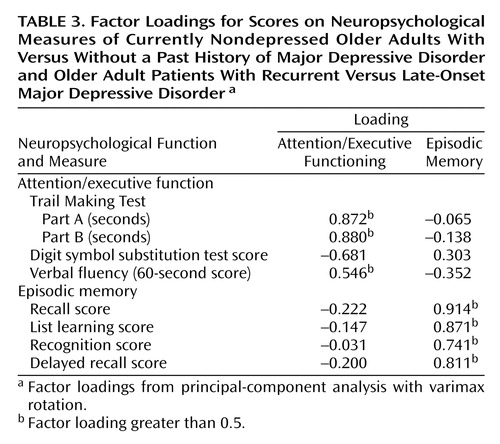
The eight neuropsychological variables (Table 2) were aggregated in a factor-analytic approach. The factorial solution over both groups generated two factors, accounting for 68.12% of the overall variance in the eight cognitive tasks. Factor loadings on the first factor were high for Trail Making Parts A and B, the digit symbol substitution test, and the verbal fluency test, suggesting a representation of attention and executive function in this factor (“attention/executive function”). The memory measures loaded high on the second factor, suggesting a representation of episodic memory (“episodic memory”). The factor loadings for the factor solution are given in Table 3.
For the attention/executive function factor, ANOVA revealed a main effect for the current presence of major depressive disorder (F=4.25, df=1, 112, p<0.05), a main effect for the presence or absence of a lifetime diagnosis of major depressive disorder (F=5.37, df=1, 112, p<0.05), and an interaction between current and lifetime major depressive disorder (F=6.24, df=1, 112, p<0.01). Post hoc tests revealed that attention/executive function differed between the older adults with and without current major depressive disorder (t=2.51, df=114, p<0.05), but not between the currently nondepressed older adults with and without a lifetime diagnosis of major depressive disorder (t=0.16, df=74, p=0.87). However, there was a significant difference among the currently depressed older adults as a function of lifetime diagnosis of major depressive disorder (t=2.78, df=38, p<0.01), indicating that the patients with late-onset major depressive disorder performed worse in attention/executive function.
For the episodic memory factor, ANOVA revealed a main effect for the current presence of major depressive disorder (F=7.15, df=1, 112, p<0.01) and a main effect for the presence of a lifetime diagnosis of major depressive disorder (F=4.36, df=1, 112, p<0.05). The interaction term, however, was not significant (F=1.95, df=1, 112, p=0.17). Post hoc tests revealed no significant differences on the episodic memory tasks between currently nondepressed older adults with and without a lifetime diagnosis of major depressive disorder (t=0.59, df=74, p=0.55). The patients with recurrent major depressive disorder, however, performed worse on the episodic memory tasks than the patients with late-onset geriatric major depressive disorder (t=2.10, df=38, p<0.05).
As Figure 1 shows, among currently depressed patients only, the mean factor scores for attention/executive function were higher in patients with recurrent major depressive disorder, whereas episodic memory scores were higher in patients with late-onset major depressive disorder. This interaction between cognitive domain and type of depression (recurrent versus late-onset) proved to be statistically significant (F=13.33, df=1, 38, p<0.001) and represented a moderate effect size (η2=0.26). This pattern of results suggests a dissociation in the neuropsychological presentation of late-onset versus recurrent geriatric major depressive disorder, with specific impairment in attention/executive function in late-onset major depressive disorder and specific impairment in episodic memory in recurrent geriatric major depressive disorder.
Furthermore, we were interested in whether the neuropsychological differences between the groups may have been driven by subgroups of patients among the currently depressed older adults. Specifically, we tested whether skewness, a statistical indicator of distribution symmetry (with a value of zero indicating normal distribution [36]), was significantly different from zero for either factor score in either group. This was not the case (all p>0.11), indicating that distributions were in fact symmetric.
Differences in Depressive Symptoms and Medical Comorbidity
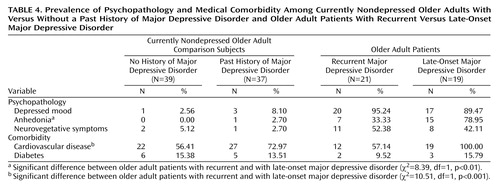
We further explored whether these differences in cognitive performance went along with differences in depressive symptoms and medical comorbidity between patients with late-onset major depressive disorder and those with recurrent geriatric major depressive disorder. Data for medical comorbidity and depressive symptoms are listed in Table 4.
There were no significant differences in depressive symptoms in the currently nondepressed groups (those with and without a lifetime diagnosis of depression) (all p>0.35). Likewise, both the number of subjects with diabetes (χ2=0.05, df=1, p=0.99) and the number of subjects with cardiovascular disease (χ2=2.27, df=1, p=0.16) were comparable in the currently nondepressed groups with and without a lifetime diagnosis of depression.
The analysis of depressive symptoms in the currently depressed patients as a function of lifetime diagnosis (late-onset versus recurrent geriatric major depressive disorder) revealed no significant differences in mood (χ2=0.48, df=39, p=0.60) and neurovegetative symptoms of depression (χ2=0.42, df=39, p=0.55). However, patients with late-onset geriatric major depressive disorder were more likely to exhibit anhedonia than patients with recurrent major depressive disorder (χ2=8.39, df=39, p<0.01). Likewise, patients with late-onset geriatric major depressive disorder were more likely to have comorbid cardiovascular disease than those with recurrent major depressive disorder (χ2=10.51, df=39, p<0.001), whereas there were no significant differences between these groups in the number of patients with diabetes mellitus (χ2=0.36, df=39, p=0.65).
Discussion
In line with our hypotheses, we found distinct differences in both the neuropsychological and clinical presentation between late-onset and recurrent geriatric major depression. The principal finding is that recurrent geriatric major depression is characterized by deficits in episodic memory, whereas late-onset geriatric major depression is characterized by specific deficits in attention/executive function. Neuropsychological performance was compromised in currently depressed older adults, relative to comparison subjects both with and without a lifetime diagnosis of depression. However, within the group of older adults with major depressive disorder, patients with recurrent geriatric major depressive disorder performed worst in tasks of episodic memory, and patients with late-onset geriatric major depressive disorder performed worst in tasks of attention/executive function. In addition, late-onset geriatric major depressive disorder was characterized by the presence of a higher degree of anhedonia and a higher rate of cardiovascular comorbidity.
This study adds to previous studies on geriatric major depressive disorder that have shown executive dysfunction, loss of motivation, an increased number of cardiovascular risk factors, and a higher prevalence of vascular brain changes in patients with late-onset geriatric major depressive disorder (9–15, 37, 38). Beyond those findings, however, the results of this study indicate that recurrent geriatric major depressive disorder may represent a distinct phenomenological subtype within geriatric major depression, in contrast to late-onset geriatric major depressive disorder. The presence of such subtypes is suggested by the dissociation between neuropsychological functions, with specific frontal lobe dysfunction in late-onset geriatric major depressive disorder and specific temporal lobe dysfunction in recurrent geriatric major depressive disorder.
Although these findings are in line with the vascular model of late-onset geriatric major depressive disorder (8–10, 12, 13), as contrasted to temporal lobe dysfunction as a consequence of recurrent depressive episodes and underlying neuronal changes (15–17), the exact mechanisms that may lead to these distinct subtypes in geriatric major depression are not known. Our data suggest that cardiovascular comorbidity may play a role in the development of specific executive dysfunction in late-onset geriatric major depressive disorder. Although the cross-sectional analysis of our data does not permit causal inference, it seems reasonable to assume that vascular changes in the frontal cortex might go along with both anhedonia and executive dysfunction. However, we cannot clearly establish this relationship from our data.
The suggestion that temporal lobe abnormalities are a potential mechanism for episodic memory dysfunction in recurrent geriatric major depressive disorder is supported by both neuropsychological and neuroimaging data (19–22). One hypothesis that can be derived from the episodic memory deficit in recurrent geriatric major depressive disorder found in this study is that recurrent depressive episodes may lead to temporal lobe dysfunction through longstanding effects of decreased hippocampal neurogenesis (17).
The clinical significance of this study is that identification of the neuropsychological profile, clinical presentation, and additional risk factors in subtypes of geriatric major depressive disorder may lead to the development of specific pharmacological or nonpharmacological intervention strategies to address the different subtypes of the disorder. Such specific interventions may be especially needed because of the high rate of treatment nonresponse in geriatric major depressive disorder (39, 40). There are data to indicate that more comprehensive treatment approaches to geriatric major depressive disorder may yield higher response rates (41), and it would be interesting to see whether differential treatment of late-onset and recurrent geriatric major depressive disorder yields similar results.
The prevalences of current major depressive disorder and lifetime history of depression in our study group of very old nursing home residents are comparable to those in representative samples. The reported point prevalence of major depressive disorder in older nursing home residents ranges from 6% to 32% (7) and is thus comparable to the prevalence of about 10% in our study. Furthermore, some studies of young adults have found lifetime prevalences comparable to those in our study (4). To our knowledge, there is only one study of the lifetime prevalence of major depressive disorder in adults ages 70 years and older (42). This study reported prevalences ranging from 23% to 45% (42), comparable to our finding of a lifetime prevalence of 33.6%. Overall, the subjects in our study represent older adults with a high level of overall cognitive functioning, as measured with the MMSE, who were able to complete a full neuropsychological assessment. Individuals with dementia were excluded in order to rule out effects of cognitive dysfunction due to neurodegenerative disorders.
This study is limited by its cross-sectional design with discrete age groupings, a feature that precludes conclusions about the timing of age effects in patients with major depression. Ideally, studies of geriatric depression would follow older adults with a known presence or absence of past major depressive disorder longitudinally, to identify both age- and disease-related changes over time and their associations with risk factors and indicators of potential underlying mechanisms. Furthermore, we could not extract reliable data on the exact time (i.e., other than before age 65 years) of the onset of the first episode of major depressive disorder in the study group. Prior studies showed that, when informants and structured interviews are used, interrater reliability may exceed 80% (43). Yet, it seems vital to further refine methods to assess lifetime history of major depressive disorder, especially in older adults. Such information would enable correlative studies of neuropsychological performance decrements in recurrent geriatric major depression.
One conceptual problem underlying research on geriatric major depressive disorder as a function of lifetime history of depression is that in fact recurrent or subthreshold depression across the lifespan may in turn increase the risk of vascular pathology. Likewise, recurrent episodes of major depressive disorder across the lifespan may have different underlying etiologies. The inclusion of behavioral, genetic, and cardiovascular variables in such studies would allow for a better understanding of common pathways and differential risk factors.
This study addressed some of the limitations of prior studies by using a two-by-two design, thus disentangling effects of a lifetime history of major depression from effects of the current presence of major depressive disorder. Assessment of depression with a state measure (Geriatric Depression Scale) assured inclusion of depressed subjects with similar levels of current depression severity and made the concurrent validation of depression diagnoses possible. Controlling for age and gender in matched randomly selected groups of subjects helped to prevent the introduction of variance that might obscure group differences.
In conclusion, the contribution of this study is the delineation of specific subtypes within geriatric major depression as a function of the presence or absence of a lifetime diagnosis of depression. Further research is needed to replicate these phenomenological differences. Findings from this study may be used clinically to guide the design of treatment interventions for specific subtypes of geriatric depression and thus may provide clinicians with more satisfying treatment options for this prevalent and disabling disorder.
 |
 |
 |
 |
Received March 7, 2004; revision received May 12, 2004; accepted May 28, 2004. From the Department of Psychiatry, Mount Sinai School of Medicine; and the Department of Psychiatry, Bronx Veterans Affairs Hospital, Bronx, N.Y. Address correspondence and reprint requests to Dr. Rapp, Department of Psychiatry, Mount Sinai School of Medicine, One Gustave L. Levy Pl., Box 1230, New York, NY 10029; [email protected] (e-mail). Supported by grants P01 AG-02219 (Dr. Mohs/Dr. Haroutunian) and P01 AG-05138 (Dr. Davis/Dr. Sano) from the National Institute on Aging.
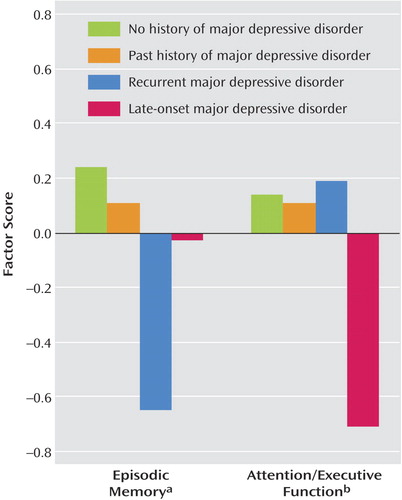
Figure 1. Cognitive Performance on Episodic Memory Tasks and Attention/Executive Function Tasks of Currently Nondepressed Older Adults With Versus Without a Past History of Major Depressive Disorder and Older Adult Patients With Recurrent Versus Late-Onset Major Depressive Disordera
aSignificant main effect for the current presence of major depressive disorder (F=7.15, df=1, 112, p<0.01) and for the presence or absence of a lifetime diagnosis of major depressive disorder (F=4.36, df=1, 112, p<0.05). In post hoc comparisons, no history of major depressive disorder = past history of major depressive disorder > late-onset major depressive disorder > recurrent major depressive disorder (t tests).
bSignificant main effect for the current presence of major depressive disorder (F=4.25, df=1, 112, p<0.05) and for the presence or absence of a lifetime diagnosis of major depressive disorder (F=5.37, df=1, 112, p<0.05). In post hoc comparisons, no history of major depressive disorder = past history of major depressive disorder = recurrent major depressive disorder > late-onset major depressive disorder (t tests).
1. Belsher G, Costello CG: Relapse after recovery from unipolar depression: a critical review. Psychol Bull 1988; 104:84–96Crossref, Medline, Google Scholar
2. Judd LL, Akiskal HS, Paulus MP: The role and clinical significance of subsyndromal depressive symptoms (SSD) in unipolar major depressive disorder. J Affect Disord 1997; 45:5–18Crossref, Medline, Google Scholar
3. Kessler RC, McGonagle KA, Nelson CB, Hughes M, Swartz M, Blazer DG: Sex and depression in the National Comorbidity Survey, II: cohort effects. J Affect Disord 1994; 30:15–26Crossref, Medline, Google Scholar
4. Waraich P, Goldner EM, Somers JM, Hsu L: Prevalence and incidence studies of mood disorders: a systematic review of the literature. Can J Psychiatry 2004; 49:124–138Crossref, Medline, Google Scholar
5. Horwath E, Johnson J, Klerman GL, Weissman MM: Depressive symptoms as relative and attributable risk factors for first-onset major depression. Arch Gen Psychiatry 1992; 49:817–823Crossref, Medline, Google Scholar
6. Judd LL, Akiskal HS, Maser JD, Zeller PJ, Endicott J, Coryell W, Paulus MP, Kunovac JL, Leon AC, Mueller TI, Rice JA, Keller MB: A prospective 12-year study of subsyndromal and syndromal depressive symptoms in unipolar major depressive disorders. Arch Gen Psychiatry 1998; 55:694–700Crossref, Medline, Google Scholar
7. Blazer DG: Depression in late life: review and commentary. J Gerontol A Biol Sci Med Sci 2003; 58:249–265Crossref, Medline, Google Scholar
8. Hickie I, Scott E: Late-onset depressive disorders: a preventable variant of cerebrovascular disease? (editorial). Psychol Med 1998; 28:1007–1013Crossref, Medline, Google Scholar
9. Taylor WD, Steffens DC, MacFall JR, McQuoid DR, Payne ME, Provenzale JM, Krishnan KR: White matter hyperintensity progression and late-life depression outcomes. Arch Gen Psychiatry 2003; 60:1090–1096Crossref, Medline, Google Scholar
10. Salloway S, Correia S, Boyle P, Malloy P, Schneider L, Lavretsky H, Sackheim H, Roose S, Krishnan KR: MRI subcortical hyperintensities in old and very old depressed outpatients: the important role of age in late-life depression. J Neurol Sci 2002; 203/204:227–233Google Scholar
11. Ballmaier M, Toga AW, Blanton RE, Sowell ER, Lavretsky H, Peterson J, Pham D, Kumar A: Anterior cingulate, gyrus rectus, and orbitofrontal abnormalities in elderly depressed patients: an MRI-based parcellation of the prefrontal cortex. Am J Psychiatry 2004; 161:99–108Link, Google Scholar
12. Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M: “Vascular depression” hypothesis. Arch Gen Psychiatry 1997; 54:915–922Crossref, Medline, Google Scholar
13. Krishnan KRR, Hays JC, Blazer DG: MRI-defined vascular depression. Am J Psychiatry 1997; 154:497–501Link, Google Scholar
14. Drevets WC: Geriatric depression: brain imaging correlates and pharmacologic considerations. J Clin Psychiatry 1994; 55(suppl A):71–81Google Scholar
15. Sheline YI, Gado MH, Kraemer HV: Untreated depression and hippocampal volume loss. Am J Psychiatry 2003; 160:1516–1518Link, Google Scholar
16. Bell-McGinty S, Butters MA, Meltzer CC, Greer PJ, Reynolds CF III, Becker JT: Brain morphometric abnormalities in geriatric depression: long-term neurobiological effects of illness duration. Am J Psychiatry 2002; 159:1424–1427Link, Google Scholar
17. Kempermann G, Kronenberg G: Depressed new neurons—adult hippocampal neurogenesis and a cellular plasticity hypothesis of major depression. Biol Psychiatry 2003; 54:499–503Crossref, Medline, Google Scholar
18. Forsell Y, Jorm AF, Winblad B: Association of age, sex, cognitive dysfunction, and disability with major depressive symptoms in an elderly sample. Am J Psychiatry 1994; 151:1600–1604Link, Google Scholar
19. Simpson SW, Baldwin RC, Burns A, Jackson A: Regional cerebral volume measurements in late-life depression: relationship to clinical correlates, neuropsychological impairment and response to treatment. Int J Geriatr Psychiatry 2001; 16:469–476Crossref, Medline, Google Scholar
20. Fossati P, Harvey PO, Le Bastard G, Ergis AM, Jouvent R, Allilaire JF: Verbal memory performance of patients with a first depressive episode and patients with unipolar and bipolar recurrent depression. J Psychiatr Res 2004; 38:137–144Crossref, Medline, Google Scholar
21. Sweeney JA, Kmiec JA, Kupfer DJ: Neuropsychologic impairments in bipolar and unipolar mood disorders on the CANTAB neurocognitive battery. Biol Psychiatry 2000; 48:674–684Crossref, Medline, Google Scholar
22. Nebes RD, Pollock BG, Houck PR, Butters MA, Mulsant BH, Zmuda MD, Reynolds CF III: Persistence of cognitive impairment in geriatric patients following antidepressant treatment: a randomized, double-blind clinical trial with nortriptyline and paroxetine. J Psychiatr Res 2003; 37:99–108Crossref, Medline, Google Scholar
23. Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO: Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1982–1983; 17:37–49Google Scholar
24. Spitzer RL, Williams JB, Gibbon M, First MB: The Structured Clinical Interview for DSM-III-R (SCID), I: history, rationale, and description. Arch Gen Psychiatry 1992; 49:624–629Crossref, Medline, Google Scholar
25. Baldessarini RJ, Finklestein S, Arana GW: The predictive power of diagnostic tests and the effect of prevalence of illness. Arch Gen Psychiatry 1983; 40:569–573Crossref, Medline, Google Scholar
26. Fleiss JL: Statistical Methods for Rates and Proportions, 2nd ed. New York, John Wiley & Sons, 1981Google Scholar
27. Milligan GW, Wong DS, Thompson PA: Robustness properties of nonorthogonal analysis of variance. Psychol Bull 1987; 101:464–470Crossref, Google Scholar
28. Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Crossref, Medline, Google Scholar
29. Wechsler D: Wechsler Adult Intelligence Scale Manual. New York, Psychological Corp, 1955Google Scholar
30. Reitan RM: The relation of the Trail Making Test to organic brain damage. J Consult Psychol 1955; 19:393–394Crossref, Medline, Google Scholar
31. Arbuthnott K, Frank J: Trail Making Test, part B as a measure of executive control: validation using a set-switching paradigm. J Clin Exp Neuropsychol 2000; 22:518–528Crossref, Medline, Google Scholar
32. Rogers CA: The structure of verbal fluency. Br J Psychol 1953; 44:368–380Medline, Google Scholar
33. Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C: The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD), I: clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 1989; 39:1159–1165Crossref, Medline, Google Scholar
34. Schulman AI: Recognition memory for targets from a scanned word list. Br J Psychol 1971; 62:335–346Crossref, Medline, Google Scholar
35. Jahnke JC: Delayed recall and the serial-position effect of short-term memory. J Exp Psychol 1968; 76:618–622Crossref, Medline, Google Scholar
36. Mandrekar SJ, Mandrekar JN: Are our data symmetric? Stat Methods Med Res 2003; 12:505–513Crossref, Medline, Google Scholar
37. Lockwood KA, Alexopoulos GS, van Gorp WG: Executive dysfunction in geriatric depression. Am J Psychiatry 2002; 159:1119–1126Link, Google Scholar
38. Lockwood KA, Alexopoulos GS, Kakuma T, Van Gorp WG: Subtypes of cognitive impairment in depressed older adults. Am J Geriatr Psychiatry 2000; 8:201–208Crossref, Medline, Google Scholar
39. Alexopoulos GS, Meyers BS, Young RC, Kalayam B, Kakuma T, Gabrielle M, Sirey JA, Hull J: Executive dysfunction and long-term outcomes of geriatric depression. Arch Gen Psychiatry 2000; 57:285–290Crossref, Medline, Google Scholar
40. Kalayam B, Alexopoulos GS: Prefrontal dysfunction and treatment response in geriatric depression. Arch Gen Psychiatry 1999; 56:713–718Crossref, Medline, Google Scholar
41. Thomas L, Mulsant BH, Solano FX, Black AM, Bensasi S, Flynn T, Harman JS, Rollman BL, Post EP, Pollock BG, Reynolds CF III: Response speed and rate of remission in primary and specialty care of elderly patients with depression. Am J Geriatr Psychiatry 2002; 10:583–591Crossref, Medline, Google Scholar
42. Palsson SP, Ostling S, Skoog I: The incidence of first-onset depression in a population followed from the age of 70 to 85. Psychol Med 2001; 31:1159–1168Crossref, Medline, Google Scholar
43. Wiener P, Alexopoulos GS, Kakuma T, Meyers BS, Rosenthal E, Chester J: The limits of history-taking in geriatric depression. Am J Geriatr Psychiatry 1997; 5:116–125Crossref, Medline, Google Scholar



