Untreated Depression and Hippocampal Volume Loss
Abstract
OBJECTIVE: The purpose of this study was to investigate the effect of antidepressant treatment on hippocampal volumes in patients with major depression. METHOD: For 38 female outpatients, the total time each had been in a depressive episode was divided into days during which the patient was receiving antidepressant medication and days during which no antidepressant treatment was received. Hippocampal gray matter volumes were determined by high resolution magnetic resonance imaging and unbiased stereological measurement. RESULTS: Longer durations during which depressive episodes went untreated with antidepressant medication were associated with reductions in hippocampal volume. There was no significant relationship between hippocampal volume loss and time depressed while taking antidepressant medication or with lifetime exposure to antidepressants. CONCLUSIONS: Antidepressants may have a neuroprotective effect during depression.
Major depression is characterized by a high rate of recurrence. Even when on long-term antidepressant regimens, approximately 20%–80% of patients experience another depressive episode within 1–5 years after responding to treatment (1). Patients not receiving medication have an even higher risk of relapse and recurrence (2). Some brain volumetric studies, but not all (3), have found significant reductions in hippocampal volumes in patients with recurrent major depression. Hippocampal volume loss related to the total duration of depression has been reported and has been associated with abnormalities on a neuropsychological measure of hippocampal function (4), supporting the idea that hippocampal volume reduction results in functionally significant damage. Differences in methodology, including resolution, boundary determination, and measurement techniques, may account for some discrepancies among magnetic resonance imaging (MRI) studies. Other factors may include depression chronicity, severity, and treatment refractoriness. However, an even more critical factor may be variability in lifetime history of antidepressant use. In this study, we examined whether taking antidepressant medication during depressive episodes predicted hippocampal volumes.
Method
We recruited 38 right-handed outpatient female subjects with recurrent depression in remission according to DSM-IV criteria and 38 comparison subjects matched for age, education, and height. The subjects with remitted depression are referred to hereafter as “depressed subjects.” Subjects were screened to exclude medical problems potentially affecting the CNS and were specifically screened to exclude incipient dementia (assessed by using Consortium to Establish a Registry for Alzheimer Disease criteria). The Clinical Dementia Rating of all subjects was 0. DSM-IV criteria were used to determine each past episode of recurrent major depression as part of a structured clinical interview, the Diagnostic Interview for Genetic Studies, and to exclude other psychiatric diagnoses. Current depressive symptoms were assessed with the 17-item Hamilton Depression Rating Scale. The life charting method of Post et al. (5) was used to anchor major depression episodes and to determine lifetime exposure to antidepressants. Corroborating information was obtained from family members or treating psychiatrists. Time course of antidepressant treatment was established to divide depressed time into “treated” and “untreated.” Subjects were interviewed twice by independent interviewers, with interviews separated in time by 3 years. The intraclass correlation coefficients were moderate to high for cumulative treated time during depressive episodes (0.92) and for depressed time untreated by antidepressant medication (0.74). Brain volumetric data for 24 of these subjects were previously reported (4).
MRI scans were obtained by using a Siemens 1.5-T imaging system (Siemens Medical Systems, Inc., Erlangen, Germany) with an identical protocol and measurement techniques to those previously described (4). Hippocampal anatomical boundaries were defined by specific rules (4, 6) that used stereological estimation methods. Means for volumes were determined from an average of two independent measures by the same rater, who was blind to subject identity. Intrarater reliabilities were determined by using the intraclass R (>0.90 for all measures).
Two-tailed paired t tests were used to compare depressed and comparison subjects on all demographic and MRI measures. Simple regression analyses determined the analysis of variance (ANOVA) for independent variables: cumulative “untreated” or “treated” time during depressive episodes. These variables were used to predict left, right, and total hippocampal gray matter volume. In addition, ANOVAs determined the relationship between lifetime exposure to antidepressants and hippocampal volume and between untreated depressed time and total cerebral cortex volumes. The Pearson correlation coefficient was used to determine the relationship between treated and untreated time depressed. In a post hoc analysis, we determined the analysis of covariance (ANCOVA) for the potentially confounding variables of age, education, and age at onset in predicting hippocampal volumes.
Results
Depressed subjects ranged in age from 23 to 86 years (mean=50.8, SD=17.1), had a mean of 15.8 years of education (SD=3.3), and were a mean height of 64.7 inches (SD=2.3). The Hamilton depression scale scores (mean=6.7, SD=4.7) were consistent with the absence of current depression. Mean brain volumetric data for the depressed subjects were as follows: 4374 mm3 (SD=601) for total hippocampal gray matter volume; 2171 mm3 (SD=316) for left hippocampal gray matter volume; and 2203 mm3 (SD=315) for right hippocampal gray matter volume. The mean total cerebral cortical volume was 1057 cm3 (SD=152). For the comparison subjects, the mean total, left, and right hippocampal gray matter volumes were 4850 mm3 (SD=631), 2421 mm3 (SD=318), and 2429 mm3 (SD=326), respectively, with a mean total cerebral cortical volume of 1054 cm3 (SD=154). Mean left, right, and total hippocampal gray matter volumes were significantly smaller in depressed subjects than in comparison subjects, with average differences of 10% (t=3.5, df=37, p=0.001), 9% (t=3.0, df=37, p=0.005), and 10% (t=3.3, df=37, p=0.002), respectively. There were no significant between-group differences in age, education, height, or total cerebral cortical volume (all p>0.50).
Patients had a mean of 5.4 episodes of major depression (range=1–20) accounting for a lifetime mean of 1,341 days depressed (SD=1,096, range=56–4,561, median=1,112). The total number of days depressed was divided into the number of days untreated (mean=824, SD=834) and treated (mean=517 days, SD=606) with antidepressants. Untreated time depressed and total hippocampal gray matter volume were significantly and inversely related (Figure 1). This was true for both left (beta=–0.21, R2=0.29; F=14.9, df=1, 36, p=0.0005) and right (beta=–0.18, R2=0.22; F=10.3, df=1, 36, p=0.003) hippocampal gray matter volume. No relationship was detected between the number of days depression went untreated and cerebral cortical volume or between the number of days a depressive episode was being treated and total hippocampal gray matter volume. The number of days of treated and untreated depression were not significantly correlated with each other (r=0.14, df=36, p=0.41). A comparison of correlated correlation coefficients (7) revealed that the number of days the depression went untreated was significantly more correlated with total hippocampal volume than was the number of treated depression days (z=1.82, p=0.03). No relationship was detected between lifetime exposure to antidepressants and total hippocampal volume. In a post hoc analysis, we conducted an ANCOVA with age, education, age at illness onset, and days of untreated depression (overall F=3.2, df=4, 32, p=0.02). In this model, there was no significant relationship between total hippocampal volume and age (p=0.67), education (p=0.15), or age at illness onset (p=0.66), whereas days of untreated depression was still significantly and inversely correlated with total hippocampal volume (p=0.008).
Discussion
The key implication of this study is that antidepressants may protect against hippocampal volume loss associated with cumulative episodes of depression. Hippocampal volume was significantly predicted by duration of untreated depression, whereas there was no relationship detected between cumulative time treated with antidepressants during depression. While these results could also be affected if those patients with a high proportion of untreated time had lower socioeconomic status or worse general health profile, the study group was fairly narrowly distributed in socioeconomic status (SD=3.3 for years of education), and the subjects were carefully screened to exclude physical health problems.
Repeated or severe stress has been reported to lead to adverse effects on hippocampal neuronal function through elevated glucocorticoid levels (8), decreases in brain-derived neurotrophic factor (9), and decreases in neurogenesis (10). While recent postmortem studies in patients who suffered from major depression have not found evidence for neuronal cell death (11, 12), they found evidence for hippocampal synaptic reorganization (11) and increased neuronal and glial cell packing density (12), suggesting a decrease in the hippocampal neuropil in major depressive disorder.
Animal studies have shown antidepressants to protect against stress-induced decrease in neurogenesis with preservation of hippocampal volume during a social stress paradigm (13). Our study suggests that in addition to their effects on mood and functioning, antidepressants may have similar neuroprotective effects in humans. Since we could not analyze volume effects by specific antidepressants, we do not know if some antidepressants are more neuroprotective than others. Nevertheless, depression-related volume loss does appear to be cumulative, suggesting that immediate recognition and treatment of depressive episodes is important in order to prevent damage occurring with repeated episodes of depression.
Received Aug. 13, 2002; revision received Jan. 22, 2003; accepted Feb. 6, 2003. From the Department of Psychiatry, the Department of Radiology, and the Mallinckrodt Institute of Radiology, Washington University School of Medicine; and the Department of Psychiatry and Behavioral Sciences, Stanford University, Stanford, Calif. Address reprint requests to Dr. Sheline, Department of Psychiatry, Campus Box 8134, Washington University School of Medicine, 660 South Euclid Ave., St. Louis, MO 63110; [email protected] (e-mail). Supported by NIMH grants MH-01370 and MH-58444 to Dr. Sheline and grant RR-00036 from the NIH Division of Research Resources to the General Clinical Research Center at Washington University School of Medicine. The authors thank Brigitte Mittler for research assistance and Kathy Bucholz, Ph.D., for comments on the manuscript.
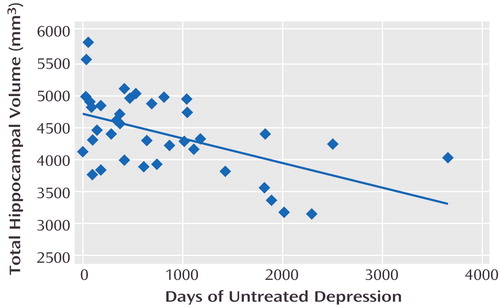
Figure 1. Relationship Between Hippocampal Volume and Days of Untreated Depression in 38 Female Outpatients With Recurrent Depression in Remissiona
aThe total time each patient had been in a depressive episode was divided into days during which the patient was receiving antidepressant medication versus days during which no antidepressant medication was being given. The regression plot shown here depicts the significant inverse relationship between total hippocampal volume and the length of time depression went untreated (beta=–0.38; R2=0.28; F=14.0, df=1, 36, p=0.0006).
1. Nierenberg AA, Alpert JE: Depressive breakthrough. Psychiatry Clin North Am 2000; 23:731–742Crossref, Medline, Google Scholar
2. Frank E, Kupfer DJ, Perel JM: Three-year outcomes for maintenance therapies in recurrent depression. Arch Gen Psychiatry 1990; 47:1093–1099Crossref, Medline, Google Scholar
3. MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, Nahmias C, Young LT: Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci USA 2003; 100:1387–1392Crossref, Medline, Google Scholar
4. Sheline YI, Sanghavi M, Mintun MA, Gado M: Depression duration but not age predicts hippocampal volume loss in women with recurrent major depression. J Neuroscience 1999; 19:5034–5043Crossref, Medline, Google Scholar
5. Post RM, Roy-Byrne PP, Uhde T: Graphic representation of the life course of illness in patients with affective disorder. Am J Psychiatry 1988; 145:844–848Link, Google Scholar
6. Bartzokis G, Mintz J, Marx P, Osborn D, Gutkind D, Chiang F, Phelan CK, Marder SR: Reliability of in vivo volume measures of hippocampus and other brain structures using MRI. Magn Reson Imaging 1993; 11:993–1006Crossref, Medline, Google Scholar
7. Meng X-L, Rosenthal R, Rubin D: Comparing correlated correlation coefficients. Psychol Bull 1992; 111:172–175Crossref, Google Scholar
8. Sapolsky RM, Krey LC, McEwen BS: The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocrinol Rev 1986; 7:284–301Crossref, Medline, Google Scholar
9. Mamounas LA, Blue ME, Siuciak JA, Altar C: BDNF promotes the survival and sprouting of serotonergic axons in the rat brain. J Neurosci 1995; 15:7929–7939Crossref, Medline, Google Scholar
10. Gould E, McEwen BS, Tanapat P, Galea LAM, Fuchs E: Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci 1997; 17:2492–2498Crossref, Medline, Google Scholar
11. Muller MB, Lucassen PJ, Yassouridis A, Hoogendijk WJ, Holsboer F, Swaab DF: Neither major depression nor glucocorticoid treatment affects the cellular integrity of the human hippocampus. Eur J Neurosci 2001; 14:1603–1612Crossref, Medline, Google Scholar
12. Stockmeier CA, Mahajan G, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY, Friedman L, Rajkowska G: Neuronal and glial density is increased and neuronal soma size is decreased in the hippocampus in major depressive disorder (MDD). Biol Psychiatry 2003; 53S:198Google Scholar
13. Czeh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, Bartolomucci A, Fuchs E: Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci USA 2001; 98:12796–12801Crossref, Medline, Google Scholar



