Association of Resolution of Major Depression With Increased Natural Killer Cell Activity Among HIV-Seropositive Women
Abstract
OBJECTIVE: Depression is a potential risk factor for morbidity and mortality among patients with numerous medical conditions, including HIV disease, and it is also associated with decrements in immune function, such as natural killer (NK) cell activity. This study examined whether improvements in the diagnostic status of major depression are related to increases in NK cell activity among HIV-seropositive women. METHOD: HIV-seropositive women were recruited as part of a longitudinal cohort study and underwent comprehensive medical and psychiatric evaluations during a 2-year period. Fifty-seven women had complete NK cell activity and depression data measured at two time points and were examined for associations between changes in depression status and alterations in NK cell activity over time. RESULTS: Among the 57 HIV-seropositive women, improvements in the diagnostic status of depression and decreases in scores on the 17-item Hamilton Depression Rating Scale were significantly associated with increases in NK cell activity over time, as measured in lytic units. Eleven women (19.3%) had a major depression diagnosis that resolved over time, and this group also had a significant increase in cell activity measured in lytic units during this period. CONCLUSIONS: This study suggests that depression may impair certain aspects of innate cellular immunity relevant to delaying the progression of HIV disease and that these alterations are reversible with the resolution of a depressive episode. These findings support an examination of NK cell activity in assessments of the relationship between depression and morbidity and mortality in HIV disease.
Depression is potential risk factor for increased morbidity and mortality across numerous medical conditions (1), including HIV and AIDS (2–4). In chronic HIV disease, depression may adversely affect quality of life and adherence to medication regimens (5, 6), which may subsequently affect disease progression and health outcomes. Our work has suggested that killer lymphocytes may be altered during depression and subsequently affect the HIV viral load (7), although the exact mechanisms involved are not yet delineated.
Depressive symptoms are frequently reported among HIV-seropositive individuals, and several studies have documented elevated rates of major depression and subclinical depressive symptoms among HIV-seropositive homosexual men (8–12), although these rates are similar to those of HIV-seronegative homosexual men in the general population. The majority of these early studies of the prevalence of depression focused almost exclusively on men because of the demographics of HIV disease at the time. However, women now account for just over 50% of those infected with HIV globally, and the rates of new HIV cases among women in the United States and many other nations are rising (13). Because medically healthy women in the general population are also diagnosed with depression more often than men (14), it is increasingly salient to study the prevalence and effects of depression among HIV-seropositive women as well.
Recent studies have reported increased rates of the prevalence of depression and other mood disturbances among HIV-seropositive women. Morrison and colleagues (15) examined the prevalence of depressive disorders among 93 HIV-seropositive and 62 HIV-seronegative women and found that HIV-seropositive women without an active substance abuse problem had a significantly higher prevalence rate of major depressive disorder (19.4%) than HIV-seronegative women (4.8%). In a prospective longitudinal cohort study of 765 HIV-seropositive women, 42% reported chronic depressive symptoms, and 35% had intermittent depressive symptoms (2). In this study, depression was also associated with markers of the progression of HIV disease and mortality. Although the exact mechanisms underlying the association between depression and disease morbidity and mortality are unknown, the effects of depression on the immune system may represent a key mechanistic pathway (16, 17).
Associations between depression and immune system impairments among medically healthy individuals have been observed. The severity of depression has been associated with decrements in several in vitro measures of immunity (e.g., lower CD8+ T [antigenic marker on suppressor/cytotoxic T] cells and natural killer [NK] cell numbers and activity) in a large-scale meta-analysis (18). Patients diagnosed with depression, especially severe depressive states, also have pronounced decrements in immunity (19). Evans et al. (20) examined the effects of major depression on peripheral blood NK cell phenotypes and NK cell activity among depressed and nondepressed comparison participants. Depressed subjects exhibited significant reductions in NK effector cell numbers, Leu-11 (CD16) and Leu-7 (HNK-1), and NK cell activity.
Studies examining the association between depression and immunity, particularly CD4+ T lymphocyte counts, in HIV disease have reported both positive and negative findings (21–26). These results may be due to differences in psychiatric assessments or the patient samples studied or perhaps to a reliance on broad indices of immune status (e.g., CD4+ T cell counts), which may not be the most sensitive or reliable indicators of immunity. Depression is associated with alterations in more specific measures of immune function among HIV-seropositive women. For example, depression was associated with decrements in killer lymphocyte function among a group of 63 HIV-seropositive women (7). These measures are a specific indicator of innate immunity, which may be relevant to the progression of HIV disease; however, to our knowledge, no study to date has examined the association between changes in depression status and NK cell activity over time among HIV-seropositive women.
Irwin and colleagues (27) assessed NK cell lytic activity at intake and at a 6-month follow-up among medically healthy depressed individuals and comparison subjects in a longitudinal case-control design and found that as depression scores decreased, NK cell activity increased in the depressed subjects, but neither changed in the comparison subjects. Frank et al. (28) examined medically healthy depressed individuals and found that 4 weeks of fluoxetine treatment was associated with augmented NK cell activity in a subgroup of subjects that initially exhibited low NK cell activity. Schleifer and colleagues (29), however, found no change in NK cell numbers or cell activity among 21 medically healthy adults with major depression after 6 weeks of antidepressant treatment, although they did find both decreased NK cell numbers and function during initial depressive states. One way to reconcile these differences is to investigate subjects over longer time intervals with more specific immune assays, which may allow for more opportunities to observe alterations in depression and more precise changes in immunity.
In the current study, we assessed the status of a diagnosis of major depression and immune system functioning in a group of HIV-seropositive women over a period of up to 2 years. We predicted that resolution of the major depression and improvement in acute depressive symptoms would be significantly associated with increases in NK cell activity over time. We further hypothesized that a subgroup of women who showed significant improvements in mood (as indicated by a resolution of a major depression diagnosis) over time would also show improvements in immune functioning, as measured by the specific functional measure of innate immunity, NK cell activity. We also predicted that a subgroup of HIV-seropositive women who did not meet the diagnostic criteria for major depression would show no significant changes in NK cell activity.
Method
The data for this study were collected from two sites (Gainesville, Fla., and Philadelphia) as part of a prospective, longitudinal cohort study investigating the neuropsychiatric, endocrine, and immune aspects of HIV infection in women (7). The current study specifically examines the association between depression and immune data obtained across a 2-year period.
Participants
HIV-seropositive women were recruited from outpatient clinics, public health departments, and other organizations focused on HIV disease and care through a combination of community presentations, clinician referrals, word of mouth, and media advertisements. The specific inclusion and exclusion criteria were detailed in a previous report (7). Fifty-seven HIV-seropositive women had complete depression and immune data measured from at least two time points during the study period and thus were available for inclusion in the current main analyses. These 57 women were comparable to women without such matched data on relevant demographic, medical, and depression data (7). To further understand whether NK cell activity, measured in lytic units (LU), changes with resolution in the clinical diagnosis of major depression, we examined participants who experienced a change in diagnosis over the follow-up period. Eleven of the 57 women were diagnosed with major depression either at study entry or early on (i.e., within the first year of the study), but they did not meet criteria for major depression at the follow-up assessment. Forty-three women did not meet the criteria for major depression at the initial and final assessment time points, and three women who were not initially depressed became depressed. We focused this part of the investigation on the women whose depression resolved over time (N=11).
HIV serostatus was confirmed by using an enzyme-linked immunosorbent assay with Western blot analysis for confirmation of the presence of anti-HIV-1 antibodies. All women were fully aware of their HIV-seropositive status at study entry.
Procedures
The institutional review boards of the University of Pennsylvania and the University of Florida approved the protocol. The participants were assessed at baseline and followed up every 6 months across the 2-year period. All participants provided written informed consent and were reimbursed for their time, travel expenses, and child-care expenses.
Psychiatric and Medical Assessments
Each participant received a thorough outpatient assessment at study entry and at follow-up over a 2-year period, which included a physical examination and a structured psychiatric interview. Current and lifetime DSM axis I diagnoses, including major depressive disorder, were assessed by a psychiatric clinician with a modified version of the Structured Clinical Interview for DSM-IIII-R (SCID) (30). Consensus diagnoses were determined at team meetings; the clinicians’ rating/diagnosing was blind to patient immune status, and all immune assessments were performed with blinding for rating/diagnostic status. We also assessed acute symptoms of depression with the 17-item Hamilton Depression Rating Scale (31). HIV medication status was coded as a categorical variable pertaining to whether or not the woman was taking antiretroviral medication or protease inhibitors.
Immune Assessments
To control for potential circadian effects on immunity, all participants were evaluated at the same time of day. Specifically, the participants were placed in a recumbent position, an intravenous line was started at approximately 9:00 a.m., and intravenous line patency was maintained with a slow normal saline drip. Blood was obtained approximately 1 hour later. Blood cell counts and flow cytometry panels were performed on peripheral blood samples, as detailed previously (7). NK cell activity was assessed by using standard techniques established in our laboratory (32). Lytic units/107 of peripheral blood mononuclear cells and lytic units/107 of NK cells were calculated with the method of Bryant et al. (33) and Friberg et al. (34). By measuring the percentage of CD16+/CD56+ cells in the preparation of peripheral blood mononuclear cells, we determined the lytic units of NK activity (LUNK) per NK cell (35). Expressing the NK data as LUNK cell adjusts for differences in the percentage of NK cells (CD16+/CD56+) in the effector cell population. Thus, our primary outcome measure of innate immunity was expressed as LUNK cell activity. We took the log of LUNK to make the distribution more symmetric and to equalize variances for group comparisons.
Viral Assessments
Serum HIV RNA viral load was determined from archived samples with the Amplicor Monitor assay (Roche Diagnostics, Branchburg, N.J.). The lower limit of quantification for this assay is 400 copies per milliliter of blood. Because approximately half the group measured at this lower limit and standard transformations, such as the log, would not normalize the distribution, we analyzed viral load as a dichotomous variable. Thus, viral load was quantified as either nondetectable (less than or equal to 400 copies/ml) or detectable (greater than 400 copies/ml) in the current study.
Statistical Analyses
Two different analyses were performed to evaluate the overall association between different depression measures as the time-varying covariate and log (LUNK) cell activity over time as the longitudinal dependent variable. First, to analyze such associations for the entire group (N=57), we employed separate linear regression models for each depression measure with subject effects included as fixed effects to account for significant heterogeneity among the subjects with respect to both LUNK and the depression measure, which would cause confounding under a random effects model. The estimate of the longitudinal correlation was obtained from the estimated regression slope divided by the model-based standard deviations for log (LUNK) cell activity and the depression measure. Second, for the analysis of the 11 women who exhibited a change in depression status, we used Wilcoxon’s signed rank tests to examine whether there were changes in LUNK cell activity during the period depression resolved. Similar tests were performed for the remaining women across an equivalent period of time. Finally, we used Spearman’s r for continuous and ordinal outcomes and chi-square tests for binary outcomes to compare different groups of subjects with respect to demographic and medical variables. In all analyses, we adjusted for baseline viral load, antiretroviral medication use, antidepressant treatment status, and time interval (in months) between assessments.
Results
The demographic and behavioral characteristics of the 57 HIV-seropositive women with depression and immune data that were available from at least two time points were obtained from each site (Gainesville, Fla., and Philadelphia) and were comparable, as reported in our earlier work (7, 15). The mean age of the women was 39.7 years (SD=7.1). About 58% of the women were African American, and 35% were Caucasian. The majority of the women (81%) were taking an established regimen of HIV medications, and half of the group had a viral load in the detectable range. CD3+/CD4+ T cell counts averaged 521.6 (SD=7.1) for the entire group. Thirty-seven (65%) of the 57 women had a prior history of major depression, as assessed by the SCID at the initial assessment. Nineteen percent of the women reported that they were given a prescription for an antidepressant medication at the initial assessment. There were no significant differences between the groups on the relevant demographic and medical variables examined in this study.
The average time interval (in months) between the initial assessment and the final assessment for the group of 57 woman was 12.74 months (SD=5.79), with an average time interval of 13.64 months (SD=6.62) for the group of 11 women who were depressed and later had a resolution and 12.28 months (SD=5.70) for the group of 43 nondepressed women. There was no significant difference in time interval between the groups (χ2=0.36, df=1, p=0.55).
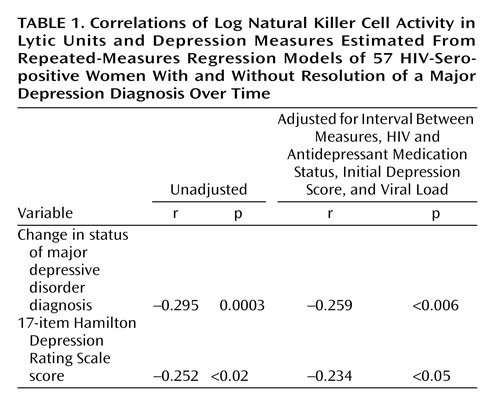
By employing linear regression with the subject as a fixed effect to evaluate associations between depression and immunity in the entire group (Table 1), improvements in the diagnostic status of depression (r=–0.259, p<0.006) and decreases in Hamilton depression scale scores (r=–0.234, p<0.05) were significantly associated with increases in log (LUNK) cell activity over time. These associations were significant, even when we controlled for initial depression diagnostic status or Hamilton depression scale scores, respectively, as well as viral load, HIV medication status, antidepressant medication status, and the time interval between assessments.
Hamilton depression scale scores paralleled major depression diagnostic status over time, improving significantly (mean=17.8, SD=6.5, to mean=5.9, SD=5.7) in the 11 women with a resolution of their major depression diagnosis (z=–32, p=0.002) and showing no significant change (mean=6.7, SD=5.9, to mean=5.7, SD=5.7, in the 43 women who were nondepressed (z=–53, p=0.42). In the group with improved depression, eight (73%) of the 11 women had remitted depression, as defined by a Hamilton depression scale score of 7 or less; one woman met criteria for dysthymic disorder but not major depression at the final assessment.
LUNK cell activity of the group with resolved depression (N=11) and the nondepressed group (N=43) of HIV-seropositive women over time is presented in Figure 1. In the resolution group (11 of 57, 19.3%), representing women whose depression diagnostic status improved, there was also a significant increase (mean=5.6, SD=1.4, to mean=7.0, SD=1.0) in log (LUNK) cell activity over time (z=28, p=0.009). In fact, 82% (nine of 11) of the women in this group had improvement in log (LUNK) cell activity that paralleled resolution of their major depression diagnostic status. If we include the three women who entered the study as nondepressed but became depressed by their final assessment, along with the 11 women with a resolution of their depression diagnostic status, there remains a significant absolute change in log (LUNK) cell activity among these 14 women (z=43.5, p=0.004) over time. Of these 14 women who experienced a change in their major depression diagnostic status in either direction, 11 (79%) had a significant parallel change in log (LUNK) cell activity over time. In the nondepressed group (43 of 57, 75.4%), however, there were no significant changes (mean=6.6, SD=1.6, to mean=6.6, SD=1.4) in log (LUNK) cell activity (z=18, p=0.83) during an equivalent period.
Discussion
This study examined the association between the diagnostic status of major depression, acute depressive symptoms, and a specific measure of innate immunity—LUNK cell activity—over time among a contemporary group of HIV-seropositive women. The women whose depression diagnostic status resolved over time also showed a significant increase in LUNK cell activity across this same period. However, the women who did not meet the diagnostic criteria for major depressive disorder did not show a significant change in immunity during an equivalent period. This study is one of the first to demonstrate that an improvement in depression diagnostic status results in a parallel change in a specific indicator of immune system functioning relevant to progression of HIV disease. These findings extend our previous work demonstrating significant inverse relationships between depression and NK cell activity in a cross-sectional study (7).
To our knowledge, this is also the first study in HIV disease to show that change in the diagnostic status of major depression and reduction in acute depressive symptoms are significantly associated with increases in LUNK cell activity over time. This association was observed even though the Hamilton depression scale scores of the women in our depressed group were in the moderate range, as observed in our earlier studies of HIV-seropositive women (7, 15), which indicates that this association may be even stronger in more severely depressed groups. Our more direct measure of NK cell functional activity (LUNK) may be an underlying mechanism of the effects of depression on HIV morbidity and mortality observed in previous studies (2–4). Prior HIV studies relating depression and immunity have reported mixed results when the authors used more global indices of immune status, such as CD4+ T cell counts (21–26). The results of the current study point to the benefits of employing more specific indicators of immunity, such as LUNK cell activity, which are potentially relevant to the progression of HIV disease.
Clinical studies of depression in subjects without other medical illness have demonstrated significant alterations in NK cells (20), a cellular immune population that may play a key role in regulating HIV infection. There is mounting evidence that NK cells exert anti-HIV effects by both classic killing activity as well as the production of HIV-suppressive factors. Specifically, NK cells may be involved in a natural resistance against viral infection and may have the capacity to lyse HIV-1 infected cells (36–40). In our studies of HIV-infected men, alterations of NK lymphocytes associated with stress and depression were observed (23, 24), suggesting that killer lymphocytes mediate the effects of depression in the earlier stages of the progression of HIV disease. Ironson et al. (41) observed that NK cell number and function are preserved among AIDS patients with low CD4+ T cell counts and stated that these immune factors may be important in maintaining the health and well-being of these individuals. Thus, the mechanism underlying the impact of depression on the progression of HIV disease may operate in part by altering NK cell numbers and activity.
There are a number of limitations to the current study. The design was a prospective cohort study and not a population-based study, and thus, a sampling bias may have resulted. Although recruitment was open to women of all races and ethnic backgrounds, African American and Caucasian participants comprised the majority of the participants. Thus, these findings may not be generalizable to Hispanic or Asian populations. We also excluded women who were currently abusing alcohol or other substances to reduce the confounding effects of these substances on depression and immunity. This may also limit the generalizability of our findings. This was an observational study, not a treatment study, so we did not have complete data on adherence to specific antidepressant treatment (either pharmacological or psychological), and thus, it is difficult to ascertain whether the alterations in depression observed were due to a specific treatment or simply to the passage of time. Regardless of how depression improved in these women, the associated enhancement in immunity is noteworthy and warrants more extensive clinical evaluation in future studies.
In conclusion, our findings provide the first evidence that resolution of major depression is associated with significant increases in NK cell activity over time in HIV-seropositive women. These results extend previous findings demonstrating depression-associated decrements in NK cell numbers and function and suggest that these alterations are reversible with the resolution of the depressive episode. Increasing evidence suggests that depression may have a negative impact on the progression of HIV disease, and chronic depression has been associated with mortality in HIV-seropositive women. Given the role that innate immunity plays in the host’s defense against HIV infection, future studies assessing antidepressant treatment effects could shed light on the relationship and underlying mechanisms of depression, immunity, and the progression of HIV disease.
 |
Received Aug. 18, 2003; revisions received June 7 and Aug. 4, 2004; accepted Sept. 9, 2004. From the Department of Psychology, University of Pennsylvania, Philadelphia; the Department of Pediatrics, the Department of Biostatistics and Epidemiology, the Department of Psychiatry, Medicine, and Neuroscience, and the Division of Allergy-Immunology, Joseph Stokes Jr. Research Institute, Children’s Hospital of Philadelphia, University of Pennsylvania School of Medicine, Philadelphia; and the Department of Psychiatry, Neuroscience, and Pharmacology, University of Florida College of Medicine, Gainesville. Address correspondence and reprint requests to Dr. Cruess, Department of Psychology, University of Connecticut, 406 Babbidge Rd., Unit 1020, Storrs, CT 06269-1020; [email protected] (e-mail). Supported by an NIMH grant (MH-55454) awarded to Dr. Evans. The authors thank the Clinical Virology Laboratory at Children’s Hospital of Philadelphia (Dr. Richard Hodinka, director) for performing the viral load determinations, the Clinical Immunology Laboratory (Dr. Donald E. Campbell, director) for performing the flow cytometry studies, and Nancy Tustin for performing the natural killer cell assays.
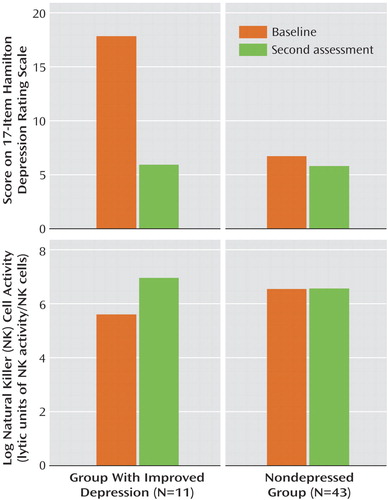
Figure 1. Change in Log Natural Killer Cell Activity Over Time in Lytic Units in HIV-Seropositive Women With and Without Resolution of a Major Depression Diagnosis
1. Evans DL, Charney DS: Mood disorders and medical illness: a major public health problem (editorial). Biol Psychiatry 2003; 54:177–180Crossref, Medline, Google Scholar
2. Ickovics JR, Hamburger ME, Vlahov D, Schoenbaum EE, Schuman P, Boland RJ, Moore J: Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women. JAMA 2001; 285:1466–1474Crossref, Medline, Google Scholar
3. Leserman J, Petitto JM, Gu H, Gaynes BN, Barroso J, Golden RN, Perkins DO, Folds JD, Evans DL: Progression to AIDS, a clinical AIDS condition and mortality: psychosocial and physiological predictors. Psychol Med 2002; 32:1059–1073Crossref, Medline, Google Scholar
4. Evans DL, Mason K, Bauer R, Leserman J, Pettito J: Neuropsychiatric manifestations of HIV-1 infection and AIDS, in Psychopharmacology: The Fifth Generation of Progress. Edited by Charney D, Coyle J, Davis K, Nemeroff C. New York, Raven Press, 2002, pp 1281–1399Google Scholar
5. Cook JA, Cohen MH, Burke J, Grey D, Anastos K, Kirstein L, Palacio H, Richardson J, Wilson T, Young M: Effects of depressive symptoms and mental health quality of life on use of highly active antiretroviral therapy among HIV-seropositive women. J Acquir Immune Defic Syndr 2002; 30:401–409Crossref, Medline, Google Scholar
6. Starace F, Ammassari A, Trotta MP, Murri R, De Longis P, Izzo C, Scalzini A, d’Arminio Monforte A, Wu AW, Antinori A: Depression is a risk factor for suboptimal adherence to highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2002; 31:S136-S139Google Scholar
7. Evans DL, Ten Have TR, Douglas SD, Gettes DR, Morrison M, Chiappini MS, Brinker-Spence P, Job C, Mercer DE, Wang YL, Cruess D, Dube B, Dalen EA, Brown T, Bauer R, Petitto JM: Association of depression with viral load, CD8 T lymphocytes, and natural killer cells in women with HIV infection. Am J Psychiatry 2002; 159:1752–1759Link, Google Scholar
8. Atkinson JJ, Grant I, Kennedy CJ, Richman DD, Spector SA, McCutchan JA: Prevalence of psychiatric disorders among men infected with human immunodeficiency virus: a controlled study. Arch Gen Psychiatry 1988; 45:859–864Crossref, Medline, Google Scholar
9. Evans DL, Perkins DO: The clinical psychology of AIDS. Curr Opin Psychiatry 1990; 3:96–102Crossref, Google Scholar
10. Rabkin JG, Ferrando SJ, Jacobsberg LB, Fishman B: Prevalence of axis I disorders in an AIDS cohort: a cross-sectional, controlled study. Compr Psychiatry 1997; 38:146–154Crossref, Medline, Google Scholar
11. Williams JB, Rabkin JG, Remien RH, Gorman JM, Ehrhardt AA: Multidisciplinary baseline assessment of homosexual men with and without human immunodeficiency virus infection: standardized clinical assessment of current and lifetime psychopathology. Arch Gen Psychiatry 1991; 48:124–130Crossref, Medline, Google Scholar
12. Perkins DO, Stern RA, Golden RN, Murphy C, Naftolowitz D, Evans DL: Mood disorders in HIV infection: prevalence and risk factors in a nonepicenter of the AIDS epidemic. Am J Psychiatry 1994; 151:233–236Link, Google Scholar
13. AIDS Epidemic Update 2002. New York, Joint United Nations Programme on HIV/AIDS, Dec 2002Google Scholar
14. Blazer DG, Kessler RC, McGonagle KA, Swartz MS: The prevalence and distribution of major depression in a national community sample: the National Comorbidity Survey. Am J Psychiatry 1994; 151:979–986Link, Google Scholar
15. Morrison MF, Petitto JM, Ten Have T, Gettes DR, Chiappini MS, Weber AL, Brinker-Spence P, Bauer RM, Douglas SD, Evans DL: Depressive and anxiety disorders in women with HIV infection. Am J Psychiatry 2002; 159:789–796Link, Google Scholar
16. Kiecolt-Glaser JK, Glaser R: Depression and immune function: central pathways to morbidity and mortality. J Psychosom Res 2002; 53:873–876Crossref, Medline, Google Scholar
17. Cruess DG, Petitto JM, Leserman J, Douglas SD, Gettes DR, Ten Have TR, Evans DL: Depression and HIV infection: impact on immune function and disease progression. CNS Spectr 2003; 8:52–58Crossref, Medline, Google Scholar
18. Herbert T, Cohen S: Depression and immunity: a meta-analytic review. Psychol Bull 1993; 113:472–486Crossref, Medline, Google Scholar
19. Weisse CS: Depression and immunocompetence: a review of the literature. Psychol Bull 1992; 111:475–489Crossref, Medline, Google Scholar
20. Evans DL, Folds JD, Pettito J, Golden RN, Pedersen CA, Corrigan M, Gilmore JH, Silva SG, Quade D, Ozer H: Circulating natural killer cell phenotypes in males and females with major depression: relation to cytotoxic activity and severity of depression. Arch Gen Psychiatry 1992; 49:388–395Crossref, Medline, Google Scholar
21. Burack JH, Barrett DC, Stall RD, Chesney MA, Ekstrand ML, Coates TJ: Depressive symptoms and CD4 lymphocyte decline among HIV-infected men. JAMA 1993; 270:2567–2573Crossref, Google Scholar
22. Lyketsos CG, Hoover DR, Guccione M, Dew MA, Wesch JE, Bing EG, Treisman GJ: Changes in depressive symptoms as AIDS develops. Am J Psychiatry 1996; 153:1430–1437Link, Google Scholar
23. Leserman J, Petitto JM, Perkins DO, Folds JD, Golden RN, Evans DL: Severe stress, depressive symptoms, and changes in lymphocyte subsets in human immunodeficiency virus-infected men: a 2-year follow-up study. Arch Gen Psychiatry 1997; 54:279–285Crossref, Medline, Google Scholar
24. Evans DL, Leserman J, Perkins DO, Stern RA, Murphy C, Tamul K, Liao D, van der Horst CM, Hall CD, Folds JD, Golden RN, Petitto JM: Stress-associated reductions of cytotoxic T lymphocytes and natural killer cells in asymptomatic HIV infection. Am J Psychiatry 1995; 152:543–550Link, Google Scholar
25. Perry S, Fishman B, Jacobsberg L, Frances A: Relationships over 1 year between lymphocyte subsets and psychosocial variables among adults with infection by human immunodeficiency virus. Arch Gen Psychiatry 1992; 49:396–401Crossref, Medline, Google Scholar
26. Rabkin JG, Williams JB, Remien RH, Goetz R, Kertzner R, Gorman JM: Depression, distress, lymphocyte subsets, and human immunodeficiency virus symptoms on two occasions in HIV-seropositive homosexual men. Arch Gen Psychiatry 1991; 48:111–119Crossref, Medline, Google Scholar
27. Irwin M, Lacher U, Caldwell C: Depression and reduced natural killer cytotoxicity: a longitudinal study of depressed patients and control subjects. Psychol Med 1992; 22:1045–1050Crossref, Medline, Google Scholar
28. Frank MG, Hendricks SE, Johnson DR, Wieseler JL, Burke WJ: Antidepressants augment natural killer cell activity: in vivo and in vitro. Neuropsychobiology 1999; 39:18–24Crossref, Medline, Google Scholar
29. Schleifer SJ, Keller SE, Bartlett JA: Depression and immunity: clinical factors and therapeutic course. Psychiatry Res 1999; 85:63–69Crossref, Medline, Google Scholar
30. Spitzer RL, Williams JBW, Gibbon M, First MB: Structured Clinical Interview for DSM-III-R—Non-Patient Edition (SCID-NP, Version 1.0). Washington, DC, American Psychiatric Press, 1990Google Scholar
31. Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
32. Douglas SD, Durako SJ, Tustin N, Houser J, Muenz L, Starr SE, Wilson C: Natural killer cell enumeration and function in HIV infected and high risk uninfected adolescents. AIDS Res Hum Retroviruses 2001; 17:543–552Crossref, Medline, Google Scholar
33. Bryant J, Day R, Whiteside TL, Herbeman RB: Calculation of lytic units for the expression of cell-mediated cytotoxicity. J Immunol Methods 1992; 146:91–103Crossref, Medline, Google Scholar
34. Friberg D, Bryant JL, Whiteside TL: Measurements of natural killer (NK) activity and NK-cell quantification. Methods 1996; 9:316–326Crossref, Medline, Google Scholar
35. Whiteside TL: Measurement of NK-cell activity in humans, in Manual of Clinical Laboratory Immunology, 6th ed. Edited by Rose NR, Hamilton RG, Detrick B. Washington, DC, ASM Press, 2001, pp 296–300Google Scholar
36. Chehimi J, Starr SE, Frank I, Rengaraju SJ, Jackson C, Llanes M, Kobayashi B, Perussia D, Young E, Nickbarg SF, Wolf SK, Trinchieri G: Natural killer (NK) cell stimulatory factor increases the cytotoxic activity of NK cells from both healthy donors and human immunodeficiency virus-infected patients. J Exp Med 1992; 175:789–796Crossref, Medline, Google Scholar
37. Whiteside TL, Herberman RB: Role of human natural killer cells in health and disease. Clin Diagn Lab Immunol 1994; 1:125–133Medline, Google Scholar
38. Levy JA: HIV and the Pathogenesis of AIDS. Washington, DC, ASM Press, 1998Google Scholar
39. Oliva A, Kinter AL, Vaccarezza M, Rubbert A, Catanzaro A, Moir S, Monaco J, Ehler L, Mizell S, Jackson R, Li Y, Romano JW, Fauci AS: Natural killer cells from human immunodeficiency virus (HIV)-infected individuals are an important source of CC-chemokines and suppress HIV-1 entry and replication in vitro. J Clin Invest 1998; 102:223–231Crossref, Medline, Google Scholar
40. Forthal DN, Landucci G, Daar ES: Antibody from patients with acute human immunodeficiency virus (HIV) infection inhibits primary strains of HIV type 1 in the presence of natural-killer effector cells. J Virol 2001; 75:6953–6961Crossref, Medline, Google Scholar
41. Ironson G, Balbin E, Solomon G, Fahey J, Klimas N, Schneiderman N, Fletcher MA: Relative preservation of natural killer cytotoxicity and number in healthy AIDS patients with low CD4 cell counts. AIDS 2001; 15:2065–2073Crossref, Medline, Google Scholar



