Pregnancy, Delivery, and Neonatal Complications in a Population Cohort of Women With Schizophrenia and Major Affective Disorders
Abstract
OBJECTIVE: This study ascertained the incidence of complications during pregnancy, labor, and delivery and the neonatal characteristics of infants born to women with schizophrenia, bipolar disorder, or major depression in a population-based cohort. METHOD: Based on records linkage across a psychiatric case register and prospectively recorded obstetric data, the study comprised women with schizophrenia or major affective disorders who had given birth to 3,174 children during 1980–1992 in Western Australia. A comparison sample of 3,129 births to women without a psychiatric diagnosis was randomly selected from women giving birth during 1980–1992. Complications were scored with the McNeil-Sjöström Scale. Odds ratios were calculated for specific reproductive events. RESULTS: Both schizophrenic and affective disorder patients had increased risks of pregnancy, birth, and neonatal complications, including placental abnormalities, antepartum hemorrhages, and fetal distress. Women with schizophrenia were significantly more likely to have placental abruption, to give birth to infants in the lowest weight/growth population decile, and to have children with cardiovascular congenital anomalies. Neonatal complications were significantly more likely to occur in winter; low birth weight peaked in spring. Complications other than low birth weight and congenital anomalies were higher in pregnancies after psychiatric illness than in pregnancies preceding the diagnosis. CONCLUSIONS: While genetic liability and gene-environment interactions may account for some outcomes, maternal risk factors and biological and behavioral concomitants of severe mental illness appear to be major determinants of increases in reproductive pathology in this cohort. Risk reduction in these vulnerable groups may be achievable through antenatal and postnatal interventions.
Maternal obstetric complications are widely regarded as environmental contributors to schizophrenia risk, although the evidence is still inconclusive. While meta-analyses of a number of case-control studies (1–3) suggest that significant associations exist between adult schizophrenia and exposure to obstetric complications, several population-based investigations (4–6) have failed to demonstrate excess occurrence of obstetric complications in individuals who developed schizophrenia compared to those who did not. The sources of discrepant findings are likely to include different definitions of obstetric complications, the use of retrospective maternal recall versus prospectively collected obstetric data, differences between rating scales, and variation in sample size and selection. A more fundamental problem may be the confounding of observed associations between maternal obstetric complications and schizophrenia by heterogeneity in the degree of maternal genetic risk within samples ascertained through an individual with schizophrenia. Since genetic susceptibility to schizophrenia may be associated with a heightened sensitivity of the developing brain to obstetric insults (7), varying degrees of transmitted parental genetic risk might result in variable thresholds for the effects of obstetric complications, dependent on their timing, severity, and points of impact. It is, therefore, important to reduce heterogeneity with regard to variables such as genetic risk, severity, and type of obstetric complications.
In this respect, a research focus on obstetric complications in mothers with schizophrenia and their offspring, termed “strategic populations” by Rosenthal (8), offers a vantage point by being more homogeneous for genetic risk transmitted to the offspring. Children of women with schizophrenia have an eight- to 10-fold higher risk of developing the disorder compared to the general population, and individuals who develop schizophrenia are more likely to have a mother, rather than a father, with schizophrenia (9). Examination of the following could help clarify whether obstetric complications are a covariant effect of the genetic predisposition to schizophrenia or a concomitant of severe mental illness:
| 1. | The incidence of obstetric complications in mothers with schizophrenia relative to comparison samples of mothers without psychiatric disorder | ||||
| 2. | The frequency of obstetric complications in pregnancies before and after the onset of overt psychotic illness | ||||
| 3. | The association of obstetric complications with maternal risk factors, such as age, parity, and lifestyle exposures, including smoking, socioeconomic status, and availability of social support | ||||
| 4. | The occurrence of obstetric complications that are specific to women with schizophrenia compared to obstetric complications in women with other psychiatric disorders | ||||
Early studies of pregnancy outcomes in women with schizophrenia showed an increased incidence of perinatal deaths and malformations among their newborns (10–12). The samples tended to be small, and the studies differed with regard to the selection of cases and comparison subjects, the definitions of obstetric complications, and the methods of assessment. A meta-analysis by Sacker et al. (13) of 14 such studies conducted before 1990 concluded that compared to births in the general population, a small but statistically significant excess could be demonstrated for obstetric complications, low birth weight, stillbirths, and fetal or neonatal deaths among the offspring of mothers (but not fathers) suffering from schizophrenia. Several recent, population-based studies of large samples have produced more clearcut evidence. Bennedsen et al. (14–16) found a significantly increased relative risk for preterm births, low birth weight and small-for-gestation babies, as well as postneonatal deaths among children born to women with schizophrenia. Nilsson et al. (17) reported significantly increased risks of low birth weight and stillbirths, particularly high in women who developed schizophrenic episodes during pregnancy.
It appears likely, therefore, that women with schizophrenia are at an increased risk for adverse reproductive outcomes. However, the extent to which such adverse outcomes are intrinsically related to schizophrenia, rather than to a nonspecific “continuum of reproductive casualty” (18) associated with severe maternal mental illness, remains unclear. Few studies to date have addressed the occurrence of obstetric complications in women with affective disorders, and the findings are inconsistent (19).
We report results from a population-based study with record linkage across several morbidity case registers and databases in Western Australia. The aims of the study were to determine the frequency, nature, and severity of obstetric complications experienced by women with schizophrenia compared to women with affective disorders and women without a diagnosed psychiatric disorder, to investigate the temporal relationship between pregnancies and the onset of maternal psychiatric illness, and to explore a range of pregnancy outcomes in relation to maternal risk factors.
Method
Study Population
The study population comprised all women with diagnoses of schizophrenia or major affective disorder (unipolar depression and bipolar disorder) who gave birth in Western Australia during 1980–1992. A comparison sample was drawn randomly from the records of women who gave birth during the same period but were not on the psychiatric case register. The data were obtained by linking the Mental Health Information System, a psychiatric case register established in 1966 that records all in- and outpatient contacts with public mental health services and all inpatient contacts with private mental health service providers, and the Maternal and Child Health Research Database, a statutory collection of structured information on all pregnancies and births since 1980. The Maternal and Child Health Research Database is linked to other population registers, including those recording deaths, birth defects, and cerebral palsy (20).
Western Australia is the habitat of one of the geographically most isolated populations (1.7 million) in the world, with a very low rate of outmigration (1.7%) (21). Demographic indicators place inhabitants of Western Australia among the healthiest populations in terms of infant, perinatal, and maternal mortality and years lost from all causes of death (22). However, health status disparities persist between the minority (3.4%) indigenous (Aboriginal) Australians and the nonindigenous majority (23).
Record Linkage
A total of 79,599 women with records on the psychiatric case register were eligible for matching against 308,022 births for the period 1980–1992. Data linkage was carried out with Automatch software (24), which employs a probabilistic matching strategy followed by clerical review to assess the accuracy of matching. The process linked 20,258 women with psychiatric diagnoses to 23,351 children born during 1980–1992. Sibships were then identified to ensure that each mother was linked to all of her births recorded on the database. The database was supplemented with information on the neonatal period through a linkage to the birth defects and cerebral palsy registers that covered the entire population (25). Maternal socioeconomic status was coded with the Index of Relative Socio-Economic Disadvantage of the Australian Bureau of Statistics (26), derived by principal components analysis of over 40 census items.
Case Definition and Diagnoses
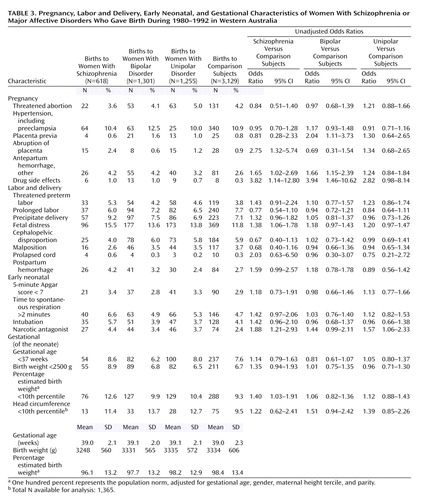
Case mothers were defined as all of those with at least one contact on the psychiatric register who had a recorded diagnosis of schizophrenia (ICD-9 295.0–295.9) or affective psychosis (ICD-9 296.0–296.9). There were 1,870 women meeting this criterion. Since 271 of them had received at different times diagnoses of both schizophrenia and affective psychoses, the records of these women were reviewed by one of the authors (A.V.J.), who assigned them (blind to the pregnancy outcome) to the diagnostic categories of schizophrenia, affective psychoses, or other diagnosis based on a hierarchical rule taking into account the number and temporal sequence of the diagnoses. The patients who could not be unequivocally classified as having either schizophrenia or affective psychoses (N=39) were excluded. The final cohort consisted of 1,831 women: 382 with ICD-9 schizophrenia and 1,449 with ICD-9 affective psychoses. The women with affective psychoses were subdivided into a unipolar depression group (N=686, ICD-9 codes 296.1, 296.6, 296.8, and 296.9) and a bipolar disorder group (N=763, ICD-9 codes 296.0 and 296.2–5). The cohort had a total of 3,174 births over 20 weeks’ gestation: 618 to mothers with schizophrenia, 1,255 to mothers with unipolar depression, and 1,301 to mothers with bipolar disorder. The nonpsychiatric comparison group included 1,831 women with 3,129 offspring. Within the study cohort, 3,844 (68.9%) of the offspring had mothers who were Australian-born, including 325 (5.2%) whose mothers were of Aboriginal descent.
Validation of Diagnoses
The concurrent validity of register diagnoses of schizophrenia and affective psychoses was evaluated in an independent sample of 180 women who were assessed with a semistructured interview covering the 90 items of the OPCRIT diagnostic algorithm (27). With reference to the interview-based OPCRIT diagnosis, a register diagnosis of schizophrenia provided a sensitivity of 0.92 and a specificity of 0.88. Similarly, a register diagnosis of affective psychosis yielded a sensitivity of 0.80 and a specificity of 0.90.
Definition and Scoring of Obstetric Complications
Obstetric complications were analyzed according to their timing in pregnancy, labor and delivery, and the early neonatal period. Within each period, we examined the frequency of specific obstetric complications and their clustering and severity in individual women with the McNeil-Sjöström Scale (28). The scale provides a scoring system for multiple specific obstetric complications and assigns severity weights reflecting the presumed likelihood of harm to the offspring, with a special focus on CNS damage. Summary scores at scale severity level 4 (clearly harmful) were used in the analysis, setting a high threshold for accepting any given set of obstetric complications as relevant. A computer algorithm was written incorporating the McNeil-Sjöström rules to score for each woman 25 obstetric complication items routinely recorded on the Maternal and Child Health Research Database. To ascertain the validity of the algorithm, 40 randomly selected patients were manually scored, and the scores were compared to those generated by the algorithm. The percent agreement was 100% for pregnancy, 95% for labor and delivery, and 82% for the early neonatal period (overall agreement=92%).
Analysis
Data were analyzed with SPSS for Windows, Version 10.0.1 (SPSS, Chicago, 1999) and STATA 6 (Stata Corp., College Station, Tex., 1999). For each variable, frequency tables were obtained and distributions examined for their general shape and the presence of outliers, missing data, and invalid entries. Unadjusted and adjusted odds ratios and their confidence intervals (CIs) were calculated by using STATA 6 with logistic regression; significance was accepted at p<0.05. Crude odds ratios with 95% CIs not containing unity were followed up by adjusting the odds ratios for maternal age, marital status, sex of the infant, plural births, parity, and Aboriginal descent of the mother. Because logistic regression assumes independence of observations, we adjusted for possible correlation within sibships by calculating robust estimates of variance. For multivariate models, we used generalized estimating equations with an exchangeable correlation structure into which a Poisson regression was fitted and again adjusted for sibship clustering to produce odds ratios with robust estimates of variance (29). All calculations use births as the unit of analysis.
Results
Maternal Characteristics
The salient characteristics distinguishing women with schizophrenia, women with unipolar depression, women with bipolar disorder, and unaffected women are listed in Table 1.
The group of women with schizophrenia was characterized by a significant excess of either very young (age ≤19) or mature (age ≥35) mothers. The women in all three diagnostic groups were more likely than the comparison women to be single mothers, the difference being most pronounced in the schizophrenia group. The partners of the women with schizophrenia were more likely to be unemployed or legally disabled (including disability due to a psychiatric condition). The women of Aboriginal descent (37 mothers, 71 offspring) were overrepresented in the schizophrenia group. There were no differences between the groups regarding migration status (born overseas versus born in Australia). The women with unipolar depression or bipolar disorder were more likely to be multiparous than either the women with schizophrenia or the unaffected women.
Complication Scores in Individual Women
The concurrent or consecutive occurrence of obstetric events in individual women was assessed with the computer algorithm to assign all recorded obstetric complications to subscales of the McNeil-Sjöström Scale (28). Separate scores were generated for obstetric complications in pregnancy, labor and delivery, and the neonatal period. An overall score was calculated for obstetric complications occurring across all phases of reproduction.
The proportion of women with aggregated scores on any obstetric complications at severity level 4 or greater (Table 2) was greater for each of the three groups of mothers with diagnoses compared with the nonpsychiatric sample, but the difference was not significant. However, all three diagnostic groups were significantly more likely to have a severity score of 4 or more for pregnancy obstetric complications, and there was an increased risk of neonatal complications in the offspring of women with schizophrenia (odds ratio=1.27, 95% CI=1.04–1.54). Relative to a baseline of 40.5% in unaffected women, a larger percentage (46.3%) of women with schizophrenia had accumulated two or more obstetric complications. The women with schizophrenia were also overrepresented (7.3% compared to 4.8% of unaffected women, 5.4% of the women with unipolar disorder, and 6.2% of the women with bipolar disorder) in the subgroup with five or more complications.
Complications in Pregnancy, Labor, and Delivery
Since aggregated obstetric complications scores do not provide information on specific adverse reproductive events, we calculated the crude odds ratios for 18 specific obstetric complications in pregnancy, delivery, and the early neonatal period (Table 3).
With reference to the comparison group, both the women with schizophrenia and the women with bipolar disorder were significantly more likely to experience placental abnormalities, antepartum hemorrhages, and drug toxic side effects (because of alcohol, tobacco and illicit substances). During labor, all three diagnostic groups were more likely to experience fetal distress (significant in schizophrenia mothers, assessed by indicators such as abnormalities of the fetal heart rate or a fresh meconium staining of the liquor in the first stages of labor) and to have a narcotic antagonist (naloxone) administered to the newborn (significant in mothers with schizophrenia and unipolar depression). After adjustment for maternal age, parity, plurality, marital status, Aboriginality, and sex (Table 4), the complications that remained significant were placental abnormalities (abruption of the placenta in the women with schizophrenia and placenta previa in the women with bipolar disorder), antepartum hemorrhages (in the women with bipolar disorder), and naloxone administration (in the women with schizophrenia and the women with unipolar depression). Fetal distress dropped marginally from significance in the women with schizophrenia but became significant in the women with unipolar depression.
With the exception of fetal distress, no specific labor or delivery obstetric complications occurred significantly more often in the women with schizophrenia or affective disorders than in unaffected women. Neither the women with schizophrenia nor the women with affective disorders had any excess of cephalopelvic disproportion, atypical presentation, or cord anomalies. The women with diagnoses did not differ from the unaffected women in the frequency of threatened preterm labor, early rupture of the membranes, or prolonged labor.
Perinatal and early neonatal complications
There was a nonsignificant tendency for a suboptimal 5-minute Apgar score in the offspring of the women with schizophrenia, as well as for delayed respiration and intubation.
Fetal growth
There were no significant differences among the four groups in mean gestational age, although the proportion of births at less than 37 weeks’ gestation was slightly higher in the women with schizophrenia than in all three other groups (Table 4). The newborns of the mothers with schizophrenia had a lower mean birth weight (3,248 g versus 3,334 g in the comparison group), a smaller head circumference (11.4% were in the lowest 10th percentile compared to 9.5% of the comparison offspring), and tended to be shorter (9.4% in the lowest 10th percentile compared to 8.7% of the offspring of unaffected mothers).
Because absolute birth weight does not always reflect the appropriateness of fetal growth, we calculated an index of percentage of expected birth weight using data for all newborns in the total population of Western Australia. Percentage of expected birth weight compares an infant’s birth weight with that of a population of infants of the same gestational age and with the same characteristics affecting fetal growth (30). Since percentage of expected birth weight is adjusted for gestational age, it allows assessment of the independent contribution of fetal growth rate to pregnancy outcomes. The percentage of expected birth weight of the offspring of the women with schizophrenia was 96.1% of the mean percentage of expected birth weight of the total population of neonates. A significantly larger proportion of babies of the mothers with schizophrenia (12.6% compared to 9.3% of the comparison group, 10.4% of the offspring of the mothers with unipolar disorder, and 9.9% of the offspring of the women with bipolar disorder) fell into the lowest 10th percentile of intrauterine growth. This corresponded to an odds ratio of 1.41 (95% CI=1.04–1.91), which dropped to an odds ratio of 1.38 (95% CI=1.00–1.90) after adjustment for maternal age, marital status, plural birth, sex of the infant, and Aboriginal descent.
Smoking during pregnancy is a risk factor for low birth weight. Since complete data on smoking in the study cohort were not available, we modeled its effects on birth weight using data from meta-analyses of studies (31) that indicate that a mean birth weight reduction of 206 g (95% CI=202–210) is attributable to smoking. Assuming a worst-case scenario, based on a recent epidemiological study (32) that found a 60.5% prevalence of smoking among women with schizophrenia and 58.3% among women with affective psychoses compared to 27% in the general population of women in the fertile age range, the model predicted a birth weight reduction of 69 g for the offspring of women with schizophrenia and a reduction of 65 g for the offspring of women with affective disorders relative to the offspring of comparison women. The observed mean difference was 86 g (women with schizophrenia) and 2 g (women with bipolar and unipolar disorders combined). The model thus fails to explain a residual difference of 17 g between the infants of mothers with schizophrenia and the infants of comparison mothers. Since the actual rate of smoking during pregnancy in women with schizophrenia might be lower than the worst-case scenario, smoking may not account fully for the intrauterine growth retardation in the offspring of women with schizophrenia.
Stillbirths and Mortality
There were no significant differences among the diagnostic groups and between case and comparison mothers with regard to stillbirths, neonatal deaths (up to 28 days), and postneonatal deaths (29–365 days). However, there was a nonsignificant increase in childhood deaths among the offspring of the women with schizophrenia (Table 5).
Abnormalities Diagnosed at Birth, in Infancy, or During Early Childhood
Cerebral palsy was a rare complication of the neonate in the entire sample (24 instances out of 6,303 births), and its incidence did not differ significantly across the four groups (Table 5). The incidence of any birth defects (including congenital malformations and chromosomal anomalies) was marginally higher in the maternal schizophrenia group (6.0%) than in the comparison group (4.9%) (odds ratio=1.24, 95% CI=0.85–1.82) but not in the bipolar group (4.8%) or the unipolar depression group (3.5%). However, significant differences occurred at the level of specific defects. There was a significant increase in the incidence of defects of the cardiovascular system (ventricular and atrial septal defects, patent ductus arteriosus, anomalies of the aorta) among the children of mothers with schizophrenia (crude odds ratio=2.55, odds ratio=2.50 after adjustment) but not among the offspring of the mothers with affective disorders. There was also a significantly increased incidence of “other” abnormalities (including mainly minor physical anomalies) in the offspring of the women with schizophrenia. The distribution of birth defect cases by the number of children per mother ruled out the possibility that the observed increases could be due to a clustering of cases within a small number of sibships.
Seasonality of Obstetric Complications
Northern hemisphere studies (33) have implicated winter birth as a possible risk factor in schizophrenia, although the results of studies in the southern hemisphere have been inconsistent (34). It has been suggested that the season of birth effect is mediated by obstetric complications occurring more frequently in the cold months of the year (35). To test for possible effects of the season of birth, McNeil-Sjöström Scale severity level ≥4 labor and delivery and neonatal obstetric complications were regressed on alternative groupings of the calendar months. Separate analyses were undertaken for the mothers with schizophrenia, unipolar disorder, and bipolar disorder and for the unaffected mothers. The odds of neonatal complications in the offspring of the women with schizophrenia was increased both in winter (June–August) compared to the other seasons (odds ratio=1.55, 95% CI=1.08–2.24) and in the third quarter (July–September) compared to the remaining three quarters (odds ratio=1.60, 95% CI=1.11–2.31). All other comparisons were not significant. At the level of specific obstetric complications, no seasonal patterns were detected in the incidence of placentation abnormalities (previa or abruption), birth defects, preterm births (<37 weeks), or fetal distress. In the women with schizophrenia, however, there was a marked peak of term low birth weight births (percentage expected birth weight <94%) in the spring months of September and October. Thus, for the month of September, the mean birth weight of the neonates born to mothers with schizophrenia was 3060 g compared to 3364 g for the offspring of comparison mothers. No comparable trend was observed in the offspring of mothers with bipolar and unipolar depression.
Complications in Aboriginal Women
Regardless of diagnosis, all Aboriginal women (including the comparison mothers), experienced a marked excess of pregnancy, labor/delivery, and neonatal obstetric complications, and their offspring had a significantly lower mean birth weight compared to the non-Aboriginal women in the study. Within the Aboriginal group, the differences in obstetric complications and low birth weight between the patient mothers and the comparison mothers were largely attenuated, with the single exception of the unipolar depression group.
Pregnancy Complications Before and After First Admission for Psychiatric Illness
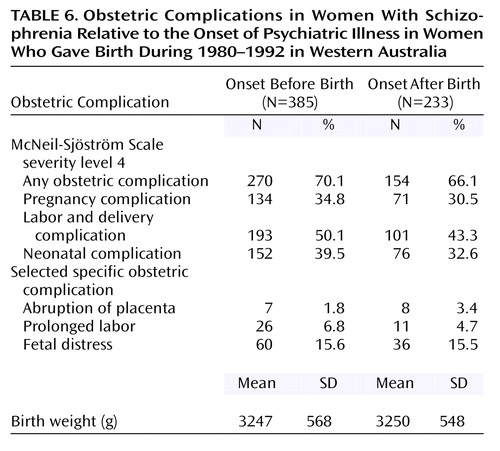
Multivariate analyses were conducted to investigate the relationship between psychiatric status at the time of the birth of the child and the likelihood of obstetric complications. The response variable was derived from the McNeil-Sjöström Scale (28) and captured counts of serious obstetric complications. The predictor variable was the case-comparison status of the mother. With the date of the first psychiatric admission as a proxy for onset, the infants born to case mothers were allocated into two groups: 1) those born before and 2) those born after the onset of their mother’s psychiatric illness (Table 6). Of the 3,174 infants born to case mothers, 1,441 (45.4%) were born before their mother’s first psychiatric admission, and 1,733 (54.6%) were born after their mother’s first psychiatric admission. Obstetric complications were regressed by general estimating equations on case-comparison status in a model adjusted for maternal age, height, parity, and marital status. Additional terms were included for maternal age and parity to adjust for potential quadratic relationships, and the entire model was adjusted for sibship clustering.
The overall fit of the model was significant (χ2=182.49, df=8, p<0.0001). Relative to the comparison sample, the mothers whose psychiatric illness commenced before the birth of the infant were significantly more likely (odds ratio=1.13, 95% CI=1.06–1.22) to experience obstetric complications. In contrast, the mothers whose psychiatric illness commenced after the birth of the infant were at no greater risk of obstetric complications than the comparison mothers (odds ratio=1.04, 95% CI=0.97–1.11). To further delineate this relationship, separate analyses compared obstetric outcomes for the comparison mothers with those of the women who had schizophrenia and then with those who had affective disorders. The pattern of results within each analysis was similar.
The overall model for the schizophrenia versus comparison women was significant (χ2=132.88, df=8, p<0.0001), showing that the mothers with onset of schizophrenia before the birth of the infant were significantly more likely to experience serious obstetric complications (odds ratio=1.13, 95% CI=1.01–1.27), while the mothers with onset after the index birth were at no greater risk than the comparison group (odds ratio=0.94, 95% CI=0.80–1.09). However, the frequency of three specific obstetric complications: abruption of the placenta, low birth weight for gestational age (percentage of expected birth weight at 96%), and cardiovascular birth defects showed no differences in pregnancies before and after the onset of the disorder in the women with schizophrenia.
The model for the women with affective disorders versus the comparison women was also significant (χ2=157.56, df=8, p<0.0001), indicating that the women with bipolar disorder whose illness onset occurred before the birth of the infant had a significantly increased risk of obstetric complications (odds ratio=1.13, 95% CI=1.02–1.25), while the women whose bipolar disorder commenced after the index birth were at no greater risk than the comparison mothers (odds ratio=1.02, 95% CI=0.92–1.12). In the group of women with unipolar depression, the risk of any obstetric complications was increased both in those with an onset before (odds ratio=1.14, 95% CI=1.02–1.27) and after (odds ratio=1.09, 95% CI=1.01–1.19) the index birth.
Complications in Women Experiencing Illness During Pregnancy
An ancillary analysis with general estimating equation regression of the pregnancy outcomes for 202 women who had a psychiatric inpatient admission in the time between their last normal menstrual period and the birth of the index child revealed no significant increases in the incidence of obstetric complications in either the women diagnosed with schizophrenia (adjusted odds ratio=1.12, CI=0.89–1.42) or in the total group of women with psychiatric disorders (adjusted odds ratio=1.11, CI=0.97–1.27).
Complications in Women With Partners Receiving Disability or Unemployed
To explore a possible dual-mating effect on the incidence of obstetric complications, we recalculated the scores for any obstetric complications at severity level ≥4, shown in Table 2, with adjustment for paternal status “receiving disability pension or unemployed” as a proxy index of likely psychiatric impairment. The adjustment resulted in a minimal change in the odds ratio and 95% CI: from 1.18 to 1.20 (in the women with schizophrenia), no change (in the women with bipolar disorder) and from 1.13 to 1.14 (in the women with unipolar depression). This negative result should be interpreted with caution in the absence of a specific paternal diagnostic breakdown (such data are currently being collected and verified, with a view to a follow-up analysis of offspring outcomes).
Discussion
This study satisfies several methodological criteria proposed by McNeil (36) for investigations into adverse reproductive events in women with psychiatric disorders:
| 1. | It is based on an entire population birth cohort, including all births to women with schizophrenia and major affective disorders. | ||||
| 2. | A comparison nonpsychiatric group was drawn at random from the same cohort. | ||||
| 3. | All study groups are derived from a geographically delimited population relatively unaffected by outmigration. | ||||
| 4. | Structured data on obstetric complications and maternal risk factors had been collected prospectively. | ||||
| 5. | Data were analyzed both in terms of aggregated scores by using a standardized scale and in terms of specific reproductive events. | ||||
Limitations of the study include a lack of exact data on smoking, which necessitated modeling smoking effects based on independently collected survey data. At this stage, we do not have data on paternal psychiatric morbidity, which could be a significant factor contributing to both genetic and environmental risks. No specific information was available on prescription medications or illicit drug use during pregnancy. Finally, the comparison sample could have included a number of women with milder disorders who never had a psychiatric inpatient or outpatient admission but may share some of the risk factors with the diagnostic groups (if anything, this would attenuate the differences between patients and comparison subjects).
With such caveats stated, the findings indicate that relative to the nonpsychiatric comparison group, the women with schizophrenia, bipolar disorder, and unipolar depression experienced an increased overall incidence of obstetric complications, reflected in the aggregated scores (severity level ≥4) on the McNeil-Sjöström Scale (28). Two specific pregnancy obstetric complications stand out in the women with schizophrenia and the women with bipolar disorder (but not in the women with unipolar depression): placentation abnormalities (abruption of the placenta in schizophrenia and placenta previa in bipolar disorder) and antepartum hemorrhages, although the proportions of women affected were small. With the exception of fetal distress and the administration of a narcotic antagonist to the newborns, significantly more frequent in schizophrenia and close to significance in women with bipolar disorder and unipolar depression, there was no significant excess of other labor or delivery obstetric complications in any one of the three diagnostic groups.
The most salient finding in the neonates was the significantly increased odds ratio (1.40) for the percentage of expected birth weight below the 10th percentile in the offspring of the women with schizophrenia. The distribution of birth weight in the maternal schizophrenia group was unimodal (Figure 1), and the overall reduction was not attributable to a very low birth weight in a particular subset of the cohort, such as the offspring of Aboriginal women. A model of birth weight reduction based on an assumption of a high rate of smoking in the women with schizophrenia explained only part of the observed difference from the comparison group. The absence of a birth weight reduction in the offspring of women with bipolar disorder, whose smoking history was unlikely to be very dissimilar to that in the maternal schizophrenia group, was notable but difficult to explain.
The frequency of any congenital malformations, diagnosed either at birth or in the first years of life, was increased only in the infants of women with schizophrenia (6.0%, compared to 4.9% in the nonpsychiatric sample) and involved in particular a significant excess of malformations of the cardiovascular system. There were no significant differences among the groups with regard to the incidence of neural tube defects, other CNS malformations, or cerebral palsy.
Among the hazards that may be contributing to the increased risk of obstetric complications in the women with schizophrenia and, to a lesser degree, in the women with affective disorders, a major factor appears to be the clustering of adverse maternal characteristics. While being a single, divorced, or separated mother was common in all three diagnostic groups, the women with schizophrenia were more likely than any other group to experience socioeconomic disadvantage and to lack adequate social support. In addition, they were significantly more likely to be either younger than 20 years or older than 34 years at the index pregnancy. The probability that a large proportion of the reproductive hazards in the women with schizophrenia (and, to a lesser degree, in the women with affective disorders) is associated with risk exposures and behaviors arising as concomitants of severe mental illness is supported by the finding that after adjustment for maternal risk factors, the incidence of adverse outcomes in all three diagnostic groups was significantly increased only in pregnancies occurring after the onset of psychiatric illness.
Among the variety of maternal obstetric complications reported in the literature as significantly more frequent in the histories of individuals who develop schizophrenia as adults (7, 37–40), only placental abruption and fetal distress with signs of hypoxia were also significantly more common in the mothers with schizophrenia in our cohort. We did not find significant increases in preeclampsia, threatened abortion, preterm births, premature rupture of the membranes, abnormal presentation, cord complications, prolonged labor, or cesarean or other emergency delivery.
Findings from recent studies on birth cohorts in Denmark (14–16) and Sweden (17) are of particular relevance because of shared features with the present study: overlapping periods of births, comparable socioeconomic and health service environment, and design attributes (record linkage between case registers and other population databases, prospectively recorded obstetric complications, use of the McNeil-Sjöström Scale [28]). Common findings across the three studies include low birth weight and infants that are small for gestational age (significant in all three studies), an excess of women with schizophrenia giving birth at age ≥35 (in the Australian cohort, there was also a significant excess of mothers age ≤19), and a marginal increase in the incidence of malformations in the newborn. While the frequencies of stillbirths and postneonatal deaths were significantly increased in the Danish and Swedish cohorts but not in the Australian cohort, there was a trend in the latter of increased childhood mortality in the offspring of the women with schizophrenia. None of the three studies found increased frequencies of preeclampsia, atypical fetal presentations, or severe complications of labor. The incidence of placental abruption was increased in both the Australian cohort and the Danish cohort but did not reach significance in the latter.
The main differences between the findings of the present study and the two Scandinavian studies concern the temporal relationship between obstetric complications and the onset of psychotic illness. The Swedish study reported a markedly increased risk of obstetric complications in the women who had a hospital admission for a psychotic episode during the index pregnancy, a finding we were unable to replicate. Our finding of a significant increase in the risk of obstetric complications for pregnancies (in both the women with schizophrenia and the women with affective disorders) occurring after the onset of psychiatric illness and no such increase for the pregnancies before the onset of the disorder had no counterpart in the Danish study (15). Replication of this finding could be important for the understanding of the causes of reproductive pathology in the women with schizophrenia and affective disorders.
Three lines of suggestive evidence generated by our study allow some speculation as to their implications in the context of a broad gene-environment model of causation in schizophrenia and major affective disorders:
| 1. | The differences in the rate of obstetric complications in pregnancies preceding and pregnancies following the onset of major psychiatric disorder | ||||
| 2. | The specificity of obstetric complications for a particular diagnostic group | ||||
| 3. | The presence or absence of seasonality in the occurrence of obstetric complications | ||||
The increase in the overall McNeil-Sjöström Scale severity score ≥4 for obstetric complications in pregnancies occurring after the onset of psychiatric illness is largely accounted for by fetal distress that was nonspecific to maternal diagnosis and likely to reflect a variety of adverse behavioral and biological concomitants of severe mental illness. In contrast, the rates of placental complications, cardiovascular birth defects, and low birth weight show no difference in the pregnancies preceding and the pregnancies following the onset of psychiatric disorder, suggesting a preexisting susceptibility that may involve both genetic and environmental components. Any combination of risk factors for placental complications, such as age (>35), multiparity, smoking, second-trimester bleeding, and breech presentation, might explain their increased incidence in both women with schizophrenia and women with bipolar disorder. The excess of cardiovascular birth defects, on the other hand, appears to be relatively specific to mothers with schizophrenia and may not be a fortuitous association. It is likely to involve parentally transmitted genes, some of which may be expressed in both the heart and brain or are in linkage disequilibrium with other genes conferring susceptibility to schizophrenia. Patent ductus arteriosus, the second most common congenital heart disease (41), shows in our sample a 10-fold increase in incidence in the newborn of the schizophrenia mothers relative to the comparison mothers. The pathogenesis of patent ductus arteriosus is thought to include a genetic component involving a disruption of the prostaglandin E2 receptor (42). Abnormal prostaglandin D2 and E2 signaling has been suggested to underlie the attenuated skin flush response to niacin in patients with schizophrenia and their relatives (43), possibly reflecting an essential fatty acids deficiency (44). Ventricular septal defect, showing in our sample a threefold increase in the newborn of schizophrenia mothers relative to the comparison mothers, is the most common heart abnormality in the velocardiofacial syndrome, which is associated with microdeletions on chromosome 22q11 and an increased risk of schizophrenia (45, 46). Finally, atrial septal defect, secundum type, which shows a twofold increase in the newborn of schizophrenia mothers, has been linked to chromosome 6p21.3 within the human leukocyte antigen region (47), close to the tumor necrosis factor alpha, a cytokine involved in inflammatory responses and implicated in the pathophysiology of schizophrenia (48).
Low birth weight, indexed by the percentage expected birth weight, is the single obstetric complication in our study that occurs only in mothers with schizophrenia in pregnancies both before and after the onset of psychotic illness and shows a seasonal variation in its incidence. Low birth weight is associated with multiple risk factors, including maternal nutritional status, smoking (transplacental exposure), alcohol abuse, maternal physique, birth order, and hypertension in pregnancy. A role of maternally (but not paternally) transmitted fetal genes has been suggested (49), but no definitive evidence has been produced. Our finding of a reduction in birth weight in southern hemisphere springtime (and, in particular, September and October) in the offspring of schizophrenic mothers tallies well with our previously reported finding of a significant September excess of schizophrenic births during 1916–1961 in the Western Australian population (34). It also parallels closely, to the point of identity in both timing and size, the springtime birth weight reduction observed by Kendell et al. (50) in a Scottish population-based sample of patients with schizophrenia who were born in April to June. But while Kendell et al. (50) suspected nutritional folate deficiency, the effects of low ambient temperatures, or increased exposure to passive smoke in enclosed spaces as contributing causes, such factors are unlikely to apply to the Western Australian environment where folate supplementation in pregnancy has been routine since the 1980s, the average winter temperatures are higher, and the seasonal variation in indoor exposures are attenuated in comparison with northern Europe. An alternative explanation, applicable to both environments, is the winter spread of common viral and bacterial infections. Maternal exposure to infection has been shown to increase the placental levels of proinflammatory cytokines, including interleukin 1β, interleukin 6, and tumor necrosis factor alpha and to affect brain development in animal studies (51). Altered placental levels or conformation changes in such proteins due to expression of variants in human leukocyte antigen genes associated with susceptibility to schizophrenia (52) may result in a compromised intrauterine environment and restricted fetal growth involving the brain.
If such maternal-fetal genetic susceptibility to environmental factors could be demonstrated in prospective studies, further research into the pathogenesis of obstetric complications might add a novel perspective to the mechanisms of transmission of schizophrenia risk and provide a unifying framework for the findings of genetic epidemiology and the molecular genetics of severe mental disorders. As more women with such disorders are now capable of living in the community and having children, research aiming at a better understanding of both the genetic and environmental reproductive risks could pave the way for the development of preventive programs ensuring optimal antenatal and postnatal care for these vulnerable groups.
 |
 |
 |
 |
 |
 |
Received Oct. 26, 2003; revision received Dec. 30, 2003; accepted Jan. 9, 2004. From the Centre for Clinical Research in Neuropsychiatry, School of Psychiatry and Clinical Neurosciences, University of Western Australia; the Centre for Child Health Research, Telethon Institute for Child Health Research, Perth, Western Australia, and the University of Western Australia; and the Division of Population Sciences, Telethon Institute for Child Health Research and Curtin University of Technology, Perth, Western Australia. Address correspondence and reprint requests to Dr. Jablensky, Centre for Clinical Research in Neuropsychiatry, School of Psychiatry and Clinical Neurosciences, University of Western Australia, Level 3 Medical Research Foundation Bldg., 50 Murray St., Perth, Western Australia 6000, Australia; [email protected] (e-mail). Supported by a Theodore and Vada Stanley Foundation Research Award (319520/31951). The authors thank Dr. Thomas McNeil for the validation of the obstetric complications scoring algorithm and Mr. T. Pinder, Mr. H. Nguyen, Dr. M. Croft, and Ms. G. Valuri for data processing; and Dr. John Newnham for comments on the manuscript.
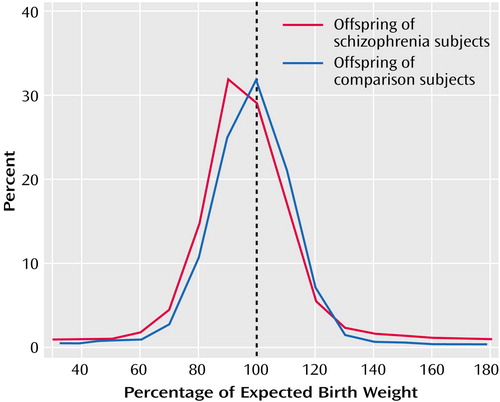
Figure 1. Distribution of Percentage of Expected Birth Weight in the Offspring of Women With Schizophrenia in Relation to the Offspring of Comparison Women During 1980–1992 in Western Australia
1. Geddes JR, Lawrie SM: Obstetric complications and schizophrenia: a meta-analysis. Br J Psychiatry 1995; 167:786–793Crossref, Medline, Google Scholar
2. Verdoux H, Geddes JR, Takei N, Lawrie SM, Bovet P, Eagles JM, Heun R, McCreadie RG, McNeil TF, O’Callaghan E, Stöber G, Willinger U, Wright P, Murray RM: Obstetric complications and age at onset in schizophrenia: an international collaborative meta-analysis of individual patient data. Am J Psychiatry 1997; 154:1220–1227Link, Google Scholar
3. Cannon M, Jones PB, Murray RM: Obstetric complications and schizophrenia: historical and meta-analytic review. Am J Psychiatry 2002; 159:1080–1092Link, Google Scholar
4. Done DJ, Johnstone EC, Frith CD, Golding J, Shepherd PM, Crow TJ: Complications of pregnancy and delivery in relation to psychosis in adult life: data from the British perinatal mortality survey sample. Br Med J 1991; 302:1576–1580Crossref, Medline, Google Scholar
5. Byrne M, Browne R, Mulryan N, Scully A, Morris M, Kinsella A, Takei N, McNeil T, Walsh D, O’Callaghan E: Labour and delivery complications in schizophrenia. Br J Psychiatry 2000; 176:531–536Crossref, Medline, Google Scholar
6. Kendell RE, McInneny K, Juszczak E, Bain M: Obstetric complications and schizophrenia. Br J Psychiatry 2000; 176:516–522Crossref, Medline, Google Scholar
7. Cannon TD, Rosso IM, Hollister JM, Bearden CE, Sanchez LE, Hadley T: A prospective cohort study of genetic and perinatal influences in the etiology of schizophrenia. Schizophr Bull 2000; 26:351–366Crossref, Medline, Google Scholar
8. Rosenthal D: The offspring of schizophrenic couples. J Psychiatr Res 1966; 4:169–188Crossref, Medline, Google Scholar
9. Gottesman II, McGuffin P, Farmer AE: Clinical genetics as clues to the “real” genetics of schizophrenia (a decade of modest gains while playing for time). Schizophr Bull 1987; 13:23–47Crossref, Medline, Google Scholar
10. Sobel DE: Infant mortality and malformations in children of schizophrenic women. Psychiatr Q 1961; 35:60–65Crossref, Google Scholar
11. Rieder RO, Rosenthal D, Wender P, Blumenthal H: The offspring of schizophrenics: fetal and neonatal deaths. Arch Gen Psychiatry 1975; 32:200–211Crossref, Medline, Google Scholar
12. Modrzewska K: The offspring of schizophrenic parents in a North Swedish isolate. Clin Genet 1980; 17:191–201Crossref, Medline, Google Scholar
13. Sacker A, Done DJ, Crow TJ: Obstetric complications in children born to parents with schizophrenia: a meta-analysis of case-control studies. Psychol Med 1996; 26:279–287Crossref, Medline, Google Scholar
14. Bennedsen BE, Mortensen PB, Olesen AV, Henriksen TB: Preterm birth and intra-uterine growth retardation among children of women with schizophrenia. Br J Psychiatry 1999; 175:239–245Crossref, Medline, Google Scholar
15. Bennedsen BE, Mortensen PB, Olesen AV, Henriksen TB, Frydenberg M: Obstetric complications in women with schizophrenia. Schizophr Res 2001; 47:167–175Crossref, Medline, Google Scholar
16. Bennedsen BR, Mortensen PB, Olesen AV, Henriksen TB: Congenital malformations, stillbirths, and infant deaths among children of women with schizophrenia. Arch Gen Psychiatry 2001; 58:674–679Crossref, Medline, Google Scholar
17. Nilsson E, Lichtenstein P, Cnattingius S, Murray RM, Hultman CM: Women with schizophrenia: pregnancy outcome and infant death among their offspring. Schizophr Res 2002; 58:221–229Crossref, Medline, Google Scholar
18. Pasamanick B, Knobloch H: Brain damage and reproductive casualty. Am J Orthopsychiatry 1956; 112:613–618Link, Google Scholar
19. Lapalme M, Hodgins S, LaRoche C: Children of parents with bipolar disorder: a metaanalysis of risk for mental disorders. Can J Psychiatry 1997; 42:623–631Crossref, Medline, Google Scholar
20. Stanley FJ, Croft ML, Gibbins J, Read AW: A population database for maternal and child health research in Western Australia using record linkage. Paediatr Perinat Epidemiol 1994; 8:433–447Crossref, Medline, Google Scholar
21. Holman CD, Bass AJ, Rouse I, Hobbs M: Population-based linkage of health records in Western Australia: development of a health services research linked database. Aust NZ J Publ Health 1999; 23:453–459Crossref, Medline, Google Scholar
22. OECD Health Data 2000: Comparative Analysis of 29 Countries. Paris, Organisation for Economic Co-Operation and Development, 2000 (CD-ROM Version, July 15, 2000)Google Scholar
23. Edwards RW, Madden R: The Health and Welfare of Australia’s Aboriginal and Torres Strait Islander Peoples, 2001: Catalogue Number 4704.0. Canberra, Australian Bureau of Statistics, 2001Google Scholar
24. Jaro MA: Automatch: Generalized Record Linkage System, Version 2.9c. Burtonsville, Md, Matchware Technologies, 1994Google Scholar
25. Stanley F, Read A, Kurinczuk J, Croft M, Bower C: A population maternal and child health research database for research and policy evaluation in Western Australia. Semin Neonatol 1997; 2:195–201Crossref, Google Scholar
26. 1996 Census of Population and Housing: Socio-Economic Indexes for Areas: Catalogue Number 2039.0. Canberra, Australian Bureau of Statistics, 1998Google Scholar
27. McGuffin P, Farmer A, Harvey I: A polydiagnostic application of operational criteria in studies of psychotic illness: development and reliability of the OPCRIT system. Arch Gen Psychiatry 1991; 48:764–770Crossref, Medline, Google Scholar
28. McNeil T, Cantor-Graae E, Sjöström K: Obstetric complications as antecedents of schizophrenia: empirical effects of using different obstetric complication scales. J Psychiatr Res 1994; 28:519–530Crossref, Medline, Google Scholar
29. Liang KY, Zeger SL: Longitudinal data analysis using generalized linear models. Biometrika 1986; 73:13–22Crossref, Google Scholar
30. Blair E: The undesirable consequences of controlling for birth weight in perinatal epidemiological studies. J Epidemiol Community Health 1996; 50:559–563Crossref, Medline, Google Scholar
31. Windham GC, Eaton A, Hopkins B: Evidence for an association between environmental tobacco smoke exposure and birthweight: a meta-analysis and new data. Paediatr Perinat Epidemiol 1999; 13:35–57Crossref, Medline, Google Scholar
32. Jablensky A, McGrath J, Herrman H, Castle D, Gureje O, Evans M, Carr V, Morgan V, Korten A, Harvey C: Psychotic disorders in urban areas: an overview of the Study on Low Prevalence Disorders. Aust NZ J Psychiatry 2000; 34:221–236Crossref, Medline, Google Scholar
33. Torrey EF, Miller J, Rawlings R, Yolken RH: seasonality of births in schizophrenia and bipolar disorder: a review of the literature. Schizophr Res 1997; 28:1–38Crossref, Medline, Google Scholar
34. Morgan VA, Jablensky AV, Castle DJ: Season of birth in schizophrenia and affective psychoses in Western Australia 1916–61. Acta Psychiatr Scand 2001; 104:138–147Crossref, Medline, Google Scholar
35. Kinney DK, Levy DL, Yurgelun-Todd DA, Medoff D, LaJonchere CM, Radford-Paregol M: Season of birth and obstetrical complications in schizophrenics. J Psychiatr Res 1994; 28:499–509Crossref, Medline, Google Scholar
36. McNeil TF: Perinatal risk factors and schizophrenia: selective review and methodological concerns. Epidemiol Rev 1995; 17:107–112Crossref, Medline, Google Scholar
37. Jones PB, Rantakallio P, Hartikainen A-L, Isohanni M, Sipila P: Schizophrenia as a long-term outcome of pregnancy, delivery, and perinatal complications: a 28-year follow-up of the 1966 North Finland general population birth cohort. Am J Psychiatry 1998; 155:355–364Link, Google Scholar
38. Zornberg GL, Buka SL, Tsuang MT: Hypoxic-ischemia-related fetal/neonatal complications and risk of schizophrenia and other nonaffective psychoses: a 19-year longitudinal study. Am J Psychiatry 2000; 157:196–202Link, Google Scholar
39. Rosso IM, Cannon TD, Huttunen T, Huttunen MO, Lönnqvist J, Gasperoni TL: Obstetric risk factors for early-onset schizophrenia in a Finnish birth cohort. Am J Psychiatry 2000; 157:801–807Link, Google Scholar
40. Dalman C, Thomas HV, David AS, Gentz J, Lewis G, Allebeck P: Signs of asphyxia at birth and risk of schizophrenia: population-based case-control study. Br J Psychiatry 2001; 179:403–408Crossref, Medline, Google Scholar
41. Mitchell SC, Korones SB, Berendes HW: Congenital heart disease in 56,109 births: incidence and natural history. Circulation 1971; 43:323–332Crossref, Medline, Google Scholar
42. Nguyen M, Camenisch T, Snouwaert JN, Hicks E, Coffman TM, Anderson PAW, Malouf NN, Koller BH: The prostaglandin receptor EP4 triggers remodelling of the cardiovascular system at birth. Nature 1997; 390:78–81Crossref, Medline, Google Scholar
43. Fabrikant S, Jansen B, Hallmayer J, Brett A, Johnston J, Jablensky A: The niacin skin-flush response in patients with schizophrenia, unaffected first degree relatives and controls, in Phospholipid Spectrum Disorders in Psychiatry and Neurology, 2nd ed. Edited by Peet M, Glen I, Horrobin D. Carnforth, UK, Marius Press, 2003, pp 333–340Google Scholar
44. Messamore E: Relationship between the niacin skin flush response and essential fatty acids in schizophrenia. Prostaglandins Leukot Essent Fatty Acids 2003; 69:413–419Crossref, Medline, Google Scholar
45. Pulver AE, Nestadt G, Goldberg R, Shprintzen RJ, Lamacz M, Wolyniec PS, Morrow B, Karagiyorgou M, Antonarakis SE, Housman D, Kucherlapati R: Psychotic illness in patients diagnosed with velo-cardio-facial syndrome and their relatives. J Nerv Ment Dis 1994; 182:476–478Crossref, Medline, Google Scholar
46. Liu H, Heath SC, Sobin C, Roos JL, Galke BL, Blundell ML, Lenane M, Robertson B, Wijsman EM, Rapoport JL, Gogos JA, Karagiyorgou M: Genetic variation at the 22q11 PRODH2/DGCR6 locus presents an unusual pattern and increases susceptibility to schizophrenia. Proc Natl Acad Sci USA 2002; 99:3717–3722Crossref, Medline, Google Scholar
47. Mohl W, Mayr WR: Atrial septal defect of the secundum type and HLA. Tissue Antigens 1977; 10:121–122Crossref, Medline, Google Scholar
48. Schwab SG, Mondabon S, Knapp M, Albus M, Hallmayer J, Borrmann-Hassenbach M, Trixler M, Gross M, Schulze TG, Rietschel M, Lerer B, Maier W, Wildenauer DB: Association of tumor necrosis factor alpha gene-G308A polymorphism with schizophrenia. Schizophr Res 2003; 65:19–25Crossref, Medline, Google Scholar
49. Basso O, Olsen J, Christensen K: Low birthweight and prematurity in relation to paternal factors: a study of recurrence. Int J Epidemiol 1999; 28:695–700Crossref, Medline, Google Scholar
50. Kendell RE, Boyd JH, Grossmith VL, Bain M: Seasonal fluctuation in birthweight in schizophrenia. Schizophr Res 2002; 57:157–164Crossref, Medline, Google Scholar
51. Urakubo A, Jarskog LF, Lieberman JA, Gilmore JH: Prenatal exposure to maternal infection alters cytokine expression in the placenta, amniotic fluid, and fetal brain. Schizophr Res 2001; 47:27–36Crossref, Medline, Google Scholar
52. Wright P, Nimgaonkar VL, Donaldson PT, Murray RM: Schizophrenia and HLA: a review. Schizophr Res 2001; 47:1–12Crossref, Medline, Google Scholar



