Obstetric Risk Factors for Early-Onset Schizophrenia in a Finnish Birth Cohort
Abstract
OBJECTIVE: Although case-control investigations have shown an association between obstetric complications and schizophrenia, particularly among patients with early onsets, cohort studies have mostly failed to confirm this effect. The authors examined whether a history of fetal hypoxia and other obstetric complications elevated risk for early-onset schizophrenia in a 1955 Helsinki birth cohort. METHOD: The subjects were 80 randomly selected patients with schizophrenia (36 with early and 44 with later onsets) representative of all available probands in the cohort, 61 of their nonschizophrenic siblings, and 56 demographically matched nonpsychiatric comparison subjects. Psychiatric diagnoses were obtained from structured clinical interviews, and obstetric data were taken from standardized, prospectively ascertained obstetric records. A score for hypoxia-associated obstetric complications was entered into logistic regression models, along with measures of prenatal infection and fetal growth retardation. RESULTS: Hypoxia-associated obstetric complications significantly increased the odds of early-onset schizophrenia but not of later-onset schizophrenia or unaffected sibling status, after prenatal infection and fetal growth retardation were taken into account. CONCLUSIONS: These findings support an association between obstetric complications and increased risk for early-onset schizophrenia. The authors advance a model whereby the neurotoxic effects of fetal hypoxia may lead to an early onset of schizophrenia due to premature cortical synaptic pruning.
Obstetric complications have been found to be associated with schizophrenia in numerous studies involving many different types of samples, including patients with childhood-onset schizophrenia (1, 2), siblings (3–5) and twins (6–8) discordant for schizophrenia, adopted individuals with schizophrenia (9), offspring of schizophrenic parents (10, 11), adult patients with schizophrenia and matched comparison subjects (12, 13), and representative birth cohorts (14–16). However, questions remain as to the nature of the mechanism(s) underlying this association and whether obstetric complications are related to a particular type or course of schizophrenia.
Most prior studies of obstetric complications in schizophrenia used general summary measures of obstetric influences, making it difficult to determine whether a narrow or broad range of neurally disruptive mechanisms is implicated. When particular types of complications have been examined, both direct and indirect indicators of fetal hypoxia have emerged as the strongest predictors, occurring in a larger proportion of patients than either exposure to infections during gestation or fetal growth retardation (reviewed in references 17 and 18). Nevertheless, since these prenatal influences could confer a heightened risk for oxygen insufficiency, it remains unclear whether the association of fetal hypoxia with schizophrenia is a direct one or is secondary to prenatal influences. To address this question it is necessary to examine the association of fetal hypoxia with schizophrenia after controlling for the effects of earlier obstetric influences statistically.
Because genetic factors play a substantial role in schizophrenia (19), it is also possible that a given obstetric risk factor is itself a consequence of genetic liability for the disorder, in which case the influences of genetic and obstetric factors on risk for schizophrenia would be confounded. Family studies provide a basis for examining this question: if such genotype-environment covariation exists, one would expect a higher rate of an obstetric factor in the first-degree relatives of schizophrenic probands than in the general population. While prior studies have produced conflicting evidence of fetal growth retardation as a cofamilial trait (20, 21), we know of no study thus far that has found evidence of a higher than normal rate of fetal hypoxia in the offspring or siblings of schizophrenic patients (18). To our knowledge, this issue has not yet been examined in regard to prenatal exposure to infection.
The association of obstetric complications and schizophrenia is one line of evidence implicating neurodevelopmental disturbances in the etiology of the disorder. Because obstetric complications suggest brain damage acquired during the pre- or perinatal period, it is reasonable to suspect that they are related to a form of schizophrenia positive for other indicators of neurodevelopmental compromise, such as delayed motor and cognitive development, poor premorbid social adjustment, and early onset. In support of this view, a meta-analysis (22) of individual patient data from 11 different research groups that used the Lewis and Murray obstetric complication scale (23) showed that schizophrenic patients with onsets before age 22 were about 52% more likely to have a history of obstetric complications than those with later onsets.
In this study we coded information from the original obstetric hospital records of 80 schizophrenic probands, ascertained so as to be representative of all such probands in a Helsinki birth cohort, along with 61 of their nonschizophrenic full siblings and 56 demographically similar comparison subjects without a personal or family history of treated psychiatric illness. On the basis of the foregoing, we hypothesized that after we controlled for prenatal infection and fetal growth retardation, complications associated with fetal hypoxia would predict increased risk for early-onset schizophrenia but would not be related to risk for later-onset schizophrenia or to sibling status.
Method
Sample Ascertainment
The study sample was formed by searching the Finnish Population Register for all individuals born in Helsinki in 1955 (N=7,840) and all their first-degree relatives (N=26,273, including 12,796 siblings). The cohort was screened for psychiatric morbidity by searching national case registries with methods previously described (19, 24). A total of 267 (1.3%) members of the offspring of this population (i.e., cohort members and their siblings) had a register diagnosis of 295.x (schizophrenia or schizoaffective disorder) according to the ICD-8 numbering scheme. Probands were randomly selected from this pool of 267 and approached initially through their treating psychiatrists. Those who expressed interest in participating were contacted by project staff, who provided a complete description of the study and obtained written informed consent from all subjects. An attempt was made to recruit at least one nonschizophrenic sibling of each studied proband who had such siblings.
Subjects for whom obstetric records were not available were excluded, such that 80 probands and 61 unaffected siblings (forming 61 proband-sibling pairs from 50 independent families) constituted the study sample. The studied probands were equivalent to the remainder of the proband population in terms of year of birth, nuclear family size, gender, history of inpatient admission, history of comorbid substance abuse, and work disability, but the studied group had a higher mean number of hospital admissions than the nonstudied group (24).
A comparison group of 56 nonschizophrenic subjects (28 sibling pairs from 28 independent families), matched to the proband-sibling pairs in terms of age, gender, gender concordance/discordance, and social class, was recruited from the same study population after exclusion of individuals with personal or family histories of treated psychosis.
Diagnostic Evaluations
All subjects were interviewed with the Structured Clinical Interview for DSM-III-R, Patient or Non-Patient edition (SCID) (25). Any subject with an axis I psychotic condition was also rated with the Scale for the Assessment of Positive Symptoms (26) and the Scale for the Assessment of Negative Symptoms (27). All other subjects were interviewed and rated on the cluster A items from the Personality Disorder Examination (28). A standard coding form was used to summarize details of the illness and treatment history of any subject with a history of inpatient admissions. The interviewer assigned diagnoses according to DSM-III-R criteria, using all available information. Clinical case reports were generated, stripped of identifying information, and independently evaluated by another diagnostician. The reliability of the primary diagnosis was excellent (i.e., kappa mean=0.94, SD=0.02) (29). Diagnostic disagreements were flagged, and another independent diagnostician rated those cases for consensus diagnoses. Of the 80 probands studied, 67 were diagnosed with schizophrenia and 13 were diagnosed as having schizoaffective disorder.
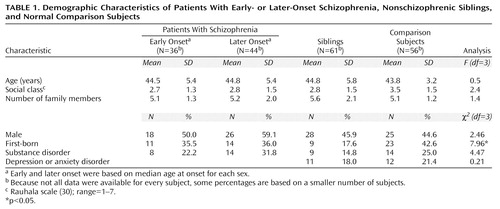
Age at onset was defined according to SCID criteria as the age at first psychotic symptoms (25). The 80 probands had mean age at first symptoms of 21.3 years (SD=5.2). The male patients were significantly younger at onset (mean age=20.1 years, SD=4.8) than the female patients (mean=22.8, SD=5.3) (one-tailed t test: t=–2.38, df=78, p=0.01). For each gender, the median age at onset (22 years for females, 19 years for males) delimited the early- and later-onset cases. Dichotomizing early- and later-onset patients by median age at onset corresponds to the method used in the aforementioned meta-analysis (22) and was preferred over use of the upper quartile (≥25 for females, ≥22 for males) and lower quartile (<20 for females, <17 for males) because of the limited sample sizes.
As shown in Table 1, the schizophrenia, sibling, and comparison groups were balanced in terms of age, gender, social class, family size, and history of substance disorder. The sibling and comparison groups were also balanced in terms of the percentages with axis I diagnoses of depression or anxiety disorder. The siblings were significantly less likely to be first-born than were the normal comparison subjects (χ2=7.70, df=1, p=0.01).
Obstetric Records and Variables
A standard form was used to code information on maternal health, fetal monitoring, prenatal and perinatal complications, and neonatal conditions from the original prenatal clinic and obstetric hospital records, without knowledge of psychiatric diagnosis. Fetal growth retardation was defined as a birth weight below the 10th percentile for the newborn’s gestational age (31). Prenatal infection was coded as either 0 or 1 depending on whether at least one of the following had been documented during pregnancy: influenza, rubella, or any other infection.
A scale for hypoxia-associated obstetric complications comprised 11 obstetric complications associated with prenatal, perinatal, or neonatal oxygen insufficiency. Two obstetric complications implicated a near certainty of hypoxia: birth asphyxia and neonatal cyanosis. Nine obstetric complications significantly related to birth asphyxia or neonatal cyanosis in the overall sample were also included in the scale: anorexia during pregnancy, anemia during pregnancy, third-trimester bleeding, preeclampsia, fetal distress, placental infarcts, breech presentation, prematurity, and an umbilical cord that was knotted or tightly wrapped around the neck.
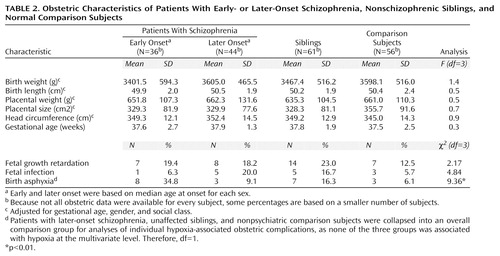
Table 2 shows that the patients with early-onset schizophrenia, patients with later onsets, siblings, and normal comparison subjects did not differ on any measure of intrauterine development. Controlling for gestational age, gender, and social class confirmed the absence of significant differences between groups on fetal growth measures.
Statistical Analyses
Our primary hypothesis was tested by entering an overall score for hypoxia-associated obstetric complications into logistic regression models predicting a single, polytomous measure for diagnostic outcome by the method of generalized logits (32), a method that guards against type I error. The outcome measure divided subjects into four groups: patients with early-onset schizophrenia (N=36), patients with later-onset schizophrenia (N=44), nonschizophrenic siblings of schizophrenic patients (N=61), and comparison subjects with no psychiatric diagnosis (N=56). The initial analysis addressed the main effects of gender, parental social class (Rauhala scale [30], varying from 1 to 7), birth order, mother’s age, prenatal infection, fetal growth retardation, and hypoxia-associated obstetric complications and the two-way interactions of hypoxia and each of the demographic variables. Terms that failed to reach a probability criterion of p£0.10 were excluded from the model on subsequent iterations. An adjusted odds ratio and 95% confidence interval (CI) were determined for each predictor, contrasting the schizophrenia and sibling groups with the nonpsychiatric comparison group. This procedure was repeated with both a prenatal and a perinatal subscale of the scale for hypoxia-associated obstetric complications to test the temporal specificity of the effects of hypoxia. When a significant group effect of any obstetric complication score was found at the multivariate level, post hoc chi-square analyses were conducted to examine which individual obstetric complications contributed to the overall effect. These univariate contrasts were Bonferroni corrected to maintain an analysis-wise alpha level of p£0.05.
A conditional logistic regression analysis was also conducted to test the association between hypoxia-associated obstetric complications and early-onset schizophrenia within families. This analysis was limited to families in which at least one proband and one sibling were available for analysis, and family of origin was used as the stratification variable, thereby controlling for dependency of multiple observations per family unit.
Results
Hypoxia-Associated Obstetric Complications
In the initial regression analysis of the score on the scale for hypoxia-associated obstetric complications as a predictor of group assignment, none of the two-way interactions between hypoxia and demographic variables met the p£0.10 criterion for inclusion, nor did the main effects of gender, birth order, or mother’s age at birth. After exclusion of these variables, there was a statistically significant effect of hypoxia-associated obstetric complications (χ2=10.83, df=3, p=0.01) and nonsignificant effects of prenatal infection (χ2=5.39, df=3, p=0.15), fetal growth retardation (χ2=1.86, df=3, p=0.60), and social class (χ2=6.64, df=3, p=0.08). Contrast analysis revealed that hypoxia-associated obstetric complications significantly increased the risk for early-onset schizophrenia (χ2=9.27, df=1, p=0.002, odds ratio=2.16, 95% CI=1.31–3.53) but were not associated with later-onset schizophrenia (χ2=0.15, df=1, p=0.70, odds ratio=0.89, 95% CI=0.48–1.63) or with being an unaffected sibling of a schizophrenic patient (χ2=0.93, df=1, p=0.33, odds ratio=1.25, 95% CI=0.80–1.96). That is, the odds of early-onset schizophrenia increased by 2.16 times per hypoxia-associated obstetric complication, such that the subjects with three or more such obstetric complications were 10 times more likely to develop early-onset schizophrenia as were those with none.
Specificity of Perinatal Hypoxia
When the previous analyses were repeated with the score on the perinatal hypoxia subscale, there was a statistically significant effect of perinatal hypoxia on group assignment (χ2=10.90, df=3, p=0.01), while the effects of prenatal infection (χ2=5.44, df=3, p=0.15) and fetal growth retardation (χ2=1.52, df=3, p=0.70) remained nonsignificant. Contrast analysis showed that perinatal hypoxia predicted early-onset schizophrenia (χ2=8.06, df=1, p=0.005, odds ratio=2.58, 95% CI=1.34–4.96) but not later-onset schizophrenia (χ2=0.78, df=1, p=0.38, odds ratio=0.66, 95% CI=0.27–1.65) or unaffected sibling status (χ2=1.84, df=1, p=0.18, odds ratio=1.51, 95% CI=0.83–2.73). Conversely, prenatal hypoxia alone was not significantly associated with group outcome (χ2=5.05, df=1, p=0.17) after we controlled for prenatal infection (χ2=5.89, df=1, p=0.12) and fetal growth retardation (χ2=1.81, df=1, p=0.61).
Individual Obstetric Complications
Since none of the later-onset, sibling, or normal comparison groups was associated with fetal hypoxia at the multivariate level, they were collapsed into a single comparison group for univariate contrasts. The individual hypoxia-associated obstetric complications found to be significantly more frequent among the patients with early-onset schizophrenia than among these comparison subjects were birth asphyxia (χ2=9.36, df=1, p=0.002, odds ratio=4.55, 95% CI=1.62–12.79), prematurity (χ2=4.10, df=1, p=0.04, odds ratio=2.13, 95% CI=1.02–4.44), and anorexia during pregnancy (χ2=7.35, df=1, p=0.007, odds ratio=8.73, 95% CI=1.38–55.25). Placental infarcts were also more frequent among the patients with early-onset schizophrenia, but this effect did not reach statistical significance (χ2=2.73, df=1, p=0.10, odds ratio=2.58, 95% CI=0.81–8.23). Only birth asphyxia retained a statistically significant association with early-onset schizophrenia after Bonferroni correction (Table 2).
Association Within Families
The odds of early-onset schizophrenia increased by 2.9 times per unit increase on the hypoxia-associated obstetric complication scale within families, although this fell slightly short of statistical significance (χ2=3.06, df=1, p=0.08, odds ratio=2.86, 95% CI=0.88–9.27).
Discussion
The principal finding of this study is that hypoxia-associated obstetric complications, but not prenatal exposure to infection or fetal growth retardation, are associated with a higher than normal risk for early-onset schizophrenia. Further, there were no differences in rates of exposure to hypoxia-associated obstetric complications or other complications between patients with later-onset schizophrenia, nonschizophrenic siblings of patients, and demographically similar comparison subjects at low genetic risk for schizophrenia.
A lack of difference in siblings indicates that the occurrence of fetal hypoxia is independent of genetic risk for schizophrenia. In other words, the current results are not consistent with hypotheses holding that hypoxia-associated obstetric complications are the consequence of an inherited predisposition to schizophrenia, as these complications were not more frequent among the patients’ unaffected biological siblings—a proportion of whom are expected to carry genes associated with susceptibility to schizophrenia (33). Rather, our findings converge with other reports of a higher incidence of obstetric complications in schizophrenia patients than in their nonpsychotic siblings (4, 5) and with evidence that the frequency of obstetric complications does not rise with increasing genetic loading for schizophrenia (3, 11, 20). Further, since the majority of subjects with a history of hypoxia-associated obstetric complications did not become schizophrenic, our results also exclude familial-sporadic theories in which obstetric complications are sufficient causes of schizophrenia acting independently from genetic risk (23). It is also important that obstetric complications were not a necessary condition for developing schizophrenia or an early-onset form of schizophrenia, as 33% of the patients with schizophrenia (22% of those with early-onset schizophrenia) had a score of 0 on the scale for hypoxia-associated obstetric complications. Because liability for schizophrenia appears to conform to a polygenic mode of inheritance (19), it is possible that the patients without hypoxia possessed a greater number of predisposing genes, such that an additional contribution of obstetric complications was not necessary for the onset of psychosis. Nonetheless, we are left with two competing etiologic models in which obstetric complications act either additively or in interaction with genetic factors in increasing liability for schizophrenia (18). While epidemiologic methods cannot differentiate these latter two alternatives, we believe that the results of studies using quantitative indicators of genetic liability are consistent with a model of schizophrenia involving interaction of genes and obstetric complications (20 and unpublished manuscript by Cannon et al.).
That most studies have shown schizophrenia to be associated with an aggregation of obstetric complications, rather than specific ones in isolation, could reflect the involvement of a common mechanism that can be produced by a variety of different conditions, or it could indicate involvement of multiple different mechanisms. In the present study, birth asphyxia was the only individual complication that was significantly associated with early-onset schizophrenia, a finding that is consistent with oxygen insufficiency as a primary obstetric mechanism. Also in accord with this interpretation are the results of a recent Swedish cohort study (16), in which three categories of obstetric complications (malnutrition, prematurity, and perinatal hypoxia) that have hypoxia as a common mechanism were significantly more prevalent among a group of schizophrenic patients with early onsets. Further, the null results of two previous cohort studies are not inconsistent with a specific involvement of hypoxia. One of these studies (14) had only eight schizophrenic patients. In the second cohort study (15) an obstetric complication scale constructed to predict stillbirth and neonatal mortality did not predict schizophrenia. However, when individual obstetric complications were examined in this cohort, bleeding during pregnancy and low birth weight, which both imply heightened risk of hypoxia, were significantly related to schizophrenia (34). Together, the results of the present and previous cohort studies therefore point to fetal hypoxia as an important and perhaps primary obstetric influence in the development of schizophrenia. We should note, however, that etiologic factors can be identified only tentatively with clinical epidemiologic methods and that research using direct biochemical indicators of blood oxygenation is needed to confirm the relationship between fetal hypoxia and schizophrenia.
Our findings are consistent with converging epidemiologic evidence that obstetric complications, hypoxia in particular, increase the risk for neurodevelopmental compromise and for a form of adult schizophrenia with an early onset (22). There have also been reports of high rates of obstetric complications in childhood-onset schizophrenia, although most come from a time period when diagnostic boundaries with the adult disorder were unclear (1, 2). In this study, by using gender-specific medians of age at onset, we were able to determine whether there was an association between obstetric complications and early-onset forms of schizophrenia in both genders, without the confound of different distributions of ages at onset in male and female patients. In fact, we found a robust association between fetal hypoxia and early-onset schizophrenia for both genders, which strengthens the hypothesis that obstetric complications relate specifically to a neurodevelopmental form of the disorder. In addition, our findings suggest that the specificity of fetal hypoxia may relate in part to its time of occurrence, since perinatal but not prenatal oxygen deprivation predicted early-onset schizophrenia. Taken together, the increasing evidence for the specificity of obstetric complications to early-onset schizophrenia suggests that data in previous studies that did not separate patients by age at onset may need to be reanalyzed.
While we can only speculate as to the mechanism(s) involved in the timing of schizophrenia onset, a prominent candidate may be the rate of cortical synaptic pruning. Some investigators (35, 36) have proposed that schizophrenia arises because of excessive pruning, such that a reduction of neuronal synapses below a certain threshold produces psychotic symptoms. Within this framework, variations in the rate of synaptic pruning would vary the age at clinical onset of schizophrenia. It is interesting that excessive pruning is consistent with several brain abnormalities documented in schizophrenia, including prominent reductions in neuropil volume (37), numbers of pyramidal dendritic spines (38), and synaptic protein levels (39). Furthermore, since synaptic pruning involves predominantly glutamatergic synapses (36), exaggerated pruning could result in glutamate receptor hypofunction, a possible cause of striatal dopaminergic hyperactivity and psychotic symptoms in schizophrenia (40, 41).
We propose that the neurotoxic effects of hypoxia-associated obstetric complications may reduce the amount of synaptic pruning required in late adolescence to cross the psychosis threshold, leading to an earlier onset of schizophrenia. This hypothesis is supported by the fact that temporal lobe brain regions particularly vulnerable to fetal hypoxia (42) have also been consistently implicated in schizophrenia (43). Thus, hippocampal neurons are reduced in number or density following fetal hypoxia (44, 45), as they appear to be in the schizophrenic brain (46, 47). These cellular findings are consistent with recent in vivo data from our laboratory indicating an association between fetal hypoxia and temporal gray matter deficits in both patients with schizophrenia and their unaffected siblings but not in comparison subjects at low genetic risk for schizophrenia (unpublished manuscript by Cannon et al.). Preschizophrenic individuals with a history of fetal hypoxia may therefore have a lower baseline number of neurons and synapses in temporal brain regions than those without such a history. With this reduced neuronal reserve, less further synaptic pruning would be required to cross the psychosis vulnerability threshold, resulting in earlier clinical onset in individuals with the schizophrenia genotype. Indeed, neuronal loss in temporal brain structures should decrease striatal glutamatergic efferents and enhance postsynaptic dopamine receptor sensitivity (48). Subsequent postnatal pruning of temporal-striatal projections would then be expected to produce exaggerated mesolimbic reactivity and psychotic symptoms at an earlier age in preschizophrenic individuals positive for fetal hypoxia.
Several strengths of this study’s design merit explicit mention. Foremost is the use of original pregnancy and birth records, which can be more accurate and reliable sources of obstetric history than retrospective parental interviews (23). In addition, the studied probands were a random and representative sample drawn from a Helsinki birth cohort and were directly interviewed according to strict diagnostic criteria. Finally, the inclusion of both unaffected siblings and a normal comparison group allowed for the evaluation of competing gene-environment etiologic models of schizophrenia.
This study was limited, however, by its inability to explore all obstetric variables that might be implicated in schizophrenia. The obstetric records used were not developed for research purposes and therefore may not have included all variables associated with schizophrenia. In addition, the statistical power was not sufficient to examine the association between obstetric complications and family history of schizophrenia, or between all obstetric complications and schizophrenia at the level of individual complications.
 |
 |
Received March 10, 1999; revisions received Sept. 28 and Nov. 16, 1999; accepted Nov. 17, 1999. From the Department of Psychology, University of Pennsylvania, Philadelphia; the Department of Psychology, University of California, Los Angeles; and the Department of Mental Health and Alcohol Research, National Public Health Institute, Helsinki. Address reprint requests to Dr. Cannon, Psychology Department, University of California, Los Angeles, 1285 Franz Hall, P.O. Box 951563, Los Angeles, CA 90095-1563; cannon@ psych.ucla.edu (e-mail). Supported by NIMH grant MH-48207 and by an Independent Scientist Award from the National Alliance for Research on Schizophrenia and Depression to Dr. Cannon. The authors thank Ulla Mustonen, M.S.W., Liisa Varonen, Ph.D., and Pirjo K㪩, M.S.W., for their contributions to subject recruitment and assessment; Antti Tanskanen, M.S., for his contributions to the register searches and database creation; and Mary O’Brien, Ph.D., for her contributions to the diagnostic procedures and reliability assessment.
1. Torrey EF, Hersh SP, McCabe KD: Early childhood psychosis and bleeding during pregnancy: a prospective study of gravid women and their offspring. J Autism Child Schizophr 1975; 5:287–297Crossref, Medline, Google Scholar
2. Rutt CN, Offord DR: Prenatal and perinatal complications in childhood schizophrenics and their siblings. J Nerv Ment Dis 1971; 152:324–331Crossref, Medline, Google Scholar
3. G�-Genta F, Bovet P, Hohlfeld P: Obstetric complications and schizophrenia: a case-control study. Br J Psychiatry 1994; 164:165–170Crossref, Medline, Google Scholar
4. DeLisi LE, Goldin LR, Maxwell ME, Kazuba DM, Gershon ES: Clinical features of illness in siblings with schizophrenia or schizoaffective disorder. Arch Gen Psychiatry 1987; 44:891–896Crossref, Medline, Google Scholar
5. Heun R, Maier W: The role of obstetric complications in schizophrenia. J Nerv Ment Dis 1993; 181:220–226Crossref, Medline, Google Scholar
6. McNeil TF, Cantor-Graae E, Torrey EF, Sj��, Bowler A, Taylor E: Obstetric complications in the histories of monozygotic twins discordant and concordant for schizophrenia. Acta Psychiatr Scand 1994; 89:196–204Crossref, Medline, Google Scholar
7. Onstad S, Skre I, Torgersen S, Kringlen E: Birthweight and obstetric complications in schizophrenic twins. Acta Psychiatr Scan 1992; 85:70–73Crossref, Medline, Google Scholar
8. Torrey EF, Taylor EH, Bracha HS, Bowler AE, McNeil TF, Rawlings RR, Quinn PO, Bigelow LB, Rickler K, Sjostrom K: Prenatal origin of schizophrenia in a subgroup of discordant monozygotic twins. Schizophr Bull 1994; 20:423–432Crossref, Medline, Google Scholar
9. Jacobsen B, Kinney D: Perinatal complications in adopted and non-adopted schizophrenics and their controls: preliminary results. Acta Psychiatr Scand Suppl 1980; 285:337–351Crossref, Google Scholar
10. Parnas J, Schulsinger F, Teasdale TW, Schulsinger H, Feldman PM, Mednick SA: Perinatal complications and clinical outcome within the schizophrenia spectrum. Br J Psychiatry 1982; 140:416–420Crossref, Medline, Google Scholar
11. Cannon TD, Mednick SA, Parnas J: Antecedents of predominantly negative- and predominantly positive-symptom schizophrenia in a high-risk population. Arch Gen Psychiatry 1990; 47:622–632Crossref, Medline, Google Scholar
12. O’Callaghan E, Gibson T, Colohan HA, Buckley P, Walshe DG, Larkin C, Waddington JL: Risk of schizophrenia in adults born after obstetric complications and their associations with early onset of illness: a controlled study. Br Med J 1992; 305:1256–1259Google Scholar
13. McNeil TF, Cantor-Graae E, Sjostrom K: Obstetric complications as antecedents of schizophrenia: empirical effects of using different obstetric complication scales. J Psychiatr Res 1994; 28:519–530Crossref, Medline, Google Scholar
14. Buka SL, Tsuang MT, Lipsitt LP: Pregnancy/delivery complications and psychiatric diagnosis: a prospective study. Arch Gen Psychiatry 1993; 50:151–156Crossref, Medline, Google Scholar
15. Done DJ, Johnstone EC, Frith CD, Golding J, Shepherd PM, Crow TJ: Complications of pregnancy and delivery in relation to psychosis in adult life: data from the British perinatal mortality survey sample. BMJ 1991; 302:1576–1580Google Scholar
16. Dalman C, Allebeck P, Cullberg J, Grunewald C, K�r M: Obstetric complications and the risk of schizophrenia: a longitudinal study of a national birth cohort. Arch Gen Psychiatry 1999; 56:234–240Crossref, Medline, Google Scholar
17. McNeil TF: Obstetric factors and perinatal injuries, in Handbook of Schizophrenia, vol. 3. Edited by Tsuang MT, Simpson JC. New York, Elsevier, 1988, pp 319–344Google Scholar
18. Cannon TD: On the nature and mechanisms of obstetric influences in schizophrenia: a review and synthesis of epidemiologic studies. Int Rev Psychiatry 1997; 9:387–397Crossref, Google Scholar
19. Cannon TD, Kaprio J, L�vist J, Huttunen MO, Koskenvuo M: The genetic epidemiology of schizophrenia in a Finnish twin cohort: a population-based modeling study. Arch Gen Psychiatry 1998; 55:67–74Crossref, Medline, Google Scholar
20. Cannon TD, Mednick SA, Parnas J, Schulsinger F, Praestholm J, Vestergaards A: Developmental brain abnormalities in the offspring of schizophrenic mothers, I: genetic and perinatal contributions. Arch Gen Psychiatry 1993; 50:551–564Crossref, Medline, Google Scholar
21. Fish B, Marcus J, Hans SL, Auerbach JG, Perdue S: Infants at risk for schizophrenia: sequelae of a genetic neurointegrative defect. Arch Gen Psychiatry 1992; 49:221–235Crossref, Medline, Google Scholar
22. Verdoux H, Geddes JR, Takei N, Lawrie SM, Bovet P, Eagles JM, Heun R, McCreadie RG, McNeil TF, O’Callaghan E, St� G, Willinger MU, Wright P, Murray RM: Obstetric complications and age at onset in schizophrenia: an international collaborative meta-analysis of individual patient data. Am J Psychiatry 1997; 154:1220–1227Google Scholar
23. Lewis SW, Murray RM: Obstetric complications, neurodevelopmental deviance, and risk of schizophrenia. J Psychiatr Res 1987; 21:413–421Crossref, Medline, Google Scholar
24. Cannon TD, van Erp TGM, Huttunen M, Lonnqvist J, Salonen O, Valanne L, Poutanen VP, Standertskjold-Nordenstam CG, Gur RE, Yan M: Regional gray matter, white matter, and cerebrospinal fluid distributions in schizophrenic patients, their siblings, and controls. Arch Gen Psychiatry 1998; 55:1084–1091Google Scholar
25. Spitzer RL, Williams JBW, Gibbon M: Structured Clinical Interview for DSM-III-R (SCID). New York, New York State Psychiatric Institute, Biometrics Research, 1987Google Scholar
26. Andreasen NC: Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, University of Iowa, 1984Google Scholar
27. Andreasen NC: Scale for the Assessment of Negative Symptoms (SANS). Iowa City, University of Iowa, 1983Google Scholar
28. Loranger AW, Susman VL, Oldham JM, Russakoff M: Personality Disorder Examination (PDE): A Structured Interview for DSM-III-R Personality Disorders. White Plains, NY, New York Hospital–Cornell Medical Center, Westchester Division, 1985Google Scholar
29. Cohen J: A coefficient of agreement for nominal scales. Educational and Psychol Measurement 1960; 20:37–46Crossref, Google Scholar
30. Rauhala U: [The quantitative strength of the social strata of Finnish society]. Sosiaalinen-Aikakauskirja 1970: 63:347–362 (Finnish)Google Scholar
31. Ott WJ: The diagnosis of altered fetal growth. Obstet Gynecol Clin North Am 1988; 15:237–263Medline, Google Scholar
32. Stokes ME, Davis CS, Koch GG: Categorical Data Analysis Using the SAS System. Cary, NC, SAS Institute, 1995Google Scholar
33. Gottesman II, Bertelsen A: Confirming unexpressed genotypes for schizophrenia: risks in the offspring of Fischer’s Danish identical and fraternal discordant twins. Arch Gen Psychiatry 1989; 46:867–872Crossref, Medline, Google Scholar
34. Sacker A, Done DJ, Crow T, Golding J: Antecedents of schizophrenia and affective illness: obstetric complications. Br J Psychiatry 1995; 166:734–741Crossref, Medline, Google Scholar
35. Feinberg I: Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res 1982; 17:319–334Google Scholar
36. Keshavan MS, Anderson S, Pettegrew JW: Is schizophrenia due to excessive synaptic pruning in the prefrontal cortex? the Feinberg hypothesis revisited. J Psychiatr Res 1994; 28:239–265Crossref, Medline, Google Scholar
37. Selemon LD, Goldman-Rakic PS: The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry 1999; 45:17–25Crossref, Medline, Google Scholar
38. Garey LJ, Ong WY, Patel TS, Kanani M, Davis A, Mortimer AM, Barnes TRE, Hirsch SR: Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J Neurol Neurosurg Psychiatry 1998; 65:446–453Crossref, Medline, Google Scholar
39. Glantz LA, Lewis DA: Reduction of synaptophysin immunoreactivity in the prefrontal cortex of subjects with schizophrenia: regional and diagnostic specificity. Arch Gen Psychiatry 1997; 54:943–952Crossref, Medline, Google Scholar
40. Olney JW, Farber NB: Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry 1995; 52:998–1007Google Scholar
41. Grace AA: Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: schizophrenia. Neuroscience 1991; 41:1–24Crossref, Medline, Google Scholar
42. Volpe JJ: Hypoxic-ischemic encephalopathy: neuropathology and pathogenesis, in Neurology of the Newborn, 3rd ed. Philadelphia, WB Saunders, 1995, pp 279–312Google Scholar
43. Cannon TD: Abnormalities of brain structure and function in schizophrenia: implications for aetiology and pathophysiology. Ann Med 1996; 28:533–539Crossref, Medline, Google Scholar
44. Yue X, Mehmet H, Penrice J, Cooper C, Cady E, Wyatt JS, Reynolds EO, Edwards AD, Squier MV: Apoptosis and necrosis in the newborn piglet brain following transient cerebral hypoxia-ischaemia. Neuropathol Appl Neurobiol 1997; 23:16–25Crossref, Medline, Google Scholar
45. Kuchna I: Quantitative studies of human newborns’ hippocampal pyramidal cells after perinatal hypoxia. Folia Neuropathol 1994; 32:9–16Medline, Google Scholar
46. Jeste DV, Lohr JB: Hippocampal pathologic findings in schizophrenia: a morphometric study. Arch Gen Psychiatry 1989; 46:1019–1024Google Scholar
47. Falkai P, Bogerts B: Cell loss in the hippocampus of schizophrenics. Eur Arch Psychiatry Neurol Sci 1986; 236:154–161Crossref, Medline, Google Scholar
48. Csernansky JG, Bardgett ME: Limbic-cortical neuronal damage and the pathophysiology of schizophrenia. Schizophr Bull 1998; 24:231–248Crossref, Medline, Google Scholar



