Three Potential Susceptibility Loci Shown by a Genome-Wide Scan for Regions Influencing the Age at Onset of Mania
Abstract
OBJECTIVE: The age at onset of bipolar disorder is associated with clinical features of the illness, including duration, severity, and pattern of comorbidity with other disorders. Age at onset is familial and heritable, and it correlates inversely with the prevalence of bipolar disorder among relatives. Because age at onset may have utility in resolving the complexity and heterogeneity of the disorder, the authors sought to identify chromosomal loci that harbor the genes influencing this trait. METHOD: A genome scan of 539 genotyped people in 97 families ascertained for the NIMH Bipolar Disorder Genetics Initiative was performed by using multipoint variance-components linkage analysis. RESULTS: The age at onset of mania was significantly heritable in these families. Three chromosomal regions yielded nonsignificant but suggestive multipoint lod scores greater than 2.5, with the strongest evidence observed at markers D12S1292, GATA31B, and GATA50C, on chromosomes 12p, 14q, and 15q, respectively. CONCLUSIONS: Although firm conclusions await an independent replication, these results suggest that three regions of the genome may contain genes influencing the age at onset of mania in bipolar disorder. To the authors’ knowledge, these regions have not been implicated previously in risk for the disorder, suggesting that separate sets of genes influence disease susceptibility and the age at which it appears.
As much as 85% of the liability for bipolar disorder may be inherited (1). Yet, although bipolar disorder has a strong genetic basis and some regions of the genome have been implicated, the genes involved in its etiology remain unknown (2, 3). In fact, even when data from several genome scans were jointly examined by two different meta-analytic methods (4, 5), discrepant findings of linked chromosomal regions emerged. Furthermore, in the more powerful of these two studies (5), the best evidence for linkage at particular loci would be considered only suggestive according to traditional criteria of genome-wide statistical significance, despite a pool of over 1,000 affected individuals.
This uncertainty may be due, at least in part, to the clinical complexity and heterogeneity of the disorder. For example, although the DSM-III-R criteria and Research Diagnostic Criteria (6) for bipolar disorder have excellent sensitivity and specificity in detecting a mental illness, the disorder may still be confused with major depression, schizophrenia, or schizoaffective disorder in as many as 10% of cases (7). Furthermore, individuals with bipolar disorder differ markedly in illness severity and duration, rates of personal and familial suicidality and mood disorder, and extent of concomitant substance abuse and neuropsychiatric abnormalities. Such complexity creates classification problems that can restrict the power of genetic studies of the condition.
Some of the clinical heterogeneity of bipolar disorder may be due to pleiotropic effects of a single set of bipolar disorder genes; alternatively, such complexity could be due to underlying etiologic heterogeneity. This latter idea is supported by the fact that some multiply affected families show evidence for autosomal dominant transmission of a single major gene for bipolar disorder, while the segregation of the illness through other pedigrees is more consistent with a multifactorial and polygenic etiology in which numerous environmental and genetic factors interact (8–12). Clarifying the source of such discrepancies has become a high priority for those working to understand the true nature of bipolar disorder because of the promise that such an approach holds for refining the phenotype(s) of the illness and the subsequent identification of risk genes for particular disease subtypes, if they exist.
The age at onset of bipolar disorder has been considered as one potentially useful variable for constructing homogeneous groups of patients (13, 14). Age at onset correlates with several clinical features, which could prove useful in explaining some of the clinical heterogeneity just discussed. An early age at onset is more often associated with a chronic course and a poorer response to mood stabilizers, while a later age at onset is associated with more severe abnormal thought content in patients selected for psychotic illness (15). Age at onset may also be a useful marker of the degree of genetic contribution to disease development. For example, Grigoroiu-Serbanescu et al. (16) found that different modes of transmission best fit the segregation of early- and late-onset bipolar disorder; early-onset forms were best explained by passage of a non-Mendelian major gene with a polygenic component, and late-onset forms showed multifactorial inheritance. Early-onset cases also show comorbidity and familial co-transmission with attention deficit and conduct disorders (13, 17–20).
Because age at onset is both familial (21) and heritable (22), it is reasonable to use linkage analysis to detect genes that regulate this trait in bipolar families. However, the age at onset of bipolar disorder as defined by the age at which any affective symptoms first appear might be too broad a phenotype to be useful for genetic study. This phenotype comprises at least two potentially etiologically distinct features: age at onset of mania and age at onset of depression. Thus, to use a composite phenotype that includes both of these aspects of the illness may introduce some degree of causal heterogeneity, which may lower inferential power and hinder gene discovery. To overcome this potential limitation, we examined the heritability of distinct components of the age at onset of bipolar disorder, i.e., the age at onset of mania or depression in pedigrees through which bipolar disorder was segregating. Because the age at onset of depression was not significantly heritable, the remainder of this work focuses on the mania age-at-onset quantitative trait.
Method
Subjects
Families were recruited by investigators at Indiana University, Johns Hopkins University, the National Institute of Mental Health (NIMH) Intramural Research Program, and Washington University as part of the NIMH Human Genetics Initiative (http://zork. wustl.edu/nimh/NIMH_initiative/NIMH_initiative_link.html). Families were selected as previously described (23) by selecting those having two or more members who met the DSM-III-R criteria for bipolar disorder, type I. Written informed consent was obtained by the data collection sites. Families were excluded if both parents had either bipolar disorder I or schizoaffective disorder, bipolar type. The subjects were separated into six diagnostic categories: bipolar disorder I, bipolar disorder II, schizoaffective disorder (bipolar), unipolar depression, other mental illness, and not mentally ill. The total study group consisted of 540 genotyped individuals in 97 families; there were 232 with bipolar disorder I, 32 with schizoaffective disorder (bipolar), 72 with bipolar disorder II, and 89 with recurrent unipolar depression. A genome scan of these families for the qualitative trait of affection status has been reported elsewhere (24–26). We gained access to the genotypes and clinical data through the NIMH Center for Collaborative Genetic Studies on Mental Disorders (http://zork.wustl.edu/nimh/).
Diagnostic Assessment
The structured interview was the Diagnostic Interview for Genetic Studies (7, 27). The test-retest reliability of diagnoses based on this interview was shown to be excellent within and across sites (7, 27). The structured interview data were supplemented by medical records and a semistructured itemized assessment of psychopathology in family members, the Family Interview for Genetic Studies (28). The Diagnostic Insterview for Genetic Studies includes a mania/hypomania section that quantifies the mania status of subjects. In this section, each participant was asked two questions about the age at onset of mania; the first question asked for the age at onset of any manic episode, and the second question asked for the age at onset of the first manic episode that could not be attributed to medical illness, medications, or substance abuse. We used the second question in our analyses because this eliminated mania episodes due to nongenetic factors; however, the correlation between the two variables was significant (r=0.62, N=433, p<0.0001). Use of the latter question excluded 37 subjects who reported at least one manic episode but no “clean” episodes (i.e., no episodes of mania that could not be attributed to medical illness, medications, or substance abuse). Only individuals diagnosed with bipolar disorder I, bipolar disorder II, or schizoaffective disorder, bipolar type, were included in the analysis.
Genotyping and Linkage Analysis
The genotyping methods have been described elsewhere (23–26, 29, 30). Allele frequencies were created previously by using the program USER13, which uses maximum likelihood methods (31). Marker distances were created by using CRIMAP (http://linkage. rockefeller.edu/soft/crimap/), and these results were compared to existing genetic databases (32). Mendelian inconsistencies were also examined previously. There were a total of 319 markers with an average interval spacing of 10 cM throughout the genome.
The distribution of the ages at onset was assessed to ensure that it met the necessary distributional assumptions for variance-components linkage analysis; this assessment was performed by using Sequential Oligogenic Linkage Analysis Routines (SOLAR) (33). Blangero et al. (34) indicated that linkage analysis using SOLAR is appropriate if the following conditions are met: 1) the quantitative trait data resemble a normal distribution, 2) the kurtosis of the distribution is less than 2.0, and 3) the t test option is specified in SOLAR. To meet the specified criteria for proper analyses, the age-at-onset trait was transformed by taking the natural logarithm.
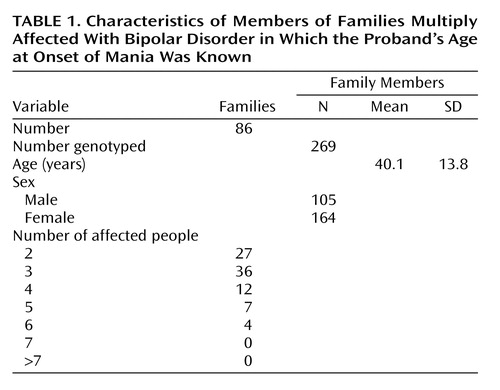
The heritability of age at onset was calculated by using SOLAR. Exact identity-by-descent (IBD) sharing proportions were calculated for each pair of relatives within each pedigree by using Genehunter 2.0 (35). Genehunter calculates exact multipoint IBD probabilities, whereas SOLAR (33) approximates IBD sharing by using Markov chain/Monte Carlo methods. Because the bipolar pedigrees were sufficiently small (2n–f<16, where n is the number of nonfounders and f is the number of founders), Genehunter was used to calculate exact IBD estimates. These IBD estimates were then transported into SOLAR, and variance-components linkage analysis was performed. Table 1 presents the number of families, number of family members, and demographic characteristics of the families used in the analysis.
Variance-components linkage analysis was used to estimate the proportion of the variance attributable to residual genetic effects, random environmental effects, or the quantitative trait loci. By fitting various models, it is possible to make inferences regarding the localization (the chromosomal regions mapped by the genetic markers, i.e., linkage) and the magnitude of effect sizes of major genes. The variance-components linkage analysis was performed on the natural logarithm of the age at onset by using the polygenic model in SOLAR. This analysis was performed only for the age-at-onset score with significant heritability (i.e., age at onset of mania).
To test for linkage at a particular locus, the likelihoods of two models, one with h2q (heritability of the quantitative trait loci) set to its maximum likelihood estimate and one with h2q set to zero, are compared. Twice the difference in the natural log of the likelihood of these two models yields a test statistic that is asymptotically distributed as a 50/50 mixture of a chi-square variable and a point-mass at zero. This is because the estimated variance due to each quantitative trait locus was fixed to a boundary in the nested model (36). The difference between the two log 10 likelihoods produces a lod score (logarithm of the odds ratio for linkage) that is equivalent to the classical lod score of linkage. Tests for linkage and for its effect are repeated throughout the genome.
The lod scores were calculated throughout the autosomal chromosomes at 2-cM intervals. Regions that had evidence for linkage (lod score greater than 0.5) were fine-mapped with points at approximately 1-cM intervals.
Results
The heritability of age at onset for mania was estimated as 0.41 (SE[h2]=0.17, p=0.004). Results of the genome screen for age at onset of mania are presented in Figure 1. The most notable results were for chromosomes 12, 14, and 15 where the lod scores were greater than 2.0. The lod scores were 2.78 near marker D12S1292 (67 cM) on chromosome 12, 3.00 near marker GATA31B (23 cM) on chromosome 14, and 3.12 near marker GATA50C (50 cM) on chromosome 15. According to widely used criteria for evaluating genome-wide statistical significance of the results of linkage analysis (37), these three results would be considered “suggestive” of linkage but not significant. Previous genome scans of this study group used three hierarchical diagnostic models to assess linkage to diagnoses of bipolar disorder (rather than age at onset of mania) but did not show findings for any of these regions (23–26, 29, 30, 38).
Discussion
Our work suggests that three regions of the genome (chromosomes 12p, 14q, and 15q) may contain genes that influence the age at onset of mania in bipolar disorder. These chromosomal regions have not been implicated as high-risk loci for bipolar disorder in either of the previously published meta-analyses of bipolar disorder (4, 5) or in previous linkage analyses of the current data set (23–26, 29, 30). This suggests that genes influencing the age at onset of mania in bipolar disorder are distinct from those influencing the liability of developing the disorder. Further studies are needed to confirm our finding and to determine whether these three loci act in concert—in either an additive or epistatic manner—to place individuals along a continuum of age at onset or whether these three loci are in some way associated with the three discrete age-at-onset classes delineated by Bellivier et al. (39). Because these three regions of interest each span several megabases, it is premature to speculate about which genes gave rise to the observed linkage signals. However, these findings can serve as the basis for future fine-mapping efforts in these regions that may elucidate the responsible polymorphisms. It is clear that none of these linkage signals is attributable to the gene coding for the serotonin transporter (SLC6A4), which has been associated with age at onset of bipolar disorder (40) but resides in chromosome region 17q11.1-q12. In addition, both of the genomic trinucleotide-repeat domains that have been associated with age at onset of bipolar disorder lie on chromosomes other than those showing the strongest linkage signals in the present study; ERDA1 (41) lies in chromosome region17q21.3 and CTG 18.1 (42) lies in region 18q21.1.
In addition to guiding subsequent work aimed at finding genes that determine age at onset generally, the present findings may also inform studies of distinct age-at-onset classes of the disorder, especially juvenile-onset bipolar disorder, which may be more genetically influenced than other forms of the illness. In fact, a growing body of evidence supports a greater genetic contribution to early-onset bipolar disorder than to later-onset forms of the disease (13, 43).
For example, in the NIMH Collaborative Program on the Psychobiology of Depression, the age at onset of bipolar disorder in probands (range=13–52 years) was found to correlate inversely with the risk of the disorder for their relatives (44). This relationship has also been demonstrated numerous times in studies that used a relatively late threshold (generally early or middle adulthood) for distinguishing between early- and later-onset bipolar disorder (43); however, the effect of age at onset on familial risk is the most pronounced among the relatives of probands with pediatric onset (i.e., before 13 years) (45). For example, Strober et al. (46) found high rates of both bipolar disorder and major depression in the first-degree relatives of all of their patients with bipolar disorder but found a much higher prevalence of bipolarity in the relatives of pediatric patients with bipolar disorder (29.4%) than in relatives of older patients (7.4%). Similarly, Neuman et al. (47) found that relatives of pediatric-onset bipolar patients were more than twice as likely to have bipolar disorder than were relatives of later-onset patients.
Such observations are consistent with a greater genetic influence on early-onset cases and suggest that pediatric cases may be more homogeneous, at least in their genetic etiology. If, as presumed, bipolar disorder has a multifactorial polygenic etiology in which numerous genes each have a small influence on total risk, then these genetically enriched early-onset cases of bipolar disorder may share a greater number of risk genes out of the total pool of responsible genes. For example, consider the hypothetical situation where bipolar disorder is caused by the combination of environmental risk factors with at least any four genes out of a pool of 10 risk genes. Among patients with a smaller genetic component (e.g., traditional cases of bipolar disorder), it may be common that the minimally sufficient number of risk genes (N=4) is possessed. Individuals in this category may share all four risk genes or, in fact, may have no overlap at all in the risk genes they possess. In an alternative scenario, among patients with a larger genetic component (e.g., patients with pediatric-onset bipolar disorder), it may be common for individuals to possess more than four of the necessary risk genes. The likelihood that one or more risk genes are shared by patients in this category (i.e., there is genetic overlap or homogeneity) increases with the number of risk genes that each individual possesses. Thus, if each individual in this category has six of the 10 total risk genes, some genetic overlap among patients is inevitable, as each case will have at least one risk gene in common with every other patient in the category; if each individual possesses all 10 risk genes, then the genetic overlap among them is absolute.
In addition to the greater risk for bipolar disorder it confers on relatives, pediatric-onset bipolar disorder is often characterized by an atypical clinical picture that often includes a chronic course, poor response to mood stabilizers, and high levels of comorbidity with attention deficit hyperactivity disorder and conduct disorder (14, 48–52). It is interesting that this pattern is seen in only about one-third of adults with bipolar disorder (53). In sum, these data suggest that individuals with an early onset of bipolar disorder are biologically different from those with a typical onset age and clinical presentation.
It is possible that the loci detected in our study of age at onset harbor genes that separate early-onset and later-onset cases. For example, these loci may contain genes whose risk alleles, if present, lead to an earlier onset of a more severe bipolar illness, while their absence merely allows the “typical” form of bipolar disorder to become manifest. This possibility cannot be powerfully examined in the present data set because we had no juvenile family members. As suggested by Todd et al. (54), linkage studies of bipolar disorder would benefit from recruiting families ascertained through juvenile probands.
Several limitations to this study should be considered. First, although the group size in this study was moderate, the power to detect genes with small effects remains limited. Second, we did not account for other forms of genetic heterogeneity that could exist in this study group. Third, to limit the testing of multiple phenotypes and improve power, we considered only one definition of mania, which may not have been optimal.
In conclusion, the present study identified three chromosomal regions that may harbor genes regulating the age at onset of mania in families that are multiply afflicted with bipolar disorder. These genes appear distinct from those that may increase the overall susceptibility to the disorder. Our results provide suggestive evidence that the age at onset of mania is controlled by multiple quantitative trait loci, which further supports the idea that age at onset is a biologically meaningful feature of the disorder that may be useful in clarifying its heterogeneity.
 |
Received March 28, 2003; revision received Sept. 9, 2003; accepted Sept. 29, 2003. From the Department of Psychiatry, Harvard Medical School, Boston; the Department of Epidemiology, Harvard School of Public Health, Boston; the Harvard Institute of Psychiatric Epidemiology and Genetics, Boston; the Department of Psychiatry, the Johnson and Johnson Center for Pediatric Psychopathology, and the Stanley Center for Pediatric Mania, Massachusetts General Hospital; and the Massachusetts Mental Health Center, Boston. Address reprint requests to Dr. Faraone, Pediatric Psychopharmacology Research, WRN 705, Massachusetts General Hospital, 55 Fruit St., Boston, MA 02114-3139; [email protected] (e-mail). The linkage analysis and manuscript preparation were supported in part by NIMH grants MH-57934 and MH-59126 and by grant HD-37694 from the National Institute of Child Health and Human Development (Dr. Faraone, principal investigator), by NIMH grants MH-43518, MH-59624, and MH-60485 (Dr. Tsuang, principal investigator), and by grants from the Stanley Foundation (J. Biederman, principal investigator) and Johnson and Johnson (J. Biederman, principal investigator). This work was completed when Dr. Glatt was a trainee in the NIMH-funded Psychiatric Genetics Training Program at the Harvard Institute of Psychiatric Epidemiology and Genetics (MH-60485, Dr. Tsuang, principal investigator). The clinical data and genotypes were provided by the NIMH Human Genetics Initiative data repository. The clinical data were collected at four sites: Indiana University, Johns Hopkins University, NIMH Intramural Research Program, and Washington University. The principal investigators at these sites were J. Nurnberger, J. DePaulo, E. Gershon, and T. Reich, respectively. The lead NIMH investigator was M. Blehar. The genotypes were generated in the laboratories of A. Goate, Ph.D., Department of Psychiatry, Washington University School of Medicine; H. Edenberg, Ph.D., Indiana University School of Medicine, S. Detera-Wadleigh, NIMH Intramural Research Program, and O. Stine, Johns Hopkins University School of Medicine.
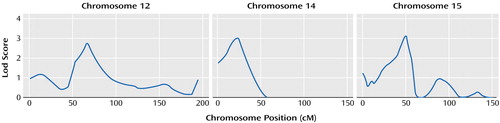
Figure 1. Three Chromosomes With the Strongest Evidence for Linkage With the Natural Log of Age at Onset of Mania in 86 Families Multiply Affected With Bipolar Disorder
1. Cardno AG, Marshall EJ, Coid B, Macdonald AM, Ribchester TR, Davies NJ, Venturi P, Jones LA, Lewis SW, Sham PC, Gottesman II, Farmer AE, McGuffin P, Reveley AM, Murray RM: Heritability estimates for psychotic disorders. Arch Gen Psychiatry 1999; 56:162–168Crossref, Medline, Google Scholar
2. Tsuang MT, Faraone SV: The genetic epidemiology of bipolar disorder, in Bipolar Disorders:100 Years After Manic-Depressive Insanity. Edited by Marneros A, Angst J. Zurich, Kluwer Academic, 2000, pp 231–242Google Scholar
3. Berrettini WH: Molecular linkage studies of bipolar disorders. Bipolar Disord 2001; 3:276–283Crossref, Medline, Google Scholar
4. Badner J, Gershon E: Meta-analysis of whole-genome linkage scans of bipolar disorder and schizophrenia. Mol Psychiatry 2002; 7:405–411Crossref, Medline, Google Scholar
5. Segurado R, Detera-Wadleigh SD, Levinson DF, Lewis CM, Gill M, Nurnberger JI Jr, Craddock N, DePaulo JR, Baron M, Gershon ES, Ekholm J, Cichon S, Turecki G, Claes S, Kelsoe JR, Schofield PR, Badenhop RF, Morissette J, Coon H, Blackwood D, McInnes LA, Foroud T, Edenberg HJ, Reich T, Rice JP, et al: Genome scan meta-analysis of schizophrenia and bipolar disorder, part III: bipolar disorder. Am J Hum Genet 2003; 73:49–62Crossref, Medline, Google Scholar
6. Spitzer RL, Endicott J, Robins E: Research Diagnostic Criteria (RDC) for a Selected Group of Functional Disorders, 3rd ed. New York, New York State Psychiatric Institute, Biometrics Research, 1978Google Scholar
7. Faraone SV, Blehar M, Pepple J, Moldin S, Norton J, Nurnberger JI, Malaspina D, Kaufmann CA, Reich T, Cloninger CR, DePaulo JR, Berg K, Gershon ES, Kirch DG, Tsuang MT: Diagnostic accuracy and confusability analyses: an application to the Diagnostic Interview for Genetic Studies. Psychol Med 1996; 26:401–410Crossref, Medline, Google Scholar
8. Smeraldi E, Negri F, Heimbuch RC, Kidd KK: Familial patterns and possible modes of inheritance of primary affective disorders. J Affect Disord 1981; 3:173–182Crossref, Medline, Google Scholar
9. Spence MA, Flodman PL, Sadovnick AD, Bailey-Wilson JE, Ameli H, Remick RA: Bipolar disorder: evidence for a major locus. Am J Med Genet 1995; 60:370–376Crossref, Medline, Google Scholar
10. Radhakrishna U, Senol S, Herken H, Gucuyener K, Gehrig C, Blouin JL, Akarsu NA, Antonarakis SE: An apparently dominant bipolar affective disorder (BPAD) locus on chromosome 20p11.2-q11.2 in a large Turkish pedigree. Eur J Hum Genet 2001; 9:39–44Crossref, Medline, Google Scholar
11. Pauls DL, Bailey JN, Carter AS, Allen CR, Egeland JA: Complex segregation analyses of old order Amish families ascertained through bipolar I individuals. Am J Med Genet 1995; 60:290–297Crossref, Medline, Google Scholar
12. Cox N, Reich T, Rice J, Elston R, Schober J, Keats B: Segregation and linkage analyses of bipolar and major depressive illnesses in multigenerational pedigrees. J Psychiatr Res 1989; 23:109–123Crossref, Medline, Google Scholar
13. Faraone SV, Glatt SJ, Tsuang MT: The genetics of pediatric-onset bipolar disorder. Biol Psychiatry 2003; 53:970–977Crossref, Medline, Google Scholar
14. Biederman J, Mick E, Faraone SV, Spencer T, Wilens TE, Wozniak J: Pediatric mania: a developmental subtype of bipolar disorder? Biol Psychiatry 2000; 48:458–466Crossref, Medline, Google Scholar
15. Sax KW, Strakowski SM, Keck PE Jr, McElroy SL, West SA, Bourne ML, Larson ER: Comparison of patients with early-, typical-, and late-onset affective psychosis. Am J Psychiatry 1997; 154:1299–1301Link, Google Scholar
16. Grigoroiu-Serbanescu M, Martinez M, Nothen MM, Grinberg M, Sima D, Propping P, Marinescu E, Hrestic M: Different familial transmission patterns in bipolar I disorder with onset before and after age 25. Am J Med Genet 2001; 105:765–773Crossref, Medline, Google Scholar
17. Faraone SV, Biederman J, Monuteaux MC: Attention deficit hyperactivity disorder with bipolar disorder in girls: further evidence for a familial subtype? J Affect Disord 2001; 64:19–26Crossref, Medline, Google Scholar
18. Faraone SV, Biederman J, Mennin D, Wozniak J, Spencer T: Attention deficit hyperactivity disorder with bipolar disorder: a familial subtype? J Am Acad Child Adolesc Psychiatry 1997; 36:1378–1387Crossref, Medline, Google Scholar
19. Biederman J, Faraone SV, Wozniak J, Monuteaux MC: Parsing the association between bipolar, conduct, and substance use disorders: a familial risk analysis. Biol Psychiatry 2000; 48:1037–1044Crossref, Medline, Google Scholar
20. Faraone SV, Biederman J, Mennin D, Russell R: Bipolar and antisocial disorders among relatives of ADHD children: parsing familial subtypes of illness. Am J Med Genet 1998; 81:108–116Crossref, Medline, Google Scholar
21. Omahony E, Corvin A, O’Connell R, Comerford C, Larsen B, Jones R, McCandless F, Kirov G, Cardno AG, Craddock N, Gill M: Sibling pairs with affective disorders: resemblance of demographic and clinical features. Psychol Med 2002; 32:55–61Crossref, Medline, Google Scholar
22. Visscher PM, Yazdi MH, Jackson AD, Schalling M, Lindblad K, Yuan QP, Porteous D, Muir WJ, Blackwood DH: Genetic survival analysis of age-at-onset of bipolar disorder: evidence for anticipation or cohort effect in families. Psychiatr Genet 2001; 11:129–137Crossref, Medline, Google Scholar
23. Genomic survey of bipolar illness in the NIMH genetics initiative pedigrees: a preliminary report. Am J Med Genet 1997; 74:227–237Crossref, Medline, Google Scholar
24. Stine OC, McMahon FJ, Chen L, Xu J, Meyers DA, MacKinnon DF, Simpson S, McInnis MG, Rice JP, Goate A, Reich T, Edenberg HJ, Foroud T, Nurnberger JI Jr, Detera-Wadleigh SD, Goldin LR, Guroff J, Gershon ES, Blehar MC, DePaulo JR: Initial genome screen for bipolar disorder in the NIMH genetics initiative pedigrees: chromosomes 2, 11, 13, 14, and X. Am J Med Genet 1997; 74:263–269Crossref, Medline, Google Scholar
25. Rice JP, Goate A, Williams JT, Bierut L, Dorr D, Wu W, Shears S, Gopalakrishnan G, Edenberg HJ, Foroud T, Nurnberger J Jr, Gershon ES, Detera-Wadleigh SD, Goldin LR, Guroff JJ, McMahon FJ, Simpson S, MacKinnon D, McInnis M, Stine OC, DePaulo JR, Blehar MC, Reich T: Initial genome scan of the NIMH genetics initiative bipolar pedigrees: chromosomes 1, 6, 8, 10, and 12. Am J Med Genet 1997; 74:247–253Crossref, Medline, Google Scholar
26. Edenberg HJ, Foroud T, Conneally PM, Sorbel JJ, Carr K, Crose C, Willig C, Zhao J, Miller M, Bowman E, Mayeda A, Rau NL, Smiley C, Rice JP, Goate A, Reich T, Stine OC, McMahon F, DePaulo JR, Meyers D, Detera-Wadleigh SD, Goldin LR, Gershon ES, Blehar MC, Nurnberger JI Jr: Initial genomic scan of the NIMH genetics initiative bipolar pedigrees: chromosomes 3, 5, 15, 16, 17, and 22. Am J Med Genet 1997; 74:238–246Crossref, Medline, Google Scholar
27. Nurnberger JI Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T (NIMH Genetics Initiative): Diagnostic Interview for Genetic Studies: rationale, unique features, and training. Arch Gen Psychiatry 1994; 51:849–859Crossref, Medline, Google Scholar
28. Maxwell ME: Manual for the FIGS (Family Interview for Genetics Studies). Bethesda, Md, National Institute of Mental Health, Clinical Neurogenetics Branch, Intramural Research Program, March 30, 1992Google Scholar
29. Foroud T, Castelluccio PF, Koller DL, Edenberg HJ, Miller M, Bowman E, Rau NL, Smiley C, Rice JP, Goate A, Armstrong C, Bierut LJ, Reich T, Detera-Wadleigh SD, Goldin LR, Badner JA, Guroff JJ, Gershon ES, McMahon FJ, Simpson S, MacKinnon D, McInnis M, Stine OC, DePaulo JR, Blehar MC, Nurnberger JI Jr: Suggestive evidence of a locus on chromosome 10p using the NIMH genetics initiative bipolar affective disorder pedigrees. Am J Med Genet 2000; 96:18–23Crossref, Medline, Google Scholar
30. Detera-Wadleigh SD, Badner JA, Yoshikawa T, Sanders AR, Goldin LR, Turner G, Rollins DY, Moses T, Guroff JJ, Kazuba D, Maxwell ME, Edenberg HJ, Foroud T, Lahiri D, Nurnberger JI Jr, Stine OC, McMahon F, Meyers DA, MacKinnon D, Simpson S, McInnis M, DePaulo JR, Rice J, Goate A, Gershon ES, et al: Initial genome scan of the NIMH genetics initiative bipolar pedigrees: chromosomes 4, 7, 9, 18, 19, 20, and 21q. Am J Med Genet 1997; 74:254–262Crossref, Medline, Google Scholar
31. Boehnke M: Allele frequency estimation from data on relatives. Am J Hum Genet 1991; 48:22–25Medline, Google Scholar
32. Lander E, Green P: Construction of multilocus genetic linkage maps in humans. Proc Natl Acad Sci USA 1987; 84:2363–2367Crossref, Medline, Google Scholar
33. Almasy L, Blangero J: Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet 1998; 62:1198–1211Crossref, Medline, Google Scholar
34. Blangero J, Williams J, Almasy L: Variance component methods for detecting complex trait loci. Adv Genet 2001; 42:151–181Crossref, Medline, Google Scholar
35. Kruglyak L, Daly MJ, Reeve-Daly MP, Lander ES: Parametric and nonparametric linkage analysis: a unified multipoint approach. Am J Hum Genet 1996; 58:1347–1363Medline, Google Scholar
36. Self SG, Liang KY: Asymptotic properties of maximum likelihood estimators and likelihood ratio tests under nonstandardized conditions. J Am Stat Assoc 1987; 82:605–610Crossref, Google Scholar
37. Lander E, Kruglyak L: Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat Genet 1995; 11:241–247Crossref, Medline, Google Scholar
38. Dick DM, Foroud T, Edenberg HJ, Miller M, Bowman E, Rau NL, DePaulo JR, McInnis M, Gershon E, McMahon F, Rice JP, Bierut LJ, Reich T, Nurnberger JI Jr: Apparent replication of suggestive linkage on chromosome 16 in the NIMH genetics initiative bipolar pedigrees. Am J Med Genet 2002; 114:407–412Crossref, Medline, Google Scholar
39. Bellivier F, Golmard JL, Henry C, Leboyer M, Schurhoff F: Admixture analysis of age at onset in bipolar I affective disorder. Arch Gen Psychiatry 2001; 58:510–512Crossref, Medline, Google Scholar
40. Bellivier F, Leroux M, Henry C, Rayah F, Rouillon F, Laplanche JL, Leboyer M: Serotonin transporter gene polymorphism influences age at onset in patients with bipolar affective disorder. Neurosci Lett 2002; 334:17–20Crossref, Medline, Google Scholar
41. Verheyen GR, Del-Favero J, Mendlewicz J, Lindblad K, Van Zand K, Aalbregtse M, Schalling M, Souery D, Van Broeckhoven C: Molecular interpretation of expanded RED products in bipolar disorder by CAG/CTG repeats located at chromosomes 17q and 18q. Neurobiol Dis 1999; 6:424–432Crossref, Medline, Google Scholar
42. Lindblad K, Nylander PO, Zander C, Yuan QP, Stahle L, Engstrom C, Balciuniene J, Pettersson U, Breschel T, McInnis M, Ross CA, Adolfsson R, Schalling M: Two commonly expanded CAG/CTG repeat loci: involvement in affective disorders? Mol Psychiatry 1998; 3:405–410Crossref, Medline, Google Scholar
43. Tsuang MT, Faraone SV: The Genetics of Mood Disorders. Baltimore, Johns Hopkins University Press, 1990Google Scholar
44. Rice J, Reich T, Andreasen NC, Endicott J, Van Eerdewegh M, Fishman R, Hirschfeld RM, Klerman GL: The familial transmission of bipolar illness. Arch Gen Psychiatry 1987; 44:441–447Crossref, Medline, Google Scholar
45. Pauls DL, Morton LA, Egeland JA: Risks of affective illness among first-degree relatives of bipolar I old-order Amish probands. Arch Gen Psychiatry 1992; 49:703–708Crossref, Medline, Google Scholar
46. Strober M, Morrell W, Burroughs J, Lampert C, Danforth H, Freeman R: A family study of bipolar I disorder in adolescence: early onset of symptoms linked to increased familial loading and lithium resistance. J Affect Disord 1988; 15:255–268Crossref, Medline, Google Scholar
47. Neuman R, Geller B, Rice J, Todd R: Increased prevalence and earlier onset of mood disorders among relatives of prepubertal versus adult probands. J Am Acad Child Adolesc Psychiatry 1997; 36:466–473Crossref, Medline, Google Scholar
48. Wozniak J, Biederman J, Kiely K, Ablon S, Faraone S, Mundy E, Mennin D: Mania-like symptoms suggestive of childhood onset bipolar disorder in clinically referred children. J Am Acad Child Adolesc Psychiatry 1995; 34:867–876Crossref, Medline, Google Scholar
49. Carlson GA, Bromet EJ, Sievers S: Phenomenology and outcome of subjects with early- and adult-onset psychotic mania. Am J Psychiatry 2000; 157:213–219Link, Google Scholar
50. Biederman J, Mick E, Bostic J, Prince J, Daly J, Wilens T, Spencer T, Garcia-Jetton J, Russell R, Wozniak J, Faraone S: The naturalistic course of pharmacologic treatment of children with manic-like symptoms: a systematic chart review. J Clin Psychiatry 1998; 59:628–637Crossref, Medline, Google Scholar
51. Faraone SV, Biederman J, Wozniak J, Mundy E, Mennin D, O’Donnell D: Is comorbidity with ADHD a marker for juvenile onset mania? J Am Acad Child Adolesc Psychiatry 1997; 36:1046–1055Crossref, Medline, Google Scholar
52. Schurhoff F, Bellivier F, Jouvent R, Mouren-Simeoni MC, Bouvard M, Allilaire JF, Leboyer M: Early and late onset bipolar disorders: two different forms of manic-depressive illness? J Affect Disord 2000; 58:215–221Crossref, Medline, Google Scholar
53. McElroy SL, Keck PE Jr, Pope HG Jr, Hudson JI, Faedda GL, Swann AC: Clinical and research implications of the diagnosis of dysphoric or mixed mania or hypomania. Am J Psychiatry 1992; 149:1633–1644Link, Google Scholar
54. Todd RD, Neuman R, Geller B, Fox LW, Hickok J: Genetic studies of affective disorders: should we be starting with childhood onset probands? J Am Acad Child Adolesc Psychiatry 1993; 32:1164–1171Crossref, Medline, Google Scholar



