Association Between Allelic Variation of Serotonin Transporter Function and Neuroticism in Anxious Cluster C Personality Disorders
Abstract
OBJECTIVE: Association between the low-activity variant of a polymorphism in the transcriptional control region of the serotonin transporter (5-HTTLPR) and neuroticism or harm avoidance was found in several but not all studies. The authors analyzed the influence of 5-HTTLPR variants on personality disorders. METHOD: Patients with personality disorders (N=320) and healthy volunteers (N=281) were studied with the Revised NEO Personality Inventory and the Tridimensional Personality Questionnaire. All were genotyped for 5-HTTLPR variants. RESULTS: No differences in 5-HTTLPR genotype distribution were detected between patients with cluster B and C personality disorders and comparison subjects. In contrast, among patients with a cluster C diagnosis, carriers of the low-activity short allele of the 5-HTTLPR exhibited higher neuroticism scores than noncarriers. CONCLUSIONS: These findings support the notion that there is no general association between the 5-HTTLPR and anxiety-related traits and that differential gene effects and/or gene-by-environment interactions are likely operative in distinct clinical subpopulations.
The human serotonin (5-HT) transporter (5-HTT), a critical regulator of serotonergic function and the initial target of antidepressant drugs, is encoded by a single-copy gene (SLC6A4) located on chromosome 17q12. 5-HTT gene transcription is modulated by a 44-bp length variation in its upstream regulatory region, termed short versus long 5-HTTLPR (1). Studies of allelic promoter activity in immortalized raphe cells (2), mRNA concentrations in the raphe complex of the human postmortem brain (3), platelet 5-HT uptake and content (4), 5-HT system responsivity elicited by pharmacological challenge tests with clomipramine and fenfluramine (5, 6), mood changes following tryptophan depletion (7), and single photon emission computed tomography of human brain 5-HTT (8) have confirmed that the 5-HTTLPR short variant restricts 5-HTT availability and ultimately 5-HT reuptake.
Allelic variation in 5-HTT function was found to account for approximately 8% of the inherited variance in anxiety- and depression-related personality traits in individuals as well as in sibships (1). In addition to studies of the low-activity short variant’s effect on personality traits, a role for 5-HTTLPR has been suggested in a variety of psychiatric diseases, including major depression and bipolar disorders (9, 10). Individuals with one or two copies of the low-activity short allele of 5-HTTLPR (long-short and short-short) exhibit greater neuronal activity of the amygdala in response to fearful stimuli when compared with individuals homozygous for the long allele, as assessed by blood-oxygen-level-dependent functional magnetic resonance imaging (11). Furthermore, the rates of major depression were found to be strongly influenced by the number of stressful life events in carriers of short alleles of the 5-HTTLPR but not in the individuals with the long-long genotype (12).
In a population- and family-based design, association between the 5-HTTLPR short allele and higher scores for neuroticism and harm avoidance was initially shown in a predominantly male group (1) and, subsequently, in an independent group of primarily female participants (4). Replications have been complicated by the use of small group sizes, heterogeneous subject populations, ethnic and sociocultural characteristics, and differing methods of personality assessment (4).
Based on the findings of previous studies suggesting an influence of allelic variation in 5-HT transporter function on anxiety-related traits, the a priori hypothesis of the present study was that there is a link between the personality traits of neuroticism and harm avoidance, anxious cluster C personality disorders, and the low-activity short allele of 5-HTTLPR. Therefore, we 1) tested for differences in 5-HTTLPR genotype frequencies between patients with personality disorders and healthy comparison subjects, 2) tested for differences in anxiety-related traits between patients with personality disorders and comparison subjects, and 3) examined whether personality differences are associated with the 5-HTTLPR genotype.
Method
A group of 320 unrelated patients (194 women and 126 men; mean age=36.9 years, SD=13.2) was compared to 281 unrelated healthy volunteers (210 women and 71 men; mean age=22.4 years, SD=5.8). All subjects were of European origin except for six comparison subjects who had at least one parent from outside of Europe. The study was approved by the Ethics Committee of the University of Wuerzburg, and written informed consent was obtained from all individuals after the procedures and aims of the study had been fully explained.
Personality disorders were assessed with the Structured Clinical Interview for DSM-IV Personality Disorders (13) and were allocated to cluster A, B, or C and operationalized as follows: cluster A (odd-eccentric) comprises paranoid, schizoid, and schizotypal personality disorders; cluster B (dramatic-emotional) encompasses antisocial, borderline, histrionic, and narcissistic personality disorders; cluster C (anxious-fearful) includes avoidant, dependent, and obsessive-compulsive personality disorders; and a category called personality disorders not otherwise specified.
The analysis of genetic contributions to personality and related disorders is conceptually and methodologically difficult. The documented heterogeneity of both genetic and environmental determinants has increasingly encouraged the pursuit of dimensional approaches to behavioral genetics (14), and the focus on gene variants with a significant impact on neurocircuit functionality associated with quantitative traits is a rational strategy. While quantitative genetics has focused on complex, quantitatively distributed traits and their origins in naturally occurring variations caused by multiple genetic and environmental factors, molecular genetics has begun to identify specific genes for quantitative traits, called quantitative trait loci (15). This perspective suggests that it may be less difficult to identify genes for psychopathology by searching for genes influencing personality and that complex traits are not attributable to single genes necessary or sufficient to cause a disorder. The concept of quantitative trait loci also implies that there may not be genes for psychiatric disorders, only genes for behavioral dimensions. Therefore, personality features were assessed with the Revised NEO Personality Inventory (16) and the Tridimensional Personality Questionnaire (17). These tests are based on a dimensional model of personality and support the quantitative trait loci approach.
DNA was extracted from whole blood (Qiagen, Hilden, Germany). The genotype of the 5-HTTLPR was determined by polymerase chain reaction amplification (1). Genotypic frequency for the patient group was long-long, N=103 (32.2%); long-short, N=162 (50.6%); short-short, N=55 (17.2%), long-long, N=101 (36.0%); long-short, N=140 (49.8%); and short-short, N=40 (14.2%) for the comparison subjects. Based on the assessment of the 5-HTTLPR short allele’s functional effect in vitro and in vivo, genotypes with one or two copies of the short alleles were combined (the short genotype group) and compared to the long genotype group (carriers of a long-long genotype) (1, 18).
Statistical analyses were carried out by using the SPSS 9.0.1. package (SPSS, Inc., Chicago). Association tests were performed by means of chi-square tests for the comparison of genotype frequencies between patients and comparison subjects and by analyses of variance (ANOVAs). The dependent variables of ANOVA were age- and gender-residualized z scores. Patients with only cluster A personality disorder (N=6) and patients with personality disorders on two or all three personality disorder clusters (N=60) were also excluded from subsequent analyses.
Results
First, no differences were detected in 5-HTTLPR genotype distributions between comparison subjects (N=281) and patients with cluster B (N=198, only cluster B), and cluster C (N=56, only cluster C) diagnoses (χ2=1.11, df=1, p=0.30, and χ2=1.74, df=1, p=0.20, respectively). There were also no differences in 5-HTTLPR genotypic frequencies between patients with cluster B and patients with cluster C diagnoses (χ2=0.42, df=1, p=0.52).
Second, a significant difference in age- and gender-residualized neuroticism scores between groups (F=10.57, df=2, 532, p=0.00005) was demonstrated by means of ANOVA. Post hoc Scheffé tests revealed that cluster C patients exhibited the highest neuroticism scores in relation to comparison subjects (p=0.00002) and to cluster B patients (p=0.001), whereas the comparison subjects did not differ significantly from cluster B patients (p=0.65). After Bonferroni correction for multiple testing, the results remained significant at the adjusted level of alpha=0.017.
Finally, in order to test whether the differences between the groups were influenced by 5-HTTLPR genotype, a two-way ANOVA was performed with diagnosis (comparison subjects versus cluster B subjects versus cluster C subjects) and 5-HTTLPR genotype group (long versus short) as independent variables. We observed significant main effects of group (F=3.97, df=2, 529, p<0.02) and of 5-HTTLPR genotype (F=6.45, df=1, 529, p<0.02). Moreover, there was a significant group-by-genotype interaction effect (F=5.06, df=2, 529, p=0.007) (Figure 1). When we accounted for multiple testing and employed a Bonferroni-adjusted level of significance of alpha=0.017, the group main effect almost reached significance. Post hoc analyses of variance with 5-HTTLPR genotype as an independent variable were performed separately for the three groups. Among patients with a cluster C diagnosis, carriers of the 5-HTTLPR short allele exhibited higher levels of neuroticism than noncarriers (F=10.31, df=1, 54, p=0.002), whereas there were no effects of genotype on neuroticism in comparison subjects and in cluster B patients (F=1.46, df=1, 196, p=0.23). Bonferroni correction was performed by dividing the adjusted level of alpha=0.017 by three to follow up on the interaction effect, resulting in an adjusted level of significance of alpha=0.006. At this level, the genotype effect among cluster C patients was still significant.
Analogous to the testing of the 5-HTTLPR genotype’s influence on neuroticism, ANOVA was repeated with age- and gender-residualized z scores for harm avoidance on the Tridimensional Personality Questionnaire, yielding comparable results: Cluster C patients with at least one copy of the 5-HTTLPR short allele exhibited higher levels of harm avoidance than cluster C patients without the short allele (F=7.07, df=1, 54, p=0.01), whereas no effects of genotype on harm avoidance were detected in comparison subjects and in cluster B subjects (F=3.52, df=1, 196, p=0.06).
It was not possible to incorporate the presence versus absence of an axis I diagnosis into the ANOVA because only five of the cluster C patients had no axis I diagnosis. When one considers the presence or absence of axis I diagnosis adjustment disorder (N=25) in the analyses, the 5-HTTLPR main effect was still significant (F=10.39, df=1, 52, p=0.002), even at an adjusted significance level of alpha=0.017, whereas the main effect for adjustment disorder (F=0.67, df=1, 52, p=0.42) and the interaction effect of 5-HTTLPR by adjustment disorder (F=0.68, df=1, 52, p=0.42) did not approach significance. There was still a significant main effect of 5-HTTLPR genotype on harm avoidance in cluster C patients (F=7.05, df=1, 52, p=0.01), but there was no main effect of adjustment disorder and no interaction effect of 5-HTTLPR by adjustment disorder (F=0.26, df=1, 52, p=0.61). The results again remained significant after correction for multiple testing.
Discussion
The present study failed to demonstrate a general association between allelic variation in 5-HTT function and anxiety-related traits in patients with personality disorders. Our major finding that just within a subgroup of patients with a cluster C diagnosis, carriers of the 5-HTTLPR short allele exhibited higher scores for neuroticism and harm avoidance than noncarriers further supports the increasingly accepted view that differential gene effects may be operative in distinct normal and clinical populations.
Fearful stimuli cause amygdala hyperresponsivity in healthy subjects with one or two low-activity 5-HTTLPR short alleles (9). The results of our study show a greater tendency to express anxiety-related traits in a subgroup with the low-activity 5-HTTLPR allele. In patients with cluster C personality disorder carrying low-activity 5-HTTLPR alleles, anxiety-provoking stimuli may lead to a further boost of genuine amygdala hyperexcitability, or physiological amygdala activity may lack the restrictive control by prefrontal cortical circuits caused by increased excitatory neurotransmission (19). The genetic cause for this limbic hyperexcitability—except for allelic variation in 5-HTT function—remains, however, elusive. Of note, genetic influences are not the only pathways that lead to individual differences in personality dimensions, behavior, and psychopathology. Complex traits like anxiety and depression are most likely to be generated by a complex interaction of environmental and experiential factors with a number of genes and their products, as documented extensively for the 5-HTT in both nonhuman primates and humans (10, 12, 18, 20).
Even pivotal regulatory proteins of neurocircuits, such as the 5-HTT, have only a modest impact, while noise from environmental and epigenetic mechanisms obstructs identification of relevant gene variants. Given the remarkable comorbidity between cluster C personality disorders, anxiety, and affective disorders (21) and the evidence for their modulation by common genetic factors (22), it is likely that among the different personality disorders predisposition to the cluster C spectrum is more strongly influenced than others by environmental and experiential factors, whose impact on the brain is under genetic control. In order to identify genetic mechanisms in the pathogenesis of personality disorders reliably, future studies will have to focus on the contribution of gene-by-environment interactions. Therefore, we conclude that additional factors, including environmental and experiential factors and ethnic diversity, as well as age and gender, interact with the effect of allelic variation of 5-HTT function on anxiety-related traits and personality disorders.
Received April 3, 2003; revision received Aug. 5, 2003; accepted Aug. 11, 2003. From the Department of Psychiatry and Psychotherapy, University of Wuerzburg. Address reprint requests to Dr. Jacob, Department of Psychiatry and Psychotherapy, University of Wuerzburg, Fuechsleinstrasse 15, 97080 Wuerzburg, Germany; [email protected] (e-mail).
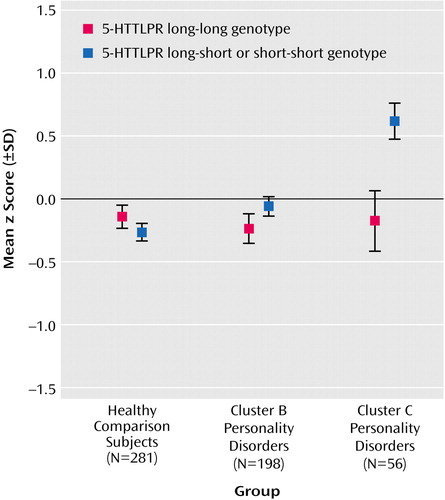
Figure 1. Age- and Gender-Residualized Scores on the Revised NEO Personality Inventory in Relation to Genotype for Serotonin Transporter Gene 5-HTTLPR in Patients With Personality Disorders and Comparison Subjects
1. Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL: Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 1996; 274:1527–1530Crossref, Medline, Google Scholar
2. Mortensen OV, Thomassen M, Larsen MB, Whittemore SR, Wiborg O: Functional analysis of a novel human serotonin transporter gene promoter in immortalized raphe cells. Brain Res Mol Brain Res 1999; 68:141–148Crossref, Medline, Google Scholar
3. Little KY, McLaughlin DP, Zhang L, Livermore CS, Dalack GW, McFinton PR, DelProposto ZS, Hill E, Cassin BJ, Watson SJ, Cook EH: Cocaine, ethanol, and genotype effects on human midbrain serotonin transporter binding sites and mRNA levels. Am J Psychiatry 1998; 155:207–213Link, Google Scholar
4. Greenberg BD, Li Q, Lucas FR, Hu S, Sirota LA, Benjamin J, Lesch KP, Hamer D, Murphy DL: Association between the serotonin transporter promoter polymorphism and personality traits in a primarily female population sample. Am J Med Genet 2000; 96:202–216Crossref, Medline, Google Scholar
5. Reist C, Mazzanti C, Vu R, Tran D, Goldman D: Serotonin transporter promoter polymorphism is associated with attenuated prolactin response to fenfluramine. Am J Med Genet 2001; 105:363–368Crossref, Medline, Google Scholar
6. Whale R, Quested DJ, Laver D, Harrison PJ, Cowen PJ: Serotonin transporter (5-HTT) promoter genotype may influence the prolactin response to clomipramine. Psychopharmacology (Berl) 2000; 150:120–122Crossref, Medline, Google Scholar
7. Neumeister A, Konstantinidis A, Stastny J, Schwarz MJ, Vitouch O, Willeit M, Praschak-Rieder N, Zach J, de Zwaan M, Bondy B, Ackenheil M, Kasper S: Association between serotonin transporter gene promoter polymorphism (5HTTLPR) and behavioral responses to tryptophan depletion in healthy women with and without family history of depression. Arch Gen Psychiatry 2002; 59:613–620Crossref, Medline, Google Scholar
8. Heinz A, Jones DW, Mazzanti C, Goldman D, Ragan P, Hommer D, Linnoila M, Weinberger DR: A relationship between serotonin transporter genotype and in vivo protein expression and alcohol neurotoxicity. Biol Psychiatry 1999; 47:643–649Crossref, Google Scholar
9. Collier DA, Stoeber G, Li T, Heils A, Catalano M, Di Bella D, Arranz MJ, Murray RM, Vallada HP, Bengel D, Müller CR, Roberts GW, Smeraldi E, Kirov G, Pak S, Lesch KP: A novel functional polymorphism within the promoter of the serotonin transporter gene: possible role in susceptibility to affective disorders. Mol Psychiatry 1996; 1:453–460Medline, Google Scholar
10. Lesch KP: Neuroticism and serotonin: a developmental genetic perspective, in Behavioral Genetics in the Postgenomic Era. Edited by Plomin R, DeFries J, Craig I, McGuffin P. Arlington, Va, American Psychiatric Press, 2003, pp 389–423Google Scholar
11. Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR: Serotonin transporter genetic variation and the response of the human amygdala. Science 2002; 297:400–403Crossref, Medline, Google Scholar
12. Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R: Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 2003; 301:386–389Crossref, Medline, Google Scholar
13. First MB, Spitzer RL, Gibbon M, Williams JBW, Benjamin L: The Structured Clinical Interview for DSM-IV Personality Disorders (SCID-II). New York, New York State Psychiatric Institute, Biometrics Research Unit, 1995Google Scholar
14. Plomin R, Owen MJ, McGuffin P: The genetic basis of complex human behaviors. Science 1994; 264:1733–1739Crossref, Medline, Google Scholar
15. Eley TC, Plomin R: Genetic analyses of emotionality. Curr Opin Neurobiol 1997; 7:279–284Crossref, Medline, Google Scholar
16. Costa PT, McCrae RR: Revised NEO Personality Inventory (NEO-PI-R) and NEO Five Factor Inventory (NEO-FFI): professional manual. Odessa, Fla, Psychological Assessment Resources, 1992Google Scholar
17. Cloninger CR, Przybeck TR, Svrakic DM: The Tridimensional Personality Questionnaire: U.S. normative data. Psychol Rep 1991; 69:1047–1057Crossref, Medline, Google Scholar
18. Bennett AJ, Lesch KP, Heils A, Long JC, Lorenz JG, Shoaf SE, Champoux M, Suomi SJ, Linnoila MV, Higley JD: Early experience and serotonin transporter gene variation interact to influence primate CNS function. Mol Psychiatry 2002; 7:118–122Crossref, Medline, Google Scholar
19. Davidson RJ: Anxiety and affective style: role of prefrontal cortex and amygdala. Biol Psychiatry 2002; 51:68–80Crossref, Medline, Google Scholar
20. Champoux M, Bennett A, Shannon C, Higley JD, Lesch KP, Suomi SJ: Serotonin transporter gene polymorphism, differential early rearing, and behavior in rhesus monkey neonates. Mol Psychiatry 2002; 7:1058–1063Crossref, Medline, Google Scholar
21. Iketani T, Kiriike N, Stein MB, Nagao K, Nagata T, Minamikawa N, Shidao A, Fukuhara H: Personality disorder comorbidity in panic disorder patients with or without current major depression. Depress Anxiety 2002; 15:176–182Crossref, Medline, Google Scholar
22. Kendler KS: Major depression and generalised anxiety disorder: same genes, (partly) different environments—revisited. Br J Psychiatry Suppl 1996; 30:68–75Medline, Google Scholar



