Anterior Cingulum Abnormalities in Male Patients With Schizophrenia Determined Through Diffusion Tensor Imaging
Abstract
OBJECTIVE: This study used diffusion tensor imaging to examine fractional anisotropy in the anterior cingulum and posterior cingulum bundles in patients with schizophrenia. METHOD: Twenty-one male patients and 20 healthy comparison men were studied. RESULTS: Reduced fractional anisotropy was seen for both sides of the anterior cingulum in the schizophrenia patients, who also exhibited less left-greater-than-right asymmetry in the anterior cingulum than was seen in the comparison subjects. CONCLUSIONS: The findings suggest structural disconnections in the anterior cingulum in patients with schizophrenia.
Disruption of a neural circuit has been hypothesized as a mechanism of schizophrenia. Disconnections between the cingulate gyrus and other regions may partly explain some of the symptoms and cognitive dysfunction in schizophrenia (1). Although the cingulate gyrus forms a single and continuous structure, it is structurally and functionally heterogeneous. The cingulate cortex has profound reciprocal connections with many other regions, and functional, anatomical, and histopathological studies have accumulated evidence that the connections between subregions of the cingulate cortex and other brain regions are disturbed in schizophrenia (2).
White matter fiber tracts are the basis for communications between regions. Diffusion tensor imaging provides access to tissue microstructure and architecture in each voxel (3). In white matter, diffusion changes usually mean the disturbance of white matter integrity.
In this study, a region of interest methodology with diffusion tensor imaging was applied to examine white matter integrity in different subregions of the cingulum bundle.
Method
Twenty-one male patients with schizophrenia (mean age=29.24, SD=5.58) who satisfied ICD-10 criteria were recruited. All patients were receiving atypical antipsychotic medication (risperidone, olanzapine, or clozapine). Twenty healthy male subjects (mean age=26.00, SD=5.99) were recruited by announcements in the local newspaper. All subjects were right-handed. None of the patients or comparison subjects had a history of head injury, organic mental disorder, neurological disorder, or alcohol or substance abuse. All subjects provided written informed consent after complete description of the study. The study was approved by the Medical Ethics Committee of Peking University.
All magnetic resonance imaging scans were performed on a 1.5-T MR scanner (GE Medical Systems, Milwaukee), which was equipped with shielded magnetic field gradients of up to 40 mT/m. A standard head coil was used for radiofrequency transmission and reception of the nuclear magnetic resonance signal. Head motion was minimized with restraining foam pads offered by the manufacturer. Diffusion weighted imaging was acquired with a single-shot echo-planar imaging sequence in alignment with the anterior-posterior commissure plane. The diffusion sensitizing gradients were applied along 25 non-collinear directions with b value=1000 seconds/mm2, together with an acquisition without diffusion weighting. A total of 12 slices were gathered, with the most caudal slice passing through the genu of corpus callosum. The acquisition parameters were as follows: TR=4000 msec, TE=85 msec, matrix=128×128, field of view=24×24 cm, number of excitations=3, slice thickness=3 mm without gap. Total scan time for diffusion tensor imaging sequence was 5 minutes 12 seconds.
The data were transferred to a dedicated computer workstation (Advantage Windows 4.0_02, GE Medical Systems) and postprocessed with Functool 2 (GE Medical Systems). Echo planar imaging distortion was corrected automatically. After diagonalization of diffusion tensor imaging, diffusion eigenvectors and corresponding eigenvalues (λ1, λ2, λ3) could be acquired. Fractional anisotropy (FA) was calculated according to the following formula:
The color-coded tensor images were acquired with Functool (GE Medical Systems). The assumption was based on the concept that the fiber orientation is in line with the eigenvector of maximum eigenvalue. Pixels were characterized as either anterior-posterior (green), left-right (red), or vertical (blue) depending on the direction of the eigenvector of the diffusion tensor. With the color-encoded tensor image, fibers in different orientations could then be easily identified, which helped localize the regions of interest more accurately.
A neuroradiologist, who was blind to subject grouping, was responsible for placing the bilateral anterior cingulum and posterior cingulum regions of interest. For the anterior cingulum and posterior cingulum, the regions of interest were placed on three contiguous slices just inferior to the most caudal slice among those that displayed the middle cingulum (Figure 1). Each region of interest contained 42 pixels. Fractional anisotropy on three slices of each side were averaged.
Repeated measure analysis of variance (ANOVA) was used to test separately for fractional anisotropy differences in the anterior and posterior cingulum, with group (schizophrenia patients, comparison subjects) as a between-subject factor and side (right, left) as a within-subject factor. The tests were considered significant when p values were less than 0.05 (two-tailed).
Results
Mean fractional anisotropy values in the patients and comparison subjects were 0.382 (SD=0.046) and 0.438 (SD=0.085), respectively, for the right anterior cingulum; 0.415 (SD=0.058) and 0.511 (SD=0.092) for the left anterior cingulum; 0.422 (SD=0.047) and 0.416 (SD=0.086) for the right posterior cingulum; and 0.420 (SD=0.049) and 0.394 (SD=0.075) for the left posterior cingulum. Repeated measure ANOVA in the anterior cingulum showed a significant group effect (F=12.943, df=1, 39, p=0.001), a significant side effect (F=47.230, df=1, 39, p<0.001), and a significant group-by-region effect (F=7.054, df=1, 39, p=0.011). Fractional anisotropy was significantly different between the right and left side in the anterior cingulum in both groups. Regarding the anterior cingulum, reduced asymmetry could be seen in the schizophrenia patients, and lower fractional anisotropy in the left anterior cingulum of patients might be the major factor causing lateralized differences.
Repeated measure ANOVA in the posterior cingulum showed no significant group effect (F=0.709, df=1, 39, p<0.41), no significant side effect (F=2.387, df=1, 39, p=0.13), and no group-by-region effect (F=1.753, df=1, 39, p<0.20).
Discussion
In this study, the anterior and posterior cingulum were investigated, and only the anterior cingulum showed significant abnormalities, which were characteristic of lower fractional anisotropy for both sides in male schizophrenia patients relative to healthy men. In addition, a left-greater-than-right asymmetry existed in both groups but was reduced in the patients. The impairment of the left anterior cingulum in schizophrenia might play a dominant role in causing the reduced asymmetry. However, these results were inconsistent with the findings in a recently published study (4).
The anterior cingulate cortex and posterior cingulate cortex have completely different connections and functions. Disconnections between the anterior cingulate cortex and other regions in schizophrenia have attracted much attention. A lesion study found that cingulotomy patients with small bilateral lesions in the anterior cingulate gyrus showed deficits of attention and executive dysfunction (2), which are prominent features of schizophrenia. Further evidence could also be found in functional and histopathological studies in these regions of schizophrenia (5). Coinciding with the findings of previous studies that used other methodologies, this study supplied direct in vivo evidence of structural disconnections between the anterior cingulate cortex and other brain regions in schizophrenia.
Our results showed reduced left-greater-than-right asymmetry in schizophrenia patients, and this is consistent with some studies that have found reduced anatomical and functional asymmetry in schizophrenia in several brain regions, including the frontal lobe, temporal lobe, and cingulate gyrus (6). Kubicki et al.’s study with diffusion tensor imaging found that uncinate fasciculus patients with schizophrenia lacked left-greater-than-right asymmetry present in normal subjects (7). A recent study reported reduced asymmetry of morphology in the anterior cingulate cortex in schizophrenia (8). These studies indicate asymmetry disturbance in schizophrenia.
However, there are some methodological limitations in this study. First, the partial volume effect was unavoidable for the curvy cingulum bundle, but thin slice thickness (3 mm) might partly counteract the effect. Second, thin slice thickness might lead to decreased signal-noise ratio, and we used the excitations with prolonged scan time to elevate the signal-noise ratio to a satisfying level.
Received April 22, 2003; revision received July 3, 2003; accepted July 10, 2003. From the Institute of Mental Health, Peking University; the Department of Radiology, People’s Hospital, Peking University; and the Department of Epidemiology & Biostatistics, Health Science Center, Peking University. Address reprint requests to Dai Zhang (Institute of Mental Health, Peking University, 51 Hua Yuan Bei Rd., Beijing 100083, China; [email protected] [e-mail]) or Nan Hong (Department of Radiology, People’s Hospital, Peking University, 11 South Xizhimen St., Beijing 100044, China; [email protected] [e-mail]). Supported by grant H010210180112 from Beijing Biomedical R & D Innovation Program and grant G1999064007 from the Major State Basic Research Development Program of the People’s Republic of China. The authors thank all subjects for their cooperation; GE Medical Systems for technical support; and Drs. Martha E. Shenton and Marek Kubicki for critical reading of this manuscript.
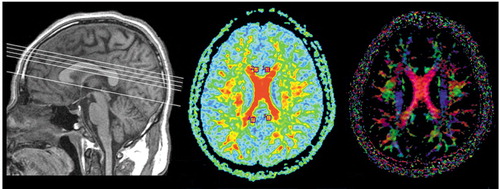
Figure 1. Localization of the Anterior Cingulum and Posterior Cingulum Through Magnetic Resonance Imaging, Fractional Anisotropy, and Diffusion Tensor Imaging in a Schizophrenia Patienta
aThe left image shows scan locations. The top line is the most caudal slice among those that displayed the middle cingulum, and the bottom line represents the anterior commissure-posterior commissure. The other three contiguous lines represent the slices on which the anterior cingulum and posterior cingulum regions of interest were placed. The middle image shows the regions of interest displayed in a median fractional anisotropy map. On the right is the corresponding color-coded tensor image.
1. Cohen RA, Kaplan RF, Moser DJ, Jenkins MA, Wilkinson H: Impairments of attention after cingulotomy. Neurology 1999; 53:819–824Crossref, Medline, Google Scholar
2. Benes FM: Neurobiological investigations in cingulate cortex of schizophrenic brain. Schizophr Bull 1993; 19:537–549Crossref, Medline, Google Scholar
3. Basser PJ, Pierpaoli C: Microstructural and physiological features tissues elucidated by quantitative diffusion tensor MRI. J Magn Reson 1996; 111:209–219Crossref, Google Scholar
4. Burns J, Job D, Bastin ME, Whalley H, Macgillivray T, Johnstone EC, Lawrie SM: Structural disconnectivity in schizophrenia: a diffusion tensor magnetic resonance imaging study. Br J Psychiatry 2003; 182:439–443Crossref, Medline, Google Scholar
5. Benes FM: Emerging principles of altered neural circuitry in schizophrenia. Brain Res Rev 2000; 31:251–269Crossref, Medline, Google Scholar
6. Berlim MT, Mattevi BS, Belmonte-de-Abreu P, Crow TJ: The etiology of schizophrenia and the origin of language: overview of a theory. Compr Psychiatry 2003; 44:7–14Crossref, Medline, Google Scholar
7. Kubicki M, Westin C-F, Maier SE, Frumin M, Nestor PG, Salisbury DF, Kikinis R, Jolesz FA, McCarley RW, Shenton ME: Uncinate fasciculus findings in schizophrenia: a magnetic resonance diffusion tensor imaging study. Am J Psychiatry 2002; 159:813–820Link, Google Scholar
8. Le Provost JB, Bartrees-Faz D, Paillere-Martinot ML, Artiges E, Pappata S, Recasens C, Perez-Gomez M, Bernardo M, Baeza I, Bayle F, Martinot JL: Paracingulate sulcus morphology in men with early-onset schizophrenia. Br J Psychiatry 2003; 182:228–232Crossref, Medline, Google Scholar



