Enhanced Dopamine Transporter Density in Psychotropic-Naive Patients With Obsessive-Compulsive Disorder Shown by [123I]β-CIT SPECT
Abstract
OBJECTIVE: The authors examine the functional anatomy of serotonergic and dopaminergic systems in obsessive-compulsive disorder in psychotropic-naive patients without comorbidity. METHOD: [123I]β-CIT binding patterns for dopamine and serotonin transporters in the brain were measured in 15 psychotropic-naive adult outpatients with OCD (no comorbidity) and in 15 pairwise-matched healthy subjects. Volumes of interest were constructed on magnetic resonance imaging scans and coregistered with single photon emission computed tomography scans. Binding ratios were compared, and possible correlations between binding patterns and obsessive-compulsive symptoms were assessed. RESULTS: There were significant differences between patients and healthy subjects in the [123I]β-CIT binding pattern for dopamine transporter in the left caudate and left putamen. Patients had higher binding ratios than healthy subjects. No differences were found in the less specific [123I]β-CIT binding pattern for serotonin transporters in the selected volumes of interest. Hemispheric within-group comparisons revealed no asymmetry effects. CONCLUSIONS: The results of our study provide direct evidence for an involvement of the dopaminergic system in the pathophysiology of OCD.
Obsessive-compulsive disorder (OCD) is characterized by recurrent, persistent, and intrusive thoughts or images that cause anxiety or distress (obsessions) and repetitive behaviors or mental acts aimed at reducing this distress or anxiety (compulsions). Patients recognize that these obsessions and compulsions are unreasonable and products of their own mind. Once considered rare, recent epidemiological data suggest prevalence rates of OCD from 1.5% to 3% (1, 2). In general, OCD symptoms can best be managed with selective serotonin reuptake inhibitors (SSRIs) or behavioral therapy. The selective response of OCD patients to SSRIs has led to the hypothesis that changes in the central serotonergic systems may be the mechanism by which these compounds exert their effect (3). Direct evidence that serotonergic perturbations are implicated in the neurobiology of OCD, however, is sparse. Serotonergic pharmacological challenges and CSF metabolite studies have yielded data that are too inconsistent—or too open to different interpretations—to serve as a valid basis for dissecting the neurobiology of OCD (3–5). Growing evidence that atypical antipsychotics, such as risperidone and quetiapine, may augment the response to SSRIs in patients with refractory OCD and those with comorbid tics, as well as studies showing an exacerbation of symptoms after administration of a dopamine agonist, would seem to point to an increase in dopaminergic system activity in OCD (6, 7). The role of dopamine in stereotypic behaviors in animal models and the preclinical evidence of important interactions between serotonergic and dopaminergic systems further strengthen the putative role of dopamine in OCD (8, 9). Studies examining the concentration of the dopamine metabolite homovanillic acid and the dopaminergic regulation of the hypothalamo-pituitary-adrenal axis lend further support to a possible role of dopamine in the pathophysiology of OCD (10, 11). Neuroimaging studies have been very influential in shaping neurobiological models of OCD. Converging data have implicated a network of brain regions, including the orbitofrontal cortex, striatum, and thalamus, in the pathophysiology of OCD. Most regions of the putatively involved network in OCD are densely innervated by serotonergic or dopaminergic neurons (12). However, despite the advent of several ligands for dopaminergic and serotonergic binding sites suitable for positron emission tomography (PET) or single photon emission computed tomography (SPECT), the functional anatomy of these neurotransmitter systems in OCD has scarcely been investigated. A commonly used ligand is 123-I-labeled 2-β-carbomethoxy-3-β-(4-iodophenyl)-tropane ([123I]β-CIT), a SPECT ligand enabling both serotonin (5-HT) and dopamine transporter visualization in the brain. [123I]β-CIT has already been used extensively to investigate several psychiatric and neuropsychiatric disorders (13). Binding of [123I]β-CIT in the striatal region has been shown to reflect mainly binding to dopamine transporter. Binding to 5-HT transporter occurs predominantly in the thalamus, midbrain, and pons, but the binding in these regions might also reflect densities of other monoamine transporters to an unknown proportion (14, 15). Differences in [123I]β-CIT binding characteristics for dopamine and 5-HT transporters also make temporal separation of the transporter occupancy possible (16, 17). Because of these characteristics, both dopamine and 5-HT transporter densities can be visualized in the same subject after a single administration of the ligand. The present study sought to examine possible differences in [123I]β-CIT binding patterns between patients with OCD and healthy comparison subjects pairwise matched by age, sex, and handedness. In order to further reduce the number of potential confounders, only OCD patients who were psychotropic naive and had no comorbid diagnoses or history of any other major psychopathology were included. We hypothesized that as a result of a putative increase in dopaminergic system activity in OCD, the binding patterns of [123I]β-CIT in OCD would reflect higher dopamine transporter density.
Method
Subjects
Psychotropic-naive patients with OCD and no comorbid diagnoses were recruited from 375 patients with OCD as their primary diagnosis who had been referred to the anxiety research unit of the Department of Psychiatry at the University Medical Center of Utrecht from 1997 to 2001. Most patients came from direct physician referrals. Healthy subjects were enrolled through advertisements in flyers and newspapers or were obtained from an existing database. Only subjects without a lifetime history of psychosis, substance abuse, recurrent major depression, bipolar disorder, eating disorders, other anxiety disorders, tics, or stuttering were included. Furthermore, all subjects had no first-degree relative with a history of a major DSM-IV axis I disorder (except for OCD in patients) or tics. All subjects had no lifetime history of illnesses with possible central nervous system sequelae and were in good physical health, as confirmed by physical and laboratory examinations. Subjects consumed less than six cups of coffee and four units of alcohol a day and smoked less than six cigarettes a day. Screening for current and prior adult psychopathology was done by administering the Mini-International Neuropsychiatric Interview (18). Diagnoses were confirmed by an experienced clinician (H.J.v.M., N.J.v.d.W.). Handedness was determined by administering the Edinburgh Handedness Scale (19). Patients had to have a minimum score of 16 on the Yale-Brown Obsessive Compulsive Scale and a maximum score of 13 on the Hamilton Depression Rating Scale (20, 22). Subjects underwent imaging procedures within 2 weeks after the screening examination. Patients had not received any form of psychotherapy, especially cognitive behavior therapy, in the 3 months preceding the study. The protocol was approved by the ethical committee of the University Medical Center of Utrecht. After complete description of the study to the subjects, written informed consent was obtained.
Image Acquisition and Analysis
On the first day of SPECT scanning, subjects received an intravenous injection of approximately 150 MBq of [123I]β-CIT (MAP Medical Technologies, Finland, radionuclidic purity [I125/I123] of at least 9.5×10–3 at calibration time and a radiochemical purity of at least 95%). We used a Picker Prism 3000 triple-headed gamma camera with ultra-high resolution fan beam collimators and a full-width at half-maximum of approximately 12 mm. Four hours after the injection the first scan was done to assess binding in the 5-HT transporter-rich regions. Between 22 and 24 hours after the injection, the second scan was performed to measure binding to dopamine transporter (16). Subjects refrained from coffee and nicotine in the 6–10 hours preceding each SPECT scan. Immediately after the first scan, subjects received 20 mg of paroxetine to displace the radiolabeled β-CIT from 5-HT transporters and enable more precise determination of binding to dopamine transporter (16). Several studies have already demonstrated that serotonin transporter occupation is virtually maximal at modest oral dosages (i.e., 10 mg) of paroxetine and other potent 5-HT reuptake blockers (14, 23). We chose the 20-mg paroxetine dose to control for possible differences in metabolism between subjects. Paroxetine was well tolerated by all subjects. During scanning, subjects were in supine position with eyes and ears open and with their head fixated in a head holder. We ensured that patients stayed awake and did not move. For an accurate determination of each subject’s volumes of interest, all subjects also underwent structural magnetic resonance imaging (MRI) (three-dimensional fast-field echo; TE/TR=4.6/30 msec; flip angle=30°; field of view=256×256 mm; matrix=128×128 by 130 mm; slice thickness=2 mm) 2 hours before the injection of [123I]β-CIT. MRIs were reoriented to the standardized coordinate system of the Montreal Standard brain (24). Volumes of interest were delineated manually on the reoriented MRI scans by a researcher (J.A.H.) blind to subject identity and diagnosis who used the Display software from Brain Imaging Center of the Montreal Neuroimaging Institute (25). Because previous studies have suggested possible hemispheric asymmetries of dopamine transporter density in healthy subjects and loss of this asymmetry in specific psychiatric populations, we decided upon examination of bilateral volumes of interest (26, 27). Volumes of interest for the 5-HT transporter-rich regions included the left and right thalamus, the midbrain, and the pons; for the dopamine transporter, volumes of interest included the left and right caudate nucleus and the left and right putamen. The cerebellum was used as a reference region, representing nonspecific binding of [123I]β-CIT.
To allow for exact coregistration of MRI and SPECT scans, fiducial markers were used. Fiducial markers were cone-shaped with cross-shaped feet and placed on the nose bridge and preauricular above the mandibular joints. The position of each marker was indicated with four dots on subjects’ skin to allow for repositioning of markers immediately before the SPECT scans. Vitamin A and Co57 were used as contrast agents for the MRI and SPECT scans, respectively. The energy peak for [123I]β-CIT was set at 160 keV with a window of 20% and at a peak of 120 keV with a window of 15% for Co57. After standard processing, brain SPECT images were resliced to isotropic voxels with dimensions of 2 mm and further treated as three-dimensional volumes to coregister within the three-dimensional orientation of the MRIs. Coregistration was performed semiautomatically based on the position of the fiducial markers, using the Register multimodality software package and additional software developed at the Brain Imaging Center of the Montreal Neurological Institute (25). The researcher performing the coregistration (N.J.v.d.W.) was blind to subject identity and diagnosis.
For each separate volume of interest, the ratio of the specific binding of [123I]β-CIT was calculated according to methods used in previously published [123I]β-CIT studies: the average radioactivity count per voxel per volume of interest minus the average radioactivity count per voxel in the cerebellum/average radioactivity count per voxel in the cerebellum.
Statistical Analyses
Age was compared by using Student’s t tests. The intrarater and interrater quality for the volumes of interest procedure was assessed by calculating intraclass correlations according to the method used by Bartko and Carpenter (28). The specific [123I]β-CIT binding ratios for each volume of interest were compared by using Mann-Whitney U tests. Within-group comparisons of hemispheric binding ratios in bilateral volumes of interest were performed by using Mann-Whitney U tests. Spearman rank correlations were calculated to assess correlations between specific binding ratios and Yale-Brown Obsessive Compulsive Scale total and subscale scores. Two-tailed significances are reported throughout.
Results
Of the 375 patients with OCD as their primary diagnosis referred to our specialized unit from 1997 to 2001, 104 had never received treatment with an SSRI (29). Unfamiliarity with OCD treatment guidelines as well as several patient-related factors may underlie this lack of adequate treatment. Fifty patients were psychotropic naive. Eighteen psychotropic-naive patients fulfilled the inclusion criteria and finally 15 patients participated. Healthy subjects were mainly recruited from the existing database. Six potential comparison subjects refused participation. All subjects completed the study. Patients and comparison subjects were perfectly matched for gender and did not differ significantly in age and handedness. Demographic and clinical characteristics are shown in Table 1. Almost all subjects were nonsmokers. Symptoms of the patient group were heterogeneous: five patients had predominantly obsessions of contamination and compulsions of washing, six had obsessions of doubt and compulsions of checking, two had predominantly aggressive obsessions and compulsions of counting, and two had mixed symptoms. Most patients had a juvenile onset (before the age of 18) of OCD. Although most patients had some aspects of depressive symptoms, as reflected in the average Hamilton depression scale score, none fulfilled criteria for a depressive episode or dysthymia or any other comorbid disorder at screening. Four patients had a prior single DSM-IV depressive episode not otherwise specified (two with dysthymic and two with more depressive features). One patient had a history of two DSM-IV depressive episodes not otherwise specified, one with dysthymic and one with more depressive features. One patient had received a form of supportive psychotherapy for his depressive symptoms.
Six patients had never received any form of treatment for their OCD. Six others had received a form of supportive or psychoanalytic therapy. Three patients had received a form of cognitive behavior therapy for a short period of time and without any noticeable effect. In these patients the cognitive behavior therapy was stopped at least 18 months before scanning.
In one patient the first SPECT scan (at 4 hours after injection) could not be reliably coregistered to the MRI because of motion artifacts, so the final analysis for the binding in the 5-HT transporter-rich regions involved 14 patients and 14 pairwise-matched healthy subjects. The intraclass correlation coefficients for the intrarater and interrater reliability of the volume of interest procedure were between 0.89 and 0.98 (mean=0.94, SD=0.04) and between 0.86 and 0.99 (mean=0.95, SD=0.05), respectively.
Mann-Whitney U tests revealed a significantly higher average binding ratio to the dopamine transporter in the left caudate and in the left putamen of patients with OCD relative to the matched healthy subjects (Figure 1, Table 2). There were no significant differences in average binding ratios to dopamine transporter in the right caudate and right putamen. Patients with juvenile onset and patients with adult onset had similar average binding ratios to dopamine transporter. There were also no significant differences in average binding ratios between the different subtypes of OCD. Hemispheric within-group comparisons for patients and healthy subjects revealed no significant asymmetry effects. No significant correlations were found between binding ratios to dopamine transporter and duration of illness or scores on the Yale-Brown Obsessive Compulsive Scale (total or subscales).
No significant differences in binding patterns were found between patients with OCD and healthy subjects in the 5-HT transporter-rich regions, i.e., the left and right thalamus, midbrain, and pons (Table 2). Hemispheric within-group comparisons revealed no asymmetry effects. No significant correlations were found for binding ratios in the thalamus, midbrain, and pons with duration of illness or scores on the Yale-Brown Obsessive Compulsive Scale (total or subscales).
Discussion
We found significantly higher [123I]β-CIT binding ratios, an index of dopamine transporter density, in the left basal ganglia of psychotropic-naive OCD patients with no comorbid diagnoses relative to healthy comparison subjects pairwise matched by age, sex, and handedness. No abnormalities in binding ratios to the 5-HT transporter-rich regions in the midbrain, thalamus, or pons were found.
To the best of our knowledge, this is the first study examining central 5-HT and dopamine transporter densities in a carefully selected group of psychotropic-naive patients with OCD. Of interest is that in a recent [123I]β-CIT SPECT study of Tourette’s syndrome, a disorder that is purported to have also basal ganglia abnormalities, no abnormalities in 5-HT or dopamine transporter densities were found in the basal ganglia, midbrain, or thalamus (30). Results obtained from functional neuroimaging studies of OCD that used other methodologies most consistently show abnormalities in the right caudate and right orbitofrontal cortex that normalize after treatment (31). Although this seems suggestive of some form of lateralized functional abnormality in OCD, this is not postulated in the prevailing neurobiological model of OCD.
We did not find hemispheric asymmetries in dopamine or 5-HT transporter binding ratios in any of the bilateral brain structures of OCD patients or healthy comparison subjects. The results of previous studies examining hemispheric asymmetry of dopamine transporter density in healthy subjects and neuropsychiatric conditions are inconsistent with these findings. Differences in radioactive ligands, neuroimaging techniques, and the number of subjects may account for these discrepancies. In a small PET study examining dopamine transporter density in patients with schizophrenia and healthy comparison subjects that used a radiolabeled form of 2-beta-carbomethoxy-3-beta-(-4-fluorophenyl)tropane ([18F]-CFT), hemispheric asymmetry was only found in the caudate of healthy comparison subjects (26). In contrast, a SPECT study with a radiolabeled cocaine analog (99Tc TRODAT) did not reveal hemispheric asymmetry in binding ratios to the dopamine transporter in the caudate and putamen of healthy volunteers (32). However, in a very large [123I]β-CIT SPECT study that examined age-related decline in dopamine transporter density in 126 healthy subjects, higher binding ratios in the left caudate and putamen were found (27). Although standard templates were used instead of volumes of interest defined on coregistered MRIs, these findings suggest that our study group may have been too small to detect hemispheric asymmetries within groups.
The data from our present study in psychotropic-naive patients clearly suggest a dopamine transporter role in the pathophysiology of OCD. Theoretically, the higher dopamine transporter density in the left basal ganglia of psychotropic-naive OCD patients, as measured by the [123I]β-CIT binding ratios, may be either due to a primary abnormality at the level of the transporter or secondary to other abnormalities. Higher transporter density may result from a higher homeostatic tone of the dopaminergic system, with lower densities of D1 and D2 receptors (33, 34). The possible role of higher dopaminergic activity in the left basal ganglia in the pathophysiology of OCD still needs to be elucidated. Both dopamine (through D1 and D2 receptors) and serotonin (e.g., through 5-HT2 receptors) are known to have a modulatory influence on the activity of excitatory (i.e., glutamate) and inhibitory (i.e., GABA) neurotransmitters in the basal ganglia and their cortico-thalamo-limbic connections. Furthermore, both the serotonergic and dopaminergic systems are known to modulate each other’s activity in parts of the fronto-thalamo-basal ganglia circuitry supposedly involved in OCD (35–37). Data on the exact nature of these interactions are still inconclusive. Finally, on the basis of the results of the present study, it is not possible to dissect whether dopaminergic abnormalities are causal or epiphenomenal to OCD.
Our study has several strong points. Patients and healthy subjects were pairwise matched, and the patient group consisted of a population with no comorbidity (including tics) that had never been exposed to psychotropic drugs or, for the most part, psychotherapy. Furthermore, SPECT data were analyzed by using coregistered MRI, allowing for more precise determination of the volumes of interest.
There are also some possible limitations to this study. The patient group was heterogeneous for OCD symptoms, reducing the chance of finding abnormalities related to a particular subtype of OCD (38). We did not match female subjects for the stage of menstrual cycle, but the stage of menstrual cycle was noted at time of scanning. Four female patients were scanned in the first 2 weeks of their menstrual cycle, the remaining four (two patients and two healthy subjects) in the last 2 weeks. Although we included several important parts of the putative disturbed fronto-thalamo-cortical circuitry in OCD in our volumes of interest, other areas could not be investigated in this study. Considering the dispersion of the data, the power of our study may have been too small to detect a bilateral increase in dopamine binding potential in the basal ganglia. Such a bilateral increase in dopamine binding potential could theoretically result from differences in the frequency of the dopamine transporter SLC6A3 genotype, associated with dopamine transporter availability in previous studies (39).
We found no abnormalities in binding ratios for the 5-HT transporter-rich regions of psychotropic-naive patients with OCD. However, [123I]β-CIT binding in these regions probably also reflects availability of other monoamine transporters to an unknown proportion. The time point for visualization may have further limited the possibility of finding abnormalities at the level of the 5-HT transporter, as was illustrated in the study by Willeit et al. (40) in seasonal affective disorder. In the latter study the 5-HT transporter was visualized at 4 hours and 24 hours after injection of the ligand, when a pseudoequilibrium state is reached. Differences were found only in the SPECT acquisitions 24 hours after the injection. In the present study, a 5-HT transporter inhibitor was administered after the first scan in order to displace [123I]β-CIT from the 5-HT transporter. Hence, the 5-HT transporter could not be visualized 24 hours after injection.
Finally, it should be mentioned that although SPECT is easier to use, has a higher safety index, and is less expensive than PET, it has also relative disadvantages like the poorer anatomical resolution and the use of semiquantitative techniques.
Notwithstanding the possible limitations, our data clearly indicate a role for dopamine in the pathophysiology of OCD. This finding needs to be replicated and should be further explored in studies examining the effect of pharmacotherapy and psychotherapy on both serotonergic and dopaminergic transporter densities in OCD and in studies further dissecting the possible involvement of neurotransmitter systems in this disorder.
 |
 |
Presented in part at the 48th annual meeting of the Society of Nuclear Medicine, Toronto, June 23–27, 2001. Received Nov. 4, 2003; revision received March 2, 2004; accepted March 17, 2004. From the Departments of Psychiatry and Nuclear Medicine, Rudolf Magnus Institute for Neuroscience, University Medical Center Utrecht, Utrecht, the Netherlands. Address correspondence and reprint requests to Dr. van der Wee, Department of Psychiatry, Leiden University Medical Center, B1-P, P.O. Box 9600, 2300 RC, Leiden, the Netherlands; [email protected] (e-mail). The authors thank Alice van Dongen, Clinical Research Assistant at the Department of Nuclear Medicine, for her assistance and ideas on this study.
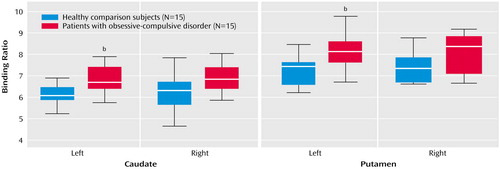
Figure 1. Specific Binding Ratios of [123I]β-CIT to Dopamine Transporter in the Caudate and Putamen of Psychotropic-Naive OCD Patients and Healthy Comparison Subjects Pairwise Matched by Age, Gender, and Handednessa
aHorizontal lines within boxes indicate median, boxes represent 75% confidence intervals, and limit lines depict the range.
bSignificant between-group difference (p<0.00625, corrected for multiple comparisons).
1. Regier DA, Narrow WE, Rae DS: The epidemiology of anxiety disorders: the Epidemiologic Catchment Area (ECA) experience. J Psychiatr Res 1990; 24(suppl 2):3–14Google Scholar
2. Stein MB, Forde DR, Anderson G, Walker JR: Obsessive-compulsive disorder in the community: an epidemiologic survey with clinical reappraisal. Am J Psychiatry 1997; 154:1120–1126Link, Google Scholar
3. Baumgarten HG, Grozdanovic Z: Role of serotonin in obsessive-compulsive disorder. Br J Psychiatry Suppl 1998; 35:13–20Medline, Google Scholar
4. Pian KL, Westenberg HG, van Megen HJ, den Boer JA: Sumatriptan (5-HT1D receptor agonist) does not exacerbate symptoms in obsessive compulsive disorder. Psychopharmacology (Berl) 1998; 140:365–370Crossref, Medline, Google Scholar
5. Khanna S, John JP, Reddy LP: Neuroendocrine and behavioral responses to mCPP in obsessive-compulsive disorder. Psychoneuroendocrinology 2001; 26:209–223Crossref, Medline, Google Scholar
6. McDougle CJ, Epperson CN, Pelton GH, Wasylink S, Price LH: A double-blind, placebo-controlled study of risperidone addition in serotonin reuptake inhibitor-refractory obsessive-compulsive disorder. Arch Gen Psychiatry 2000; 57:794–801Crossref, Medline, Google Scholar
7. Denys D, van Megen H, Westenberg H: Quetiapine addition to serotonin reuptake inhibitor treatment in patients with treatment-refractory obsessive-compulsive disorder: an open-label study. J Clin Psychiatry 2002; 63:700–703Crossref, Medline, Google Scholar
8. Tiihonen J, Kuoppamaki M, Nagren K, Bergman J, Eronen E, Syvalahti E, Hietala J: Serotonergic modulation of striatal D2 dopamine receptor binding in humans measured with positron emission tomography. Psychopharmacology (Berl) 1996; 126:277–280Crossref, Medline, Google Scholar
9. McGrath MJ, Campbell KM, Burton FH: The role of cognitive and affective processing in a transgenic mouse model of cortical-limbic neuropotentiated compulsive behavior. Behav Neurosci 1999; 113:1249–1256Crossref, Medline, Google Scholar
10. Swedo SE, Leonard HL, Kruesi MJ, Rettew DC, Listwak SJ, Berrettini W, Stipetic M, Hamburger S, Gold PW, Potter WZ: Cerebrospinal fluid neurochemistry in children and adolescents with obsessive-compulsive disorder. Arch Gen Psychiatry 1992; 49:29–36Crossref, Medline, Google Scholar
11. Brambilla F, Bellodi L, Perna G, Arancio C, Bertani A: Dopamine function in obsessive-compulsive disorder: growth hormone response to apomorphine stimulation. Biol Psychiatry 1997; 42:889–897Crossref, Medline, Google Scholar
12. Insel TR: Toward a neuroanatomy of obsessive-compulsive disorder. Arch Gen Psychiatry 1992; 49:739–744Crossref, Medline, Google Scholar
13. Kasper S, Tauscher J, Willeit M, Stamenkovic M, Neumeister A, Kufferle B, Barnas C, Stastny J, Praschak-Rieder N, Pezawas L, de Zwaan M, Quiner S, Pirker W, Asenbaum S, Podreka I, Brucke T: Receptor and transporter imaging studies in schizophrenia, depression, bulimia and Tourette’s disorder—implications for psychopharmacology. World J Biol Psychiatry 2002; 3:133–146Crossref, Medline, Google Scholar
14. Pirker W, Asenbaum S, Kasper S, Walter H, Angelberger P, Koch G, Pozzera A, Deecke L, Podreka I, Brucke T: beta-CIT SPECT demonstrates blockade of 5HT-uptake sites by citalopram in the human brain in vivo. J Neural Transm Gen Sect 1995; 100:247–256Crossref, Medline, Google Scholar
15. Laruelle M, Baldwin RM, Malison RT, Zea-Ponce Y, Zoghbi SS, al Tikriti MS, Sybirska EH, Zimmermann RC, Wisniewski G, Neumeyer JL: SPECT imaging of dopamine and serotonin transporters with [123I]beta-CIT: pharmacological characterization of brain uptake in nonhuman primates. Synapse 1993; 13:295–309Crossref, Medline, Google Scholar
16. Kuikka JT, Tiihonen J, Bergstrom KA, Karhu J, Hartikainen P, Viinamaki H, Lansimies E, Lehtonen J, Hakola P: Imaging of serotonin and dopamine transporters in the living human brain. Eur J Nucl Med 1995; 22:346–350Crossref, Medline, Google Scholar
17. Brucke T, Kornhuber J, Angelberger P, Asenbaum S, Frassine H, Podreka I: SPECT imaging of dopamine and serotonin transporters with [123I]beta-CIT: binding kinetics in the human brain. J Neural Transm Gen Sect 1993; 94:137–146Crossref, Medline, Google Scholar
18. Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC: The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998; 59(suppl 20):22–33Google Scholar
19. Oldfield RC: The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia 1971; 9:97–113Crossref, Medline, Google Scholar
20. Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Heninger GR, Charney DS: The Yale-Brown Obsessive Compulsive Scale, I: development, use, and reliability. Arch Gen Psychiatry 1989; 46:1006–1011Crossref, Medline, Google Scholar
21. Goodman WK, Price LH, Rasmussen SA, Mazure C, Delgado P, Heninger GR, Charney DS: The Yale-Brown Obsessive Compulsive Scale, II: validity. Arch Gen Psychiatry 1989; 46:1012–1016Crossref, Medline, Google Scholar
22. Hamilton M: Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol 1967; 6:278–296Crossref, Medline, Google Scholar
23. Meyer JH, Wilson AA, Ginovart N, Goulding V, Hussey D, Hood K, Houle S: Occupancy of serotonin transporters by paroxetine and citalopram during treatment of depression: a [11C]DASB PET imaging study. Am J Psychiatry 2001; 158:1843–1849Link, Google Scholar
24. Collins DL, Neelin P, Peters TM, Evans AC: Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr 1994; 18:192–205Crossref, Medline, Google Scholar
25. MacDonald JD: Register, McGill University. Quebec, Montreal Neurological Institute, McConnell Brian Imaging Centre, 1993Google Scholar
26. Laakso A, Vilkman H, Alakare B, Haaparanta M, Bergman J, Solin O, Peurasaari J, Räkköläinen V, Syvälahti E, Hietala J: Striatal dopamine transporter binding in neuroleptic-naive patients with schizophrenia studied with positron emission tomography. Am J Psychiatry 2000; 157:269–271Link, Google Scholar
27. van Dyck CH, Seibyl JP, Malison RT, Laruelle M, Zoghbi SS, Baldwin RM, Innis RB: Age-related decline in dopamine transporters: analysis of striatal subregions, nonlinear effects, and hemispheric asymmetries. Am J Geriatr Psychiatry 2002; 10:36–43Crossref, Medline, Google Scholar
28. Bartko JJ, Carpenter WT Jr: On the methods and theory of reliability. J Nerv Ment Dis 1976; 163:307–317Crossref, Medline, Google Scholar
29. Denys D, van Megen H, Westenberg H: The adequacy of pharmacotherapy in outpatients with obsessive-compulsive disorder. Int Clin Psychopharmacol 2002; 17:109–114Crossref, Medline, Google Scholar
30. Heinz A, Knable MB, Wolf SS, Jones DW, Gorey JG, Hyde TM, Weinberger DR: Tourette’s syndrome: [I-123]beta-CIT SPECT correlates of vocal tic severity. Neurology 1998; 51:1069–1074Crossref, Medline, Google Scholar
31. Saxena S, Brody AL, Schwartz JM, Baxter LR: Neuroimaging and frontal-subcortical circuitry in obsessive-compulsive disorder. Br J Psychiatry Suppl 1998; 35:26–37Medline, Google Scholar
32. Mozley LH, Gur RC, Mozley PD, Gur RE: Striatal dopamine transporters and cognitive functioning in healthy men and women. Am J Psychiatry 2001; 158:1492–1499Link, Google Scholar
33. Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG: Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc Natl Acad Sci USA 1998; 95:4029–4034Crossref, Medline, Google Scholar
34. Jaber M, Jones S, Giros B, Caron MG: The dopamine transporter: a crucial component regulating dopamine transmission. Mov Disord 1997; 12:629–633Crossref, Medline, Google Scholar
35. Smith GS, Dewey SL, Brodie JD, Logan J, Vitkun SA, Simkowitz P, Schloesser R, Alexoff DA, Hurley A, Cooper T, Volkow ND: Serotonergic modulation of dopamine measured with [11C]raclopride and PET in normal human subjects. Am J Psychiatry 1997; 154:490–496Link, Google Scholar
36. Di Giovanni G, Di Matteo V, Di Mascio M, Esposito E: Preferential modulation of mesolimbic vs nigrostriatal dopaminergic function by serotonin(2C/2B) receptor agonists: a combined in vivo electrophysiological and microdialysis study. Synapse 2000; 35:53–61Crossref, Medline, Google Scholar
37. Gervais J, Rouillard C: Dorsal raphe stimulation differentially modulates dopaminergic neurons in the ventral tegmental area and substantia nigra. Synapse 2000; 35:281–291Crossref, Medline, Google Scholar
38. Leckman JF, Grice DE, Boardman J, Zhang H, Vitale A, Bondi C, Alsobrook J, Peterson BS, Cohen DJ, Rasmussen SA, Goodman WK, McDougle CJ, Pauls DL: Symptoms of obsessive-compulsive disorder. Am J Psychiatry 1997; 154:911–917Link, Google Scholar
39. Heinz A, Goldman D, Jones DW, Palmour R, Hommer D, Gorey JG, Lee KS, Linnoila M, Weinberger DR: Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology 2000; 22:133–139Crossref, Medline, Google Scholar
40. Willeit M, Praschak-Rieder N, Neumeister A, Pirker W, Asenbaum S, Vitouch O, Tauscher J, Hilger E, Stastny J, Brucke T, Kasper S: [123I]-beta-CIT SPECT imaging shows reduced brain serotonin transporter availability in drug-free depressed patients with seasonal affective disorder. Biol Psychiatry 2000; 47:482–489Crossref, Medline, Google Scholar



