A Longitudinal Study of Hippocampal Volume, Cortisol Levels, and Cognition in Older Depressed Subjects
Abstract
OBJECTIVE: This study determined whether cognitive impairments and structural brain changes in older depressed subjects, especially in the hippocampus, are related to hypercortisolemia. METHOD: Sixty-one depressed subjects over age 60 who met DSM-IV criteria for major depression and 40 healthy comparison subjects underwent structural magnetic resonance imaging, neuropsychological testing, apolipoprotein E (APOE) genotyping, and salivary cortisol assessment (over 3 days) with follow-up 6 months later. Hippocampal volume was measured by manual segmentation that was blind to diagnosis. Average area under the curve for salivary cortisol over the 3 days was calculated. Cognitive function was assessed by using a combined memory z score. RESULTS: Depressed subjects showed multiple impairments in attention, working memory, visual memory, verbal memory, new learning, and executive function in relation to comparison subjects. They had hypercortisolemia (53% increase in area under the curve) and a reduction in right hippocampal volume (6% decrease). Hippocampal volume reduction was not associated with increased cortisol levels but was significantly correlated with continuing memory deficits at 6 months. Persisting “mild cognitive impairment” was seen in 20 (41%) of 49 subjects at 6 months and was associated with reduced hippocampal volume but not severity of depression, cortisol levels, or APOE genotype. CONCLUSIONS: Older depressed subjects have persisting cognitive impairments associated with hippocampal volume reduction, but the results do not support cortisol-mediated hippocampal neurotoxicity as the major etiological mechanism. Neuropathological studies are required to investigate the basis for hippocampal changes, while follow-up will determine whether hippocampal atrophy is a risk factor for cognitive decline.
Major depression remains a common disorder in late life (prevalence of 3%) associated with substantial morbidity and increased mortality. Cognitive impairments, especially in attention, learning and memory, and executive function, are frequent (1–3) and associated with poor outcome (3, 4), and depression is a risk factor for subsequent dementia (5). Impairments frequently persist, despite recovery from depression (1, 2). The causes and associations of impaired cognitive function during depression remain uncertain. An influential hypothesis suggests that hypercortisolemia during depression may be important (6). Studies in animals and humans, involving both exogenous steroid administration and conditions such as Cushing’s syndrome and stress, which raise endogenous levels (7–9), have shown a relationship between raised cortisol levels and neuropsychological dysfunction, especially memory impairment. Both glucocorticoid and mineralocorticoid steroid receptors are present in high concentrations in the hippocampus and frontal cortex, and prolonged and raised cortisol levels can produce neuronal dysfunction with decreased glucose uptake, reduced dendritic arborization, and, ultimately, neuronal death and cell loss in the hippocampus in animals (6, 10, 11). It has, therefore, been proposed that raised cortisol levels during depression might be associated with cognitive impairments, especially in functions subserved by medial temporal lobe structures, and that persisting hypercortisolemia might cause hippocampal damage, explaining why impairments persist in depressed subjects, even when affective symptoms have resolved (6, 11). Older subjects might be particularly susceptible to this process, since hypercortisolemia in depression is more common with advancing age (12) and the aged hippocampus appears especially vulnerable (13).
However, currently there is only limited direct evidence to support this hypothesis. A relationship between hypercortisolemia and impaired cognition has been described in some (14), although by no means all (15), studies. Hippocampal atrophy, sometimes unilateral, has been described (16–21), but other reports have found no evidence of atrophy in younger (22) or older (23, 24) subjects with depression. In a study of middle-aged depressed women, hippocampal volume reduction was related to total lifetime duration of depression (18), a finding compatible with cortisol-induced neurotoxicity, although cortisol levels were not measured in that study. Others have found that hippocampal atrophy is present only after multiple depressive episodes (25), although changes in first-episode patients may occur (20). Genotype may be important, since hippocampal changes in depression have been linked to the apolipoprotein E-4 (APOE4) genotype (26). Few previous investigators have examined hypothalamic-pituitary-adrenal (HPA) axis function in relation to these hippocampal changes, although there may be an age-related relationship between ventricular enlargement and hypercortisolemia (27). Axelson and colleagues (28) reported a relationship between lower hippocampal volume and higher cortisol levels with the dexamethasone suppression test, although overall they found no reduction in hippocampal volume in their depressed subjects.
Because of these inconsistencies in the literature, we performed a longitudinal study to test the hypothesis that hippocampal volume reduction occurred in older subjects with depression, with volume reduction that would be related to hypercortisolemia during depression and persisting cognitive impairments at follow-up.
Method
Subjects
Sixty-one subjects ages 60 and over who fulfilled DSM-IV criteria for major depression (and scored 20 or more on the Montgomery-Åsberg Depression Rating Scale [29]) were recruited from clinical old-age general psychiatry services covering geographically based catchment areas and included referrals from day hospitals, inpatient units, and outpatient clinics. A comparison group (N=40) of similar-aged older people (also all over 60 years of age) with no past history of depression or current depression (Montgomery-Åsberg Depression Rating Scale score of less than 8) were recruited from community sources such as the Royal British Legion and from the spouses of patients attending the same hospital units. Both subjects and comparison subjects with a history of prior cognitive impairment, history or evidence of stroke or transient ischemic attack, severe or unstable physical illness (e.g., insulin-dependent diabetes mellitus, untreated hypothyroidism, uncontrolled heart failure, cancer), or a cognitive section of the Cambridge Examination for Mental Disorders of the Elderly (30) score of <75 (<80 for comparison subjects) were excluded from the study. The cognitive section of the Cambridge Examination for Mental Disorders of the Elderly is an extended cognitive screen (maximum score=107) that includes all of the items within the 30-point Mini-Mental State Examination (MMSE). Other exclusion criteria were history or current evidence of substance/alcohol abuse; long-term use (>2 months) of steroids at any point during lifetime; any use within the last 3 months of steroid or other medication thought to interfere with the HPA axis; ECT in last 3 months; use of medication that might significantly affect cognition (for example, use of benzodiazepines except short-acting ones, such as hypnotics, antipsychotics, sedative tricyclic antidepressants, or anticholinergic medication); or the presence of other neurological diagnosis. Use of newer antidepressants (e.g., selective serotonin reuptake inhibitors [SSRIs] and venlafaxine) and lithium was permitted. Fifty-one subjects were taking antidepressants at the time of testing, seven were taking lithium, and 19 had a past history of treatment with ECT (although they had had none in the last 3 months). The study was approved by the local ethics committee, and all patients and comparison subjects gave written informed consent to participate.
Assessment
Depressed subjects underwent a comprehensive psychiatric assessment, including a history, a mental status workup, a cognitive screen (cognitive section of the Cambridge Examination for Mental Disorders of the Elderly), and a physical examination. Subsequent investigations involved screening blood tests, including thyroid function, B12, and folate levels. Comprehensive demographic information was collected from all subjects and included past and current medical and psychiatric histories, including detailed analysis of medication taken, family history, education, and social class. For each episode of depression, case notes were searched and/or general practitioner records accessed, along with detailed patient and informant accounts to determine the number of previous episodes, age at onset, and the total lifetime duration of depression. Rating scales administered included the Montgomery-Åsberg Depression Rating Scale and the cognitive section of the Cambridge Examination for Mental Disorders of the Elderly and a neuropsychological battery to be detailed. A 10-ml venous blood sample was taken for genotyping.
Neuropsychological Assessment
The test battery was primarily designed to test attention, memory, and executive function and included both traditional pen-and-paper and computerized tasks (31, 32). Tests administered were as follows:
| 1. | A computerized continuous performance task (VIGIL) (33). Errors of commission and omission and response latency were recorded. This is primarily an attentional task. | ||||
| 2. | The FAS verbal fluency test, a task sensitive to frontal lobe impairment. | ||||
| 3. | Difference in performance between the Trail Making A and B tasks, a measure of executive function. | ||||
| 4. | Forward and reverse digit span. This task assesses attention and working memory. | ||||
| 5. | Rey Auditory Verbal Learning Test. This is a test of immediate and delayed verbal memory and of learning. | ||||
| 6. | Rey Visual Design Learning Test. This is a test of nonverbal memory. | ||||
| 7. | Memory tests from the Cambridge Automated Neuropsychological Test Battery (CeNeS Ltd., Cambridge, U.K.) (32). This is a nonverbal battery (using abstract designs) that is automated in delivery, operates by using a touch-sensitive screen, and has been used in a number of previous studies of neuropsychiatric disorders (34–36), including in younger (37) and older (1, 2) subjects with depression. In the pattern recognition task, the subject has to recognize a series of 24 patterns; in spatial recognition, the subject has to recognize the position of a series of 20 squares; and in delayed matching to sample, the subject has to pick out a previously shown shape from three distracters after a delay ranging from 0 to 12 seconds. | ||||
| 8. | Cambridge Automated Neuropsychological Test Battery spatial memory span task. | ||||
| 9. | The Cambridge Automated Neuropsychological Test Battery spatial working memory test in which subjects search a number of boxes on a screen for hidden tokens. This task tests spatial working memory and also the need for a search strategy and thus central executive function. | ||||
| 10. | The Cambridge Automated Neuropsychological Test Battery Tower of London (Stockings of Cambridge) task. Subjects are presented with three “pockets” that can hold one, two, and three colored balls, respectively, and have to match one set with another by moving balls one at a time. This task has an attentional component but focuses on information manipulation and planning; thus it tests executive function and has been shown to activate the dorsolateral prefrontal cortex. | ||||
Salivary Cortisol Analysis
Assessment of cortisol levels was undertaken by using salivary samples collected by using Salivettes (Sarstedt, Nümbrecht, Germany). A Salivette consists of a plastic tube with a wool plug on which the subject chews to produce saliva. Samples were collected at four time points (8:00 a.m., 12:00 noon, 4:00 p.m., 8:00 p.m.) over 3 consecutive days in an attempt to obtain a comprehensive measure of cortisol production. For inpatients, samples were not taken within 1 week of admission, and for day- and outpatients, samples were taken in the subjects’ natural environment (i.e., at home). The Salivette tubes were centrifuged and the samples stored at –20°C until assayed. Salivary cortisol was measured by using an I125 disequilibrium assay, the radioactive cortisol for which was supplied by Amersham Health, Amersham, U.K., and the primary antibody Ab1002 and the solid phase antirabbit serum by IDS, Tyne and Wear, U.K. The intra- and interassay coefficients of variation for 7.0, 47, and 87 nmol/liter cortisol samples were 13.0% and 14.6%, 10.7% and 9.8%, and 9.4% and 10.2%, respectively. Average area under the curve for the 3 days was calculated.
Genotyping
APOE genotypes were analyzed by using polymerase chain reaction (38).
Magnetic Resonance Imaging (MRI) Scanning Protocol
MRI scans were undertaken by using a 1.0 Tesla Siemens Magnetom Impact Expert System (Siemens Medical, Erlangen, Germany). Whole brain T1-weighted three-dimensional MPRAGE (magnetization prepared rapid-acquisition gradient echo) turbo flash data sets were acquired in the sagittal plane (TR=11.4 msec, TE=4.4 msec, TI=400 msec, flip angle=15°, matrix=256×256, slice thickness=1 mm, cubic voxels of 1 mm). The images acquired thus consisted of truly isotropic voxels of 1×1×1 mm. The sequences were chosen to give good gray-white matter contrast.
MRI Analysis
Images were transferred to a Sun Ultra 10 work station (Sun Microsystems, Mountain View, Calif.) running Solaris 2.7 and analyzed by a single operator (who was blind to diagnosis) using the commercially available software package AnalyzeAVW-3.0 (AnalyzeDirect.com, Lenexa, Kan.; Mayo Foundation Biomedical Imaging Resource, Rochester, Minn.). Images were reoriented along the long axis of the hippocampus to ensure consistent slicing of the hippocampus normal to its long axis in coronal sections. Hippocampal volume was assessed by using a region-of-interest approach with manual segmentation by use of a mouse. All identification of anatomical structures was carried out with reference to standard neuroanatomical and neuroradiological atlases and specific anatomical reviews (39, 40). Hippocampal boundaries were based on previous descriptions (41). The anterior limit was taken as the first slice in which the head of the hippocampus was visible. The alveus was used to aid demarcation of hippocampal gray matter from that of the amygdala (which is superior and anterior to it). The posterior limit was the slice on which the fornix was visible in its longest length. The superior boundary was defined by the choroid fissure, the medial boundary by CSF; the lateral boundary was the parahippocampal gyrus (for the head), the temporal horn of the lateral ventricle (for the body), the fornix (for the tail), and the inferior boundary by the subiculum (which was included in the measurement). A full protocol is available upon request from the first author. Whole brain volume was calculated by using the ANALYSE method, which uses a seed-growing thresholding approach with dilations and erosions. Final rendered brain volumes were compared with original scans for each subject to check accuracy. To assess intrarater reliability, repeat measurements were performed on 10 randomly selected scans on two occasions. Reliability for the volume of the segmented whole brain was excellent, with an intraclass correlation coefficient of α=0.99, and was very good for hippocampal volume (left hippocampus, α=0.97; right hippocampus, α=0.99).
Follow-Up
Depressed subjects and comparison subjects were reassessed at 6 months, with a repeat psychiatric assessment, administration of ratings scales, a neuropsychological assessment, and salivary cortisol tests.
For the purposes of analysis, depressed patients were considered fully recovered if they no longer met DSM-IV criteria for major depression and their Montgomery-Åsberg Depression Rating Scale scores were <8.
Statistical Analysis
Data were analyzed with SPSS version 10 (SPSS, Chicago). Differences between groups were assessed by using independent t tests or analysis of covariance when covariates (e.g., age) were included. Paired t tests were used to investigate differences over time within groups. To deal with the potential problem of multiple comparisons (particularly when investigating correlations between cognitive test results), hippocampal volume, cortisol level, and cognitive tests involving memory were combined into a single z score. For the five tests chosen to reflect memory/hippocampal function (the Rey Auditory Verbal Learning Test, the Rey Visual Design Learning Test, pattern recognition, spatial recognition, delayed matching to sample), the mean for the comparison subjects at that particular time point (baseline or the 6-month test) was subtracted from the raw score for each subject, and the result was divided by the comparison’s standard deviation. This created z scores for the comparison subjects (mean=0, SD=1) for each of the five tasks; performance of the depressed patients was measured relative to this. The five memory z scores were then summed to create an overall memory z score (zMem), which was used as the primary variable for correlational analysis. zMem scores were calculated for memory at both baseline and 6 months. Bivariate correlations were undertaken using Pearson’s r, with partial correlations in some cases for age adjustment. Linear regression was used to investigate the predictors of continued cognitive impairment at 6 months in depressed subjects. The significance level was set at p<0.05.
Results
Subject Characteristics
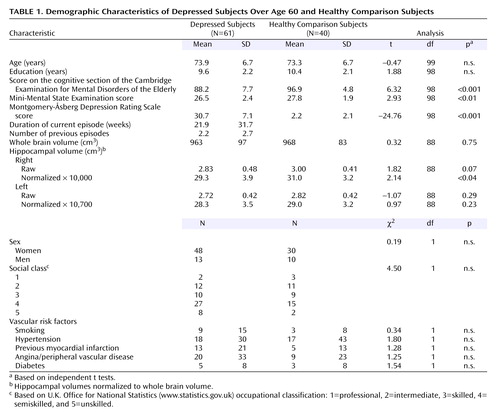
The demographic characteristics are shown in Table 1. Groups did not differ in age, sex, social class, or education. There was no difference between groups in terms of clinical vascular risk factors. Depressed subjects scored significantly lower on the MMSE and the cognitive section of the Cambridge Examination for Mental Disorders of the Elderly in relation to the comparison subjects and, as expected, had significantly higher depression scores. Of the 61 depressed subjects, 34 (56%) had a late onset (after age 60) of depression, two were bipolar (currently depressed), 34 (56%) had a melancholic subtype of depression, and 12 (20%) had psychotic features. There was no difference in the proportion of subjects with APOE4 genotypes between comparison subjects (35%) and depressed subjects (27%) (χ2=0.65, df=1, p=0.42). At baseline, 51 of the 61 subjects consented to and successfully underwent MRI, 39 consented to and underwent salivary cortisol estimation, and 37 consented to and successfully completed the full neuropsychological battery (27 successfully completed all procedures). All 40 comparison subjects completed the neuropsychological battery; one could not tolerate MRI and salivary cortisol sampling (39 successfully completed all procedures). At 6 months, 49 depressed subjects (80%) were reassessed (12 declined to participate). Of these, all completed salivary cortisol measurement, and 48 completed the neuropsychological battery. No subject underwent repeat cognitive testing within 3 months of the last ECT. All 40 comparison subjects completed the 6-month cognitive testing, and 39 had salivary cortisol measurement.
At the follow-up, mean scores on the cognitive section of the Cambridge Examination for Mental Disorders of the Elderly were 98.7 (SD=3.5) for the comparison subjects and 90.6 (SD=7.9) for the depressed subjects; MMSE scores were 28.4 (SD=1.7) for the comparison subjects and 26.7 (SD=2.7) for the depressed subjects; Montgomery-Åsberg Depression Rating Scale scores were 2.3 (SD=2.9) for the comparison subjects and 11.7 (SD=10.9) for the depressed subjects. There was a significant improvement in Montgomery-Åsberg Depression Rating Scale scores in the depressed group between baseline and 6 months (t=10.1, df=48, p<0.0001) and significant improvements for both comparison subjects and depressed subjects for scores on the cognitive section of the Cambridge Examination for Mental Disorders of the Elderly (comparison subjects: t=–3.27, df=39, p<0.01; depressed subjects: t=–2.34, df=48, p<0.05) but not on the MMSE. At 6 months, 26 depressed subjects were in remission (and all had Montgomery-Åsberg Depression Rating Scale scores <8), 11 still fulfilled criteria for major depression, and the remainder had significant residual depressive symptoms (Montgomery-Åsberg Depression Rating Scale scores >8) but did not fulfill criteria for major depression.
Salivary Cortisol Levels
Salivary cortisol levels for each time point and for the mean area under the curve are presented in Table 2. As shown, the levels were increased at baseline in depressed subjects in relation to comparison subjects, with the mean area under the curve increased by 53% (t=–4.29, df=78, p<0.0001). At 6 months, cortisol levels were not significantly different between groups at any time point, and areas under the curve were similar.
Neuropsychological Testing
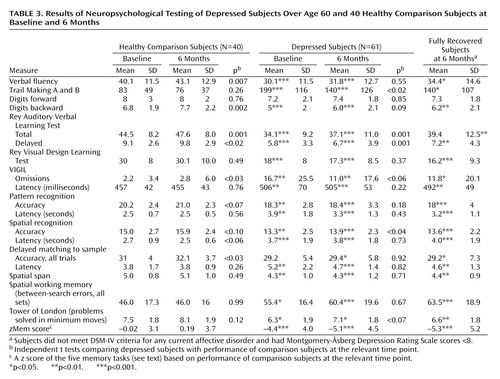
Results of cognitive testing are shown in Table 3. As can be seen, depressed subjects showed a broad range of cognitive deficits, with impairments on most tasks in relation to comparison subjects. They had significant deficits on the VIGIL continuous performance task, verbal fluency, the Rey Auditory Verbal Learning Test, digit span backward (but not forward), the Rey Visual Design Learning Test, the Trail Making tests, pattern and spatial recognition, spatial span, spatial working memory, and the Tower of London. They were also significantly slowed, with latencies on all tasks significantly longer than those of comparison subjects. At 6 months, the comparison subjects showed significant (although numerically small) improvements in some tasks, presumably reflecting a combination of learning effects and task familiarity. Depressed subjects also showed improvements in some tasks, although they only improved in four tasks in relation to six for comparison subjects. The striking observation was that despite substantial improvements in depression ratings, depressed subjects remained impaired on almost all tasks in relation to comparison subjects. Although depressed subjects had significantly improved (mean Montgomery-Åsberg Depression Rating Scale score=11.7), and hypercortisolemia had reversed, it was still possible that results were confounded by the inclusion of ill or only partially recovered subjects. As such, results are also shown separately for the 26 subjects who were in remission (Montgomery-Åsberg Depression Rating Scale score <8 points) as well as for all subjects. As shown, analysis of the fully remitted group revealed exactly the same profile of results as for the whole sample.
MRI Analysis
Results are presented in Table 1. There were no significant differences in whole brain volume between depressed subjects and comparison subjects. Hippocampal volumes were normalized to whole brain volumes to account for premorbid differences in brain size (normalized volume=hippocampal volume/whole brain volume × 10,000). Normalized right hippocampal volume was significantly reduced in depressed subjects in relation to comparison subjects (t=2.14, df=88, p<0.04), although differences for the left hippocampus were not significant (t=0.97, df=88, p=0.23) (Table 1). Groups were of similar age, and covarying for age did not alter the results.
Correlations Within Depressed Subjects
Within the depressed subjects, there was a significant correlation between advancing age and area-under-the-curve cortisol levels (r=0.43, p<0.01), but there were no significant correlations between hippocampal volume and area-under-the-curve cortisol levels at baseline (left hippocampus: r=0.01, p=0.70; right hippocampus: r=–0.04, p=0.80) or any correlations with individual cortisol measures (8:00 a.m., 12:00 noon, 4:00 p.m., 8:00 p.m.) or with the number of previous depressive episodes or total lifetime duration of depression (r<0.20, p>0.40). There was no significant correlation between area-under-the-curve cortisol and zMem score at baseline (r=–0.12, p=0.56) or 6 months (r=–0.23, p=0.24) or between zMem score at baseline and hippocampal volume (left hippocampus: r=0.02, p=0.94; right hippocampus: r=0.01, p=0.98). However, there was a significant correlation between zMem score at 6 months and hippocampal volume (left hippocampus: r=0.40, p<0.01; right hippocampus: r=0.44, p<0.005), indicating that memory impairments at recovery but not at baseline were associated with reduced hippocampal volume (Figure 1). In a secondary exploratory analysis, to confirm that important associations were not being missed, the five individual memory tests at baseline and at 6 months were correlated with area-under-the-curve cortisol. No significant correlations were seen. To clarify the relationship between variables of interest and cognitive deficits, regression analysis was performed for depressed subjects with zMem at 6 months as the dependent variable and age, hippocampal volume (right), area-under-the-curve cortisol, and current Montgomery-Åsberg Depression Rating Scale score as the independent variables. Only hippocampal volume was a significant predictor of zMem (β=0.415, t=3.51, df=40, p=0.001), explaining about 17% of the variance in memory performance at 6 months.
Persisting Mild Cognitive Impairments
To facilitate comparison with studies of nondepressed subjects, subjects at 6 months were categorized as to whether they had “mild cognitive impairment” according to the widely accepted definition of Petersen and colleagues (42) by using a Rey Auditory Verbal Learning Test score less than 1.5 standard deviations below that for the comparison subjects. Applying this cutoff identified 20 (41%) of 49 depressed subjects at 6 months as having mild cognitive impairment. Patients with mild cognitive impairment were older than cognitively normal depressed subjects (age: mean=77.2 years, SD=6.3, versus mean=71.7, SD=6.1) (t=–0.38, df=49, p<0.05), but the level of depressive symptoms was equivalent (Montgomery-Åsberg Depression Rating Scale score: mean=11.3, SD=9.9, versus mean=11.3, SD=11.1) (t=0.37, df=48, p=0.98). Hippocampal volume was reduced bilaterally in cases of mild cognitive impairment (left hippocampus: mean=26.8 cm3, SD=3.9, versus mean=29.6 cm3, SD=2.8; t=2.74, df=43, p<0.01) (right hippocampus: mean=27.1 cm3, SD=4.0, versus mean=31.0 cm3, SD=3.2; t=3.67, df=43, p<0.001). Repeat analysis covarying for age showed that significant differences remained for the right hippocampus (F=7.2, df=2, 45, p<0.01), with a near-significant difference for the left hippocampus (F=5.01, df=2, 45, p=0.15). There were no differences between patients with mild cognitive impairment and patients without mild cognitive impairment in the proportion of APOE4 alleles, and depressed subjects with and without APOE4 did not differ in hippocampal volume (t=–1.05, df=41, p>0.20).
Discussion
The main results of this study were that 1) in relation to similar-aged comparison subjects, older depressed subjects had multiple cognitive deficits in attention, learning and memory, and executive function; 2) deficits persisted at 6 months, even in subjects who were in full remission from illness, with 41% fulfilling criteria for mild cognitive impairment; 3) depressed subjects exhibited hypercortisolemia during depression, as demonstrated by raised salivary cortisol levels, with values returning to normal by 6 months; 4) depressed subjects had significant volume reduction of the right hippocampus on MRI; 5) continuing deficits in memory were associated with bilateral hippocampal volume reduction, not cortisol levels, APOE genotype, or current level of depressive symptoms.
Our first hypothesis—that depressed subjects would have reduction in hippocampal volume on MRI—was confirmed for the right hippocampus. Hippocampal volume reduction has been found in previous studies in younger and older depressed patients (16, 19–21, 25, 43, 44), although negative results have also been reported (22, 45–47). Unilateral change has also been reported in many of these studies. Intriguingly, studies of younger subjects tend to find preferential involvement of the left hippocampus (19, 44, 48), while studies of older subjects, in keeping with our results, find more involvement of the right hippocampus (16, 21). Unilateral changes would tend not to support the glucocorticoid toxicity hypothesis, since there would be no good reason why a systemic toxic process would cause unilateral brain damage. Right hippocampal volume is larger than left and so potentially may be more susceptible to age-related atrophy, although a more likely explanation may be that changes are indeed bilateral but studies may lack the power to demonstrate bilateral changes. In support, most studies, including this one, that find unilateral changes find reductions, but to a lesser degree, on the other side. The finding of hippocampal atrophy in depression receives some support from a recent neuropathological study that, while not finding neuronal loss, did find increased cell packing, consistent with loss of neuronal processes (49).
As expected, we found clear evidence of increased cortisol production (53%) in depressed subjects in relation to comparison subjects, with levels returning to comparison values by 6 months, paralleling the substantial improvement in depression. Despite reversal of hypercortisolemia and improvement in depression, significant impairments in multiple cognitive domains persisted at 6 months. The possibility that such impairments were the result of residual depressive symptoms was refuted by finding that results were essentially unchanged when only fully remitted subjects were examined (Table 1). Previous studies have emphasized the broad nature of cognitive impairments in depression, and impairments on the same or similar tasks to those included here have been described by many others (1–3, 50–52). Although most of our subjects were taking medication, we do not think that medication effects accounted for these deficits for several reasons. First, prescriptions for tricyclic antidepressants and sedative benzodiazepines were exclusion criteria; in comparison, SSRIs have few adverse effects on cognition (53). Second, the relationship between impairments and structural brain changes would not support drug-induced impairments. Third, a study of entirely drug-free depressed subjects reported an almost identical profile of neuropsychological impairment to that found here (54).
Most authors find some cognitive improvement on recovery from depression, although impairments persist in many domains (1, 2, 51, 55). Few previous studies have retested comparison subjects to account for learning effects. The current investigation did, and we found surprising improvements in many tests of comparison subjects. These not only were in a greater number of tests than for depressed subjects but made the lack of improvement in cognition after improvement in depression all the more striking. In patients, deficits remained in attention, executive function, working memory, verbal and visual memory, new learning, and speed of response. Our main aim was to investigate the correlates of this continued impairment, with the hypothesis that impairments in learning and memory would be associated with hypercortisolemia during depression and with hippocampal volume reduction. We did find a significant association between hippocampal volume and persisting impairments, whether examined as a continuous measure (Figure 1) or by dividing subjects into those with and without mild cognitive impairment. In both cases, the relationship remained after adjustment for age. Hippocampal volume reduction has also been described in nondepressed subjects with mild cognitive impairment and may predict cognitive decline (41, 56). However, psychiatric illnesses, such as depression, have to date excluded subjects from the label of mild cognitive impairment (42). We found that 41% of depressed subjects at 6 months fulfilled criteria for mild cognitive impairment, which appeared to be associated with hippocampal volume reduction, not residual depressive symptoms. It will be important to undertake longer-term follow-up of depressed patients with mild cognitive impairment described here to determine whether persisting impairments and/or hippocampal volume reduction predicts subsequent dementia. In a prospective study, Steffens and colleagues (57) found that left hippocampal volume reduction was associated with subsequent cognitive decline in older subjects with depression. Further similar studies will enable outcome of depressed patients with impairments to be compared with those with amnestic impairments in the absence of depression, who are known to be at a higher risk of cognitive decline and dementia (42).
Contrary to our second hypothesis, we did not find evidence of a relationship between raised cortisol levels during depression (or at recovery) and cognitive performance or hippocampal volume reduction. Our results, therefore, did not provide support for the glucocorticoid toxicity hypothesis of hippocampal damage in depression. Despite the studies cited describing hippocampal volume reduction in depression, there has not been a clear demonstration that this relationship is mediated by glucocorticoids. Moreover, cognitive deficits in depression have been well described, even in first-episode patients (18, 25) who might be expected to have had relatively low exposure to raised glucocorticoids compared with those with multiple episodes. Hippocampal volume reductions have been described in many psychiatric disorders, including dementia, schizophrenia (58), and posttraumatic stress disorder (59). It is unlikely that glucocorticoid toxicity underlies such changes in all disorders. In addition, in other studies, hippocampal atrophy was found to be associated with late age at onset and APOE4 genotype (16, 26, 57), suggesting that mechanisms other than raised cortisol levels might be important. Although we found no relation with APOE4 genotype, the absence of a relationship between hippocampal volume and either cortisol levels, number of previous depressive episodes, or lifetime duration of depression argues against cortisol toxicity.
There are a number of possible reasons for our negative findings. The limitations of our study include a relatively modest-sized and heterogeneous study group, as any subtype-specific correlations (for example, with psychotic or melancholic type) might have been diluted by the inclusion of other subjects. Our cortisol assessment, although rigorous, may not have accurately reflected total glucocorticoid exposure over a lifetime, which may be the real variable of importance. Individuals may vary in their susceptibility to raised cortisol levels, with such differences masked by the use of mean values for analysis. There may be other steroids that are important but were not assessed in this study, for example, dehydroepiandrosterone (DHEA). Age-related decrements in the hippocampal glucocorticoid receptors occur, and it may be that such changes are adaptive, actually protecting the aging hippocampus against glucocorticoid toxicity (13). Finally, it may be that older subjects with depression differ from those at other ages. For example, the role of vascular factors in the etiology of late-onset depression has recently been recognized (60, 61), while the increasing importance of vascular factors and cerebrovascular disease in underpinning cognitive impairments in a variety of disorders is recognized within the term “vascular cognitive impairment” (62). It may be that vascular factors, rather than raised cortisol levels, may underpin structural brain changes and enduring cognitive impairments. However, in the current study, we found no differences between groups in the presence of established vascular risk factors, such as diabetes, smoking, and hypertension, although it remains possible that, as demonstrated in autopsy studies, depressed subjects might still have had more vascular disease than comparison subjects (63).
In conclusion, we found evidence in a substantial number of older depressed subjects of persisting cognitive impairments that were linked to hippocampal volume reduction rather than current or past depressive episodes or hypercortisolemia. Longer-term follow-up is required to determine if such changes do indeed robustly predict subsequent cognitive decline, while neuropathological studies are required to determine the pathological substrates underlying hippocampal atrophy and persisting cognitive impairments in older depressed subjects.
 |
 |
 |
Received Sept. 23, 2003; revision received Jan. 13, 2004; accepted Feb. 12, 2004. From the School of Neurology, Neurobiology, and Psychiatry and the Institute for Ageing and Health, University of Newcastle Upon Tyne; the School of Neurology, Neurobiology, and Psychiatry, Royal Victoria Infirmary, Newcastle Upon Tyne; and the Department of Neuroradiology, Newcastle General Hospital, Newcastle Upon Tyne. Address reprint requests to Dr. O’Brien, Wolfson Research Centre, Institute for Ageing and Health, Newcastle General Hospital, Westgate Rd., Newcastle Upon Tyne NE4 6BE, U.K.; [email protected] (e-mail). The authors thank the Wellcome Trust and the Stanley Foundation for financial support; Nicky Barnett and Liz McGuckin for help with subject recruitment, assessment, and database management; and Philip English for expert radiographer advice and support.
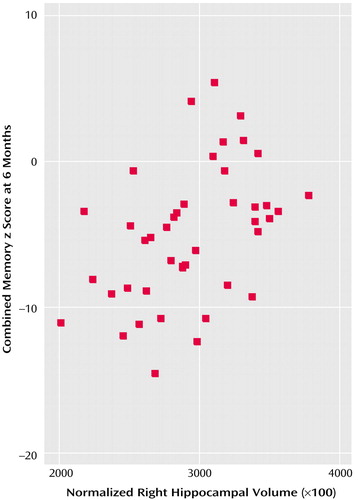
Figure 1. Scatterplot of Normalized Right Hippocampal Volume (×100) Against Combined Memory z Score for Depressed Subjects Over Age 60 at 6 Monthsa
aPearson’s r=0.40, p=0.009.
1. Abas MA, Sahakian BJ, Levy R: Neuropsychological deficits and CT scan changes in elderly depressives. Psychol Med 1990; 20:507–520Crossref, Medline, Google Scholar
2. Beats BC, Sahakian BJ, Levy R: Cognitive performance in tests sensitive to frontal lobe dysfunction in the elderly depressed. Psychol Med 1996; 26:591–603Crossref, Medline, Google Scholar
3. Alexopoulos GS, Meyers BS, Young RC, Kalayam B, Kakuma T, Gabrielle M, Sirey JA, Hull J: Executive dysfunction and long-term outcomes of geriatric depression. Arch Gen Psychiatry 2000; 57:285–290Crossref, Medline, Google Scholar
4. Simpson S, Talbot PR, Snowden JS, Neary D: Subcortical vascular disease in elderly patients with treatment resistant depression (letter). J Neurol Neurosurg Psychiatry 1997; 62:196–197Crossref, Medline, Google Scholar
5. Jorm AF: Is depression a risk factor for dementia or cognitive decline? a review. Gerontology 2000; 46:219–227Crossref, Medline, Google Scholar
6. Sapolsky RM: Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry 2000; 57:925–935Crossref, Medline, Google Scholar
7. Starkman MN, Giordani B, Berent S, Schork MA, Schteingart DE: Elevated cortisol levels in Cushing’s disease are associated with cognitive decrements. Psychosom Med 2001; 63:985–993Crossref, Medline, Google Scholar
8. Newcomer JW, Selke G, Melson AK, Hershey T, Craft S, Richards K, Alderson AL: Decreased memory performance in healthy humans induced by stress-level cortisol treatment. Arch Gen Psychiatry 1999; 56:527–533Crossref, Medline, Google Scholar
9. Kirschbaum C, Wolf OT, May M, Wippich W, Hellhammer DH: Stress- and treatment-induced elevations of cortisol levels associated with impaired declarative memory in healthy adults. Life Sci 1996; 58:1475–1483Crossref, Medline, Google Scholar
10. Landfield PW, Eldridge JC: Evolving aspects of the glucocorticoid hypothesis of brain aging: hormonal modulation of neuronal calcium homeostasis. Neurobiol Aging 1994; 15:579–588Crossref, Medline, Google Scholar
11. O’Brien JT: The “glucocorticoid cascade” hypothesis in man. Br J Psychiatry 1997; 170:199–201Crossref, Google Scholar
12. O’Brien JT, Ames D, Schweitzer I, Colman P, Desmond P, Tress B: Clinical and magnetic resonance imaging correlates of hypothalamic-pituitary-adrenal axis function in depression and Alzheimer’s disease. Br J Psychiatry 1996; 168:679–687Crossref, Medline, Google Scholar
13. Seckl JR, Olsson T: Glucocorticoid hypersecretion and the age impaired hippocampus: cause or effect? J Endocrinol 1995; 145:201–211Crossref, Medline, Google Scholar
14. Mitchell AJ, Dening TR: Depression-related cognitive impairment: possibilities for its pharmacological treatment. J Affect Disord 1996; 36:79–87Crossref, Medline, Google Scholar
15. Adler G, Jajcevic A: Post-dexamethasone cortisol level and memory performance in elderly depressed patients. Neurosci Lett 2001; 298:142–144Crossref, Medline, Google Scholar
16. Steffens DC, Byrum CE, McQuoid DR, Greenberg DL, Payne ME, Blitchington TF, MacFall JR, Krishnan KR: Hippocampal volume in geriatric depression. Biol Psychiatry 2000; 48:301–309Crossref, Medline, Google Scholar
17. Sheline YI: Hippocampal atrophy in major depression: a result of depression-induced neurotoxicity? Mol Psychiatry 1996; 1:298–299Medline, Google Scholar
18. Sheline YI, Sanghavi M, Mintun MA, Gado MH: Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci 1999; 19:5034–5043Crossref, Medline, Google Scholar
19. Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS: Hippocampal volume reduction in major depression. Am J Psychiatry 2000; 157:115–117Link, Google Scholar
20. Frodl T, Meisenzahl EM, Zetzsche T, Born C, Groll C, Jäger M, Leinsinger G, Bottlender R, Hahn K, Möller H-J: Hippocampal changes in patients with a first episode of major depression. Am J Psychiatry 2002; 159:1112–1118Link, Google Scholar
21. Bell-McGinty S, Butters MA, Meltzer CC, Greer PJ, Reynolds CF III, Becker JT: Brain morphometric abnormalities in geriatric depression: long-term neurobiological effects of illness duration. Am J Psychiatry 2002; 159:1424–1427Link, Google Scholar
22. Vakili K, Pillay SS, Lafer B, Fava M, Renshaw PF, Bonello-Cintron CM, Yurgelun-Todd DA: Hippocampal volume in primary unipolar major depression: a magnetic resonance imaging study. Biol Psychiatry 2000; 47:1087–1090Crossref, Medline, Google Scholar
23. Ashtari M, Greenwald BS, Kramer-Ginsberg J, Hu H, Wu M, Patel P, Pollack S: Hippocampal/amygdala volumes in geriatric depression. Psychol Med 1999; 29:629–638Crossref, Medline, Google Scholar
24. Pantel J, Schroder J, Essig M, Popp D, Dech H, Knopp MV, Schad LR, Eysenbach K, Backenstrass M, Friedlinger M: Quantitative magnetic resonance imaging in geriatric depression and primary degenerative dementia. J Affect Disord 1997; 42:69–83Crossref, Medline, Google Scholar
25. MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, Nahmias C, Young LT: Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci USA 2003; 100:1387–1392Crossref, Medline, Google Scholar
26. Kim do H, Payne ME, Levy RM, MacFall JR, Steffens DC: APOE genotype and hippocampal volume change in geriatric depression. Biol Psychiatry 2002; 51:426–429Crossref, Medline, Google Scholar
27. Coffey CE, Wilkinson WE, Weiner RD, Ritchie JC, Aque M: The dexamethasone suppression test and quantitative cerebral anatomy in depression. Biol Psychiatry 1993; 33:442–449Crossref, Medline, Google Scholar
28. Axelson DA, Doraiswamy PM, McDonald WM, Boyko OB, Tupler LA, Patterson LJ, Nemeroff CB, Ellinwood EH, Krishnan KRR: Hypercortisolaemia and hippocampal changes in depression. Psychiatry Res 1993; 47:163–173Crossref, Medline, Google Scholar
29. Montgomery SA, Åsberg M: A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134:382–389Crossref, Medline, Google Scholar
30. Roth M, Tym E, Mountjoy CQ, Huppert FA, Hendrie H, Verma S, Goddard R: CAMDEX: a standardised instrument for the diagnosis of mental disorder in the elderly with special reference to the early detection of dementia. Br J Psychiatry 1986; 149:698–709Crossref, Medline, Google Scholar
31. Lezak M: Neuropsychological Assessment, 3rd ed. Oxford, UK, Oxford University Press, 1995Google Scholar
32. Robbins TW, James M, Owen AM, Lange KW, Lees AJ, Leigh PN, Marsden CD, Quinn NP, Summers BA: Cognitive deficits in progressive supranuclear palsy, Parkinson’s disease, and multiple system atrophy in tests sensitive to frontal lobe dysfunction. J Neurol Neurosurg Psychiatry 1994; 57:79–88Crossref, Medline, Google Scholar
33. Cegalis J, Bowlin J: VIGIL: Software for the Assessment of Attention. Nashua, NH, Forthought, 1991Google Scholar
34. Owen AM, Herrod NJ, Menon DK, Clark JC, Downey SP, Carpenter TA, Minhas PS, Turkheimer FE, Williams EJ, Robbins TW, Sahakian BJ, Petrides M, Pickard JD: Redefining the functional organization of working memory processes within human lateral prefrontal cortex. Eur J Neurosci 1999; 11:567–574Crossref, Medline, Google Scholar
35. Owen AM, Sahakian BJ, Semple J, Polkey CE, Robbins TW: Visuo-spatial short-term recognition memory and learning after temporal lobe excisions, frontal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia 1995; 33:1–24Crossref, Medline, Google Scholar
36. Owen AM, Doyon J, Petrides M, Evans AC: Planning and spatial working memory: a positron emission tomography study in humans. Eur J Neurosci 1996; 8:353–364Crossref, Medline, Google Scholar
37. O’Brien JT, Sahakian BJ, Checkley SA: Cognitive impairments in patients with seasonal affective disorder. Br J Psychiatry 1993; 163:338–343Crossref, Medline, Google Scholar
38. Wenham PR, Newton CR, Price WH: Analysis of apolipoprotein E genotypes by the Amplification Refractory Mutation System. Clin Chem 1991; 37:241–244Medline, Google Scholar
39. Talairach J, Tournoux P: Co-Planar Stereotaxic Atlas of the Human Brain: Three-Dimensional Proportional System. New York, Thieme Medical, 1988Google Scholar
40. Jackson GD, Duncan JS: MRI Neuroanatomy: A New Angle on the Brain. Edinburgh, Churchill Livingstone, 1996Google Scholar
41. Jack CR, Petersen RC, Xu YC, O’Brien PC, Smith GE, Ivnik RJ, Boeve BF, Waring SC, Tangalos EG, Kokmen E: Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology 1999; 52:1397–1403Crossref, Medline, Google Scholar
42. Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E: Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999; 56:303–308Crossref, Medline, Google Scholar
43. Vythilingam M, Heim C, Newport J, Miller AH, Anderson E, Bronen R, Brummer M, Staib L, Vermetten E, Charney DS, Nemeroff CB, Bremner JD: Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry 2002; 159:2072–2080Link, Google Scholar
44. Mervaala E, Fohr J, Kononen M, Valkonen-Korhonen M, Vainio P, Partanen K, Partanen J, Tiihonen J, Viinamaki H, Karjalainen AK, Lehtonen J: Quantitative MRI of the hippocampus and amygdala in severe depression. Psychol Med 2000; 30:117–125Crossref, Medline, Google Scholar
45. Pantel J, Schroder J, Essig M, Popp D, Dech H, Knopp MV, Schad LR, Eysenbach K, Bakcenstrass M, Friedlinger M: Quantitative magnetic resonance imaging in geriatric depression and primary degenerative dementia. J Affect Disord 1997; 42:69–83Crossref, Medline, Google Scholar
46. Posener JA, Wang L, Price JL, Gado MH, Province MA, Miller MI, Babb CM, Csernansky JG: High-dimensional mapping of the hippocampus in depression. Am J Psychiatry 2003; 160:83–89Link, Google Scholar
47. Ashtari M, Greenwald BS, Kramer-Ginsberg E, Hu J, Wu H, Patel M, Aupperle P, Pollack S: Hippocampal/amygdala volumes in geriatric depression. Psychol Med 1999; 29:629–638Crossref, Medline, Google Scholar
48. Shah PJ, Ebmeier KP, Glabus MF, Goodwin GM: Cortical grey matter reductions associated with treatment-resistant chronic unipolar depression. Br J Psychiatry 1998; 172:527–532Crossref, Medline, Google Scholar
49. Stockmeier CA, Mahajan G, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY, Friedman L, Rajkowska G: Neuronal and glial density is increased and neuronal soma size is decreased in hippocampus in major depressive disorder (MDD) (abstract). Biol Psychiatry 2003; 53:S198Google Scholar
50. Austin M-P, Mitchell P, Parker G, Hickie I, Brodaty H, Chan J, Eyers K, Milic M, Hadzi-Pavlovic D: Cognitive function in depression: a distinct pattern of frontal impairment? Psychol Med 1999; 29:73–85Crossref, Medline, Google Scholar
51. Dahabra S, Ashton CH, Bahrainian M, Britton PG, Ferrier IN, McAllister VA, Marsh VR, Moore PB: Structural and functional abnormalities in elderly patients clinically recovered from early- and late-onset depression. Biol Psychiatry 1998; 44:34–46Crossref, Medline, Google Scholar
52. Lockwood KA, Alexopoulos GS, van Gorp WG: Executive dysfunction in geriatric depression. Am J Psychiatry 2002; 159:1119–1126Link, Google Scholar
53. van Laar MW, Volkerts ER, Verbaten MN, Trooster S, van Megen HJ, Kenemans JL: Differential effects of amitriptyline, nefazodone and paroxetine on performance and brain indices of visual selective attention and working memory. Psychopharmacology (Berl) 2002; 162:351–363Crossref, Medline, Google Scholar
54. Porter RJ, Gallagher P, Thompson JM, Young AH: Neurocognitive impairment in drug-free patients with major depressive disorder. Br J Psychiatry 2003; 182:214–220Crossref, Medline, Google Scholar
55. Nebes RD, Butters MA, Mulsant BH, Pollock BG, Zmuda MD, Houck PR, Reynolds CF III: Decreased working memory and processing speed mediate cognitive impairment in geriatric depression. Psychol Med 2000; 30:679–691Crossref, Medline, Google Scholar
56. de Leon MJ, Convit A, Wolf OT, Tarshish CY, DeSanti S, Rusinek H, Tsui W, Kandil E, Scherer AJ, Roche A, Imossi A, Thorn E, Bobinski M, Caraos C, Lesbre P, Schlyer D, Poirier J, Reisberg B, Fowler J: Prediction of cognitive decline in normal elderly subjects with 2-[(18)F]fluoro-2-deoxy-D-glucose/positron-emission tomography (FDG/PET). Proc Natl Acad Sci USA 2001; 98:10966–10971Crossref, Medline, Google Scholar
57. Steffens DC, Payne ME, Greenberg DL, Byrum CE, Welsh-Bohmer KA, Wagner HR, MacFall JR: Hippocampal volume and incident dementia in geriatric depression. Am J Geriatr Psychiatry 2002; 10:62–71Crossref, Medline, Google Scholar
58. Velakoulis D, Pantelis C, McGorry PD, Dudgeon P, Brewer W, Desmond P, Bridle N, Tierney P, Murrie V, Singh B, Copolov D: Hippocampal volume in first-episode psychoses and chronic schizophrenia. Arch Gen Psychiatry 1999; 56:133–140Crossref, Medline, Google Scholar
59. Bremner JD: Neuroimaging studies in post-traumatic stress disorder. Curr Psychiatry Rep 2002; 4:254–263Crossref, Medline, Google Scholar
60. Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M: “Vascular depression” hypothesis. Arch Gen Psychiatry 1997; 54:915–922Crossref, Medline, Google Scholar
61. Baldwin B, O’Brien J: The vascular basis of late life depressive disorder. Br J Psychiatry 2002; 180:157–161Crossref, Medline, Google Scholar
62. O’Brien JT, Erkinjuntti T, Reisberg B, Roman G, Sawada T, Pantoni L, Bowler JV, Ballard C, DeCarli C, Gorelick PB, Rockwood K, Burns A, Gauthier S, DeKosky ST: Vascular cognitive impairment. Lancet Neurol 2003; 2:89–98Crossref, Medline, Google Scholar
63. Thomas AJ, Ferrier IN, Kalaria RN, Perry RH, Brown A, O’Brien JT: A neuropathological study of vascular factors in late-life depression. J Neurol Neurosurg Psychiatry 2001; 70:83–87Crossref, Medline, Google Scholar



