Smaller Cerebellar Vermis But Not Hemisphere Volumes in Patients With Chronic Schizophrenia
Abstract
OBJECTIVE: The authors previously reported that men with chronic schizophrenia had a smaller vermian subregion than did healthy men. In this study, they tested whether posterior superior vermis reduction would be seen in a larger group of schizophrenia patients, both male and female. METHOD: Brain volumetric analyses were performed with magnetic resonance imaging (MRI) in 59 male and female patients with chronic schizophrenia and 57 male and female healthy comparison subjects. RESULTS: The men as well as the women with schizophrenia had significantly smaller total vermis volume and smaller vermian subregions than did the healthy subjects. Total intracranial volume and cerebellar hemisphere volumes did not differ between schizophrenic and healthy subjects. CONCLUSIONS: The findings support the previous finding that in patients with chronic schizophrenia, there is a selective volume reduction of the cerebellar vermis within the cerebellum.
A growing literature supports the presence of cerebellar vermal abnormalities in patients with schizophrenia. In the majority of earlier studies, the area of the cerebellar vermis was measured only in the midsagittal plane (1–6). However, there have been several MRI volumetric studies of the vermis in schizophrenia (e.g., references 7, 8), and the results have been controversial, with increased (7), decreased (4, 9), or unchanged vermal volume reported in patients with schizophrenia relative to healthy subjects (8). The more recent studies have reported decreased volume of the cerebellar vermis in schizophrenia (e.g., references 10, 11). A previous study from our group showed that the volume of the posterior superior vermis was reduced in men with chronic schizophrenia (12). In none of the aforementioned studies were volumes of the vermian subregions measured separately in women with schizophrenia. To test the hypothesis that the volume of the vermis is reduced in both male and female patients with schizophrenia, we used magnetic resonance imaging (MRI) to measure and compare volumes of vermian subregions in men and women with schizophrenia and healthy men and women.
Method
The protocol was approved by the Institutional Review Board at the Karolinska Hospital, Stockholm. All subjects gave written informed consent.
Subject Characteristics
Fifty-nine patients (38 men and 21 women) with chronic schizophrenia according to DSM-IV criteria and 57 healthy volunteers (35 men and 22 women), all Caucasians, were included in this study (Table 1). All subjects lived within the area of northern Stockholm. They were recruited at the Karolinska Hospital Department of Clinical Neuroscience, where they were investigated between August 1999 and April 2002. At the time of the investigation, 37 of the male and 19 of the female patients were receiving antipsychotic medication. Dose of current neuroleptic treatment was converted into haloperidol units (13). All subjects were found to be healthy according to physical examination and blood and urinary tests. All subjects underwent a psychiatric interview (Structured Clinical Interview for DSM-III-R—Non-Patient Version) (14) to confirm schizophrenia in the patients and a lack of mental disorder in the comparison subjects. The interview was conducted by an experienced psychiatrist well trained in the administration of this instrument. Exclusion criteria for the healthy volunteers were current or past treatment for a psychiatric disorder or any history of alcohol or drug addiction, head trauma with loss of consciousness for more than 5 minutes, or severe somatic disorder.
Magnetic Resonance Scans
The subjects were examined with a 1.5-Tesla GE Signa (Milwaukee) system at the MR Research Center, Karolinska Hospital, Stockholm. T1-weighted images, using a spoiled gradient/recall acquisition in the steady state sequence, were acquired with the following parameters: 1.5-mm coronal slices, no gap, flip angle=35°, TR=24 msec, TE=6.0 msec, number of excitations=2, field of view=24 cm, acquisition matrix=256×192. T2-weighted images were acquired with the following parameters: 2.0-mm coronal slices, no gap, TE=84 msec, TR=6000 msec, number of excitations=2, field of view=24 cm, acquisition matrix=256×192. From visual inspection, all scans were judged to be excellent without any obvious motion artifact. We rated the scan according to motion artifact and did not find a difference between patients and comparison subjects. All scans were found to lack gross clinical pathology as evaluated by a neuroradiologist. The quantitative analysis was performed blind to subject diagnosis and gender until the final statistical analysis.
Automated Segmentation
MRI data analysis was performed with BRAINS software (15, 16). Detailed information about the segmentation procedure and evaluation of validity has been described by Harris et al. (17). Reproducibility and reliability of the segmentation procedure have also been ascertained previously (17, 18).
Tracing of Cerebellar Structures
The detailed delineation of the cerebellar structures was described in the previous report (12). The cerebellar vermis was manually parcellated into anterior vermis (lobules I–V), posterior superior vermis (lobules VI–VII), and posterior inferior vermis (lobules VIII–X) in sagittal planes on the segmented images (Figure 1). The lateral extent of the vermis was determined in the following way. The vermian subregions were delineated from the sagittal slice in which the vermian subregions appeared and until the slice where the anterior vermis, posterior superior vermis, or posterior inferior vermis disappeared. The outer contour of the total vermis on a coronal projection is shown in Figure 2.
Cerebellar hemispheres and cerebellar tonsils were traced manually on all sagittal slices. The volume estimate of the cerebellar hemispheres included the cerebellar hemispheres and the tonsils but not the vermis.
Statistical Analysis
Ten scans were randomly selected and evaluated blindly for inter- and intraoperator reliability. Intraclass correlation coefficients (19) were used to establish reliability to measure volumes of the cerebellar hemispheres, anterior vermis, posterior superior vermis, and posterior inferior vermis.
Absolute volume of cerebellar vermis was the volume of anterior vermis, posterior superior vermis, and posterior inferior vermis added together. Absolute volumes of cerebellar hemispheres, anterior vermis, posterior superior vermis, and posterior inferior vermis were independent factors. In the first analysis, we assessed diagnostic difference in absolute volumes of cerebellar hemispheres, anterior vermis, posterior superior vermis, and posterior inferior vermis by using the two-way multivariate analysis of variance (MANOVA), which included diagnosis, gender, and the diagnosis-by-gender interaction.
In the final analysis, the intracranial volume was used to correct for individual differences in head size. We assessed diagnostic differences for relative volumes (ratio of volume of interest to intracranial volume) of cerebellar subregions by using the two-way analysis of variance (ANOVA) model, which included diagnosis, gender, and the diagnosis-by-gender interaction. Differences were considered to be statistically significant if p<0.01 (i.e., 0.05/five regions of interest). Spearman’s correlation was used to test the correlation between volume measures and current dose of medication converted into haloperidol units or age or duration of illness in the patient group.
Results
Subject Characteristics
There were no significant differences in age, age at onset, duration of illness, or dose of medication between the subject groups (Table 1). There were significant differences in intracranial volume between male and female patients with schizophrenia (F=18.7, df=1, 57, p<0.001) and between male and female comparison subjects (F=18.0, df=1, 55, p<0.001). Intracranial volumes did not differ between schizophrenic and healthy male subjects (F=0.06, df=1, 71, p=0.80) or between schizophrenic and healthy female subjects (F=0.12, df=1, 41, p=0.73).
Reliability
Intraclass correlations (ICCs) were used to establish interoperator reliability for the manual tracing of the cerebellar subregions by two experienced operators (I.A. and G.O.) on 10 scans. Intraoperator (G.O.) reliability was established on another set of 10 scans. Excellent inter- and intraoperator reliabilities were seen for volume measurements of anterior vermis, posterior superior vermis, posterior inferior vermis, and cerebellar hemispheres (all ICCs >0.95).
Volume Measurement
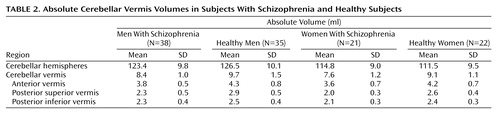
Table 2 presents the absolute volumes of cerebellar subregions in schizophrenic subjects and healthy subjects. Two-way MANOVA revealed that there was significant difference related to diagnosis (F=10.9, df=4, 109, p<0.001) and gender (F=8.2, df=4, 109, p<0.001), but there was no diagnosis-by-gender interaction (F=0.69, df=4, 109, p=0.60).
Table 3 presents the relative volumes of cerebellar subregions in schizophrenic subjects and healthy subjects. Significant differences were found for total vermis (F=26.3, df=1, 112, p<0.001), anterior vermis (F=13.5, df=1, 112, p<0.001), posterior superior vermis (F=38.7, df=1, 112, p<0.001), and posterior inferior vermis (F=9.04, df=1, 112, p=0.003), with smaller volumes seen in the schizophrenic subjects for both men and women. No significant difference related to diagnosis was found in the cerebellar hemisphere volumes. There were no gender differences for the relative volumes of cerebellar subregions. No significant gender-by-diagnosis interaction was found for any of the regions.
Absolute and relative volumes of the vermal subregions were not significantly correlated with current neuroleptic dosage. Neither was there any correlation between volume measures and age or duration of illness in the patient group.
Discussion
In the present material, both men and women with schizophrenia had significantly smaller relative volumes of the total vermis and the three subregions (anterior vermis, posterior superior vermis, and posterior inferior vermis) compared with healthy subjects. When we studied the absolute volumes, schizophrenic subjects had significantly smaller cerebellar subregions compared with healthy subjects, and female subjects had significantly smaller cerebellar subregions compared with male subjects. There were no significant correlations between current neuroleptic dosage and volumes of cerebellar subregions.
We previously reported that the volume of the posterior superior vermis was selectively reduced within the cerebellum of male subjects with schizophrenia. In the current study, with a larger number of subjects of both genders, we demonstrated that the volumes of all the vermis subregions are reduced and that the volume reduction was most prominent for the posterior superior vermis among the vermian subregions.
Volume reduction of the total vermis was found in 20 male subjects with schizophrenia (11). In another MRI study, Loeber et al. (10) described volume reduction of inferior vermis and total vermis in schizophrenic patients (15 men and four women) relative to the same number of healthy subjects. In this study, we found relative volume reduction of all the vermian subregions and total vermis within the cerebellum in female as well as male subjects with chronic schizophrenia.
In other MRI studies, structural changes of the cerebellar hemispheres were reported for patients with schizophrenia (7, 20, 21). Volume changes of the cerebellar hemispheres in schizophrenia were not found in another study, and this was also the case for the present results (4, 22). DeLisi et al. (23) found that the cerebellar hemispheric volumes become smaller over time in male and female subjects with schizophrenia relative to healthy subjects.
In conclusion, the result of the present study confirmed the hypothesis that within the cerebellum, the vermis volume is selectively reduced in male and female subjects with chronic schizophrenia. Since the volume measures were not correlated with neuroleptic dose, age of patients, or duration of disease, it seems possible that the vermian changes represent a significant feature of the pathophysiology in at least some patients with schizophrenia. Longitudinal studies are necessary to determine whether there is also a progressive change in the vermis.
 |
 |
 |
Presented in part at the seventh annual meeting of the Organization for Human Brain Mapping, Brighton, U.K., June 10–14, 2001; and the 11th congress of the Association of European Psychiatrists, Stockholm, May 4–8, 2002. Received Oct. 21, 2002; revision received March 11, 2003; accepted March 19, 2003. From the Department of Clinical Neuroscience, Human Brain Informatics Center (HUBIN), Karolinska Institute and Hospital, Stockholm; and the Department of Neuropsychiatry, Kansai Medical University. Address reprint requests to Dr. Okugawa, Department of Neuropsychiatry, Kansai Medical University, 10-15 Fumizonocho Moriguchi Osaka, 570-8506, Japan; [email protected] (e-mail). Supported by NIMH grant MH-44814 and by funding from the Wallenberg Foundation and the HUBIN project. The authors thank Lena Brandt and Kazuhiko Kuribayashi for statistical advice; Dr. Erik Jönsson for administering the Structured Clinical Interview for DSM-III-R; and Monica Hellberg for subject recruitment at HUBIN.
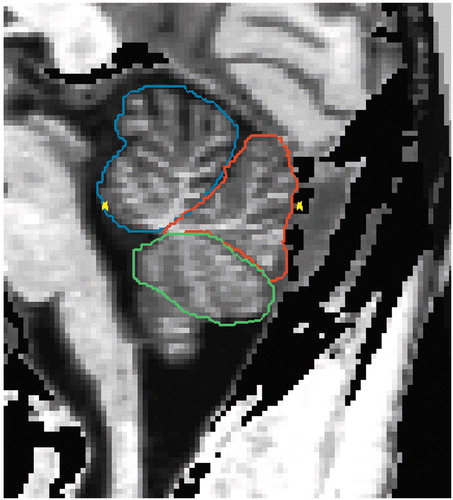
Figure 1. Sagittal MRI Slice of Cerebellar Vermis Subregionsa
aSubregions delineated are the anterior (blue), posterior superior (red), and posterior inferior (green) vermis.
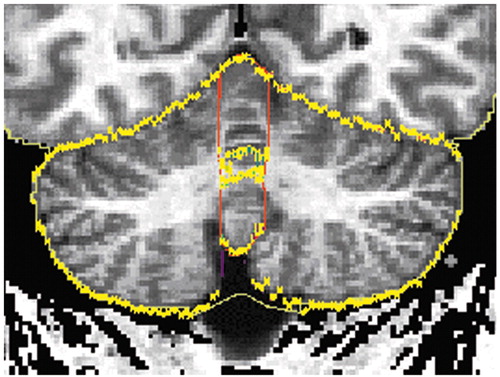
Figure 2. Coronal MRI Slice of the Total Vermisa
aThe outer contour of the total vermis is delineated in red. The yellow line delineates the intersections between the coronal plane and the traces of the cerebellar structures drawn in the sagittal projection (Figure 1).
1. Aylward EH, Reiss A, Barta PE, Tien A, Han W, Lee J, Pearlson GD: Magnetic resonance imaging measurement of posterior fossa structures in schizophrenia. Am J Psychiatry 1994; 151:1448–1452Link, Google Scholar
2. Mathew RJ, Partain CL: Midsagittal sections of the cerebellar vermis and fourth ventricle obtained with magnetic resonance imaging of schizophrenic patients. Am J Psychiatry 1985; 142:970–971Link, Google Scholar
3. Nasrallah HA, Schwarzkopf SB, Olson SC, Coffman JA: Perinatal brain injury and cerebellar vermal lobules I-X in schizophrenia. Biol Psychiatry 1991; 29:567–574Crossref, Medline, Google Scholar
4. Nopoulos PC, Ceilley JW, Gailis EA, Andreasen NC: An MRI study of cerebellar vermis morphology in patients with schizophrenia: evidence in support of the cognitive dysmetria concept. Biol Psychiatry 1999; 46:703–711Crossref, Medline, Google Scholar
5. Rossi A, Stratta P, Mancini F, de Cataldo S, Casacchia M: Cerebellar vermal size in schizophrenia: a male effect. Biol Psychiatry 1993; 33:354–357Crossref, Medline, Google Scholar
6. Uematsu M, Kaiya H: Cerebellar vermal size predicts drug response in schizophrenic patients: a magnetic resonance imaging (MRI) study. Prog Neuropsychopharmacol Biol Psychiatry 1988; 12:837–849Crossref, Medline, Google Scholar
7. Levitt JJ, McCarley RW, Nestor PG, Petrescu C, Donnino R, Hirayasu Y, Kikinis R, Jolesz FA, Shenton ME: Quantitative volumetric MRI study of the cerebellum and vermis in schizophrenia: clinical and cognitive correlates. Am J Psychiatry 1999; 156:1105–1107Abstract, Google Scholar
8. Sullivan EV, Deshmukh A, Desmond JE, Mathalon DH, Rosenbloom MJ, Lim KO, Pfefferbaum A: Contribution of alcohol abuse to cerebellar volume deficits in men with schizophrenia. Arch Gen Psychiatry 2000; 57:894–902Crossref, Medline, Google Scholar
9. Jacobsen LK, Giedd JN, Berquin PC, Krain AL, Hamburger SD, Kumra S, Rapoport JL: Quantitative morphology of the cerebellum and fourth ventricle in childhood-onset schizophrenia. Am J Psychiatry 1997; 154:1663–1669Link, Google Scholar
10. Loeber RT, Cintron CMB, Yurgelun-Todd DA: Morphometry of individual cerebellar lobules in schizophrenia. Am J Psychiatry 2001; 158:952–954Link, Google Scholar
11. Ichimiya T, Okubo Y, Suhara T, Sudo Y: Reduced volume of the cerebellar vermis in neuroleptic-naive schizophrenia. Biol Psychiatry 2001; 49:20–27Crossref, Medline, Google Scholar
12. Okugawa G, Sedvall G, Nordström M, Andreasen N, Pierson R, Magnotta V, Agartz I: Selective reduction of the posterior superior vermis in men with chronic schizophrenia. Schizophr Res 2002; 55:61–67Crossref, Medline, Google Scholar
13. American Psychiatric Association: Practice Guideline for the Treatment of Patients With Schizophrenia. Am J Psychiatry 1997; 154(April suppl)Google Scholar
14. Spitzer RL, Williams JBW, Gibbon M, First MB: Structured Clinical Interview for DSM-III-R—Non-Patient Version (SCID-NP). New York, New York State Psychiatric Institute, Biometrics Research, 1989Google Scholar
15. Andreasen NC, Cizadlo T, Harris G, Swayze V II, O’Leary DS, Cohen G, Ehrhardt J, Yuh WT: Voxel processing techniques for the antemortem study of neuroanatomy and neuropathology using magnetic resonance imaging. J Neuropsychiatry Clin Neurosci 1993; 5:121–130Crossref, Medline, Google Scholar
16. Andreasen NC, Cohen G, Harris G, Cizadlo T, Parkkinen J, Rezai K, Swayze VW II: Image processing for the study of brain structure and function: problems and programs. J Neuropsychiatry Clin Neurosci 1992; 4:125–133Crossref, Medline, Google Scholar
17. Harris G, Andreasen NC, Cizadlo T, Bailey JM, Bockholt HJ, Magnotta VA, Arndt S: Improving tissue classification in MRI: a three-dimensional multispectral discriminant analysis method with automated training class selection. J Comput Assist Tomogr 1999; 23:144–154Crossref, Medline, Google Scholar
18. Agartz I, Okugawa G, Greiz D, Nordström M, Greitz D, Magnotta V, Sedvall G: Reliability and reproducibility of brain tissue volumetry from segmented MR scans. Eur Arch Psychiatry Clin Neurosci 2001; 251:255–261Crossref, Medline, Google Scholar
19. Shrout P, Fleiss J: Intraclass correlations: uses in assessing rater reliability. Psychol Bull 1979; 86:420–429Crossref, Medline, Google Scholar
20. Flaum M, Swayze VW II, O’Leary DS, Yuh WTC, Ehrhardt JC, Arndt SV, Andreasen NC: Effects of diagnosis, laterality, and gender on brain morphology in schizophrenia. Am J Psychiatry 1995; 152:704–714Link, Google Scholar
21. Gaser C, Volz HP, Kiebel S, Riehemann S, Sauer H: Detecting structural changes in whole brain based on nonlinear deformations—application to schizophrenia research. Neuroimage 1999; 10:107–113Crossref, Medline, Google Scholar
22. Andreasen NC, Flashman L, Flaum M, Arndt S, Swayze V II, O’Leary DS, Ehrhardt JC, Yuh WTC: Regional brain abnormalities in schizophrenia measured with magnetic resonance imaging. JAMA 1994; 272:1763–1769Crossref, Medline, Google Scholar
23. DeLisi LE, Sakuma M, Tew W, Kushner M, Hoff AL, Grimson R: Schizophrenia as a chronic active brain process: a study of progressive brain structural change subsequent to the onset of schizophrenia. Psychiatry Res 1997; 74:129–140Crossref, Medline, Google Scholar



