Familial Aggregation of Delusional Proneness in Schizophrenia and Bipolar Pedigrees
Abstract
OBJECTIVE: Clinical, familial, and, more recently, genetic linkage studies suggest that overlapping genetic susceptibility might contribute to both schizophrenia and bipolar disorder. To identify a potential psychotic dimension common to families of both bipolar and schizophrenia probands, the authors tested if delusional proneness was observed among first-degree relatives of bipolar and schizophrenia probands. METHOD: The authors included 32 schizophrenia probands and 61 bipolar probands and their respective first-degree relatives (N=63 and N=59). They were all interviewed with the Diagnostic Interview for Genetic Studies, and delusional proneness was assessed with a self-report questionnaire, the Peters et al. Delusions Inventory. Schizophrenia and bipolar probands were subdivided into subgroups according to the intensity of delusional symptoms assessed by Peters et al. Delusions Inventory scores, and the authors compared delusional proneness in their respective first-degree relatives. RESULTS: Familial aggregation of delusional proneness was demonstrated, since Peters et al. Delusions Inventory scores were higher among nonschizophrenic first-degree relatives of schizophrenia probands with productive symptoms and among first-degree relatives of bipolar probands with psychotic features during their affective episodes. The authors also found an intrafamilial correlation of delusional proneness scores in nonaffected siblings of schizophrenia and bipolar probands. CONCLUSIONS: Delusional proneness appears to be an inherited predisposition common to both schizophrenia and bipolar disorder. In the future, this dimension might be valuable when used as a quantitative phenotype in linkage and association studies.
Ambiguities in identifying phenotypes may be the rate-limiting step in psychiatric genetic studies (1). Consequently, new strategies are being tested to identify elemental components of the phenotype more closely related to susceptibility alleles than are complex clinical phenotypes, such as schizophrenia or bipolar disorders. These intermediate phenotypes might have a simple genetic architecture, and if so, they could be used to enhance the power of linkage and association studies of complex disorders. Intermediate phenotypes may also help solve the problem of the threshold definition of spectrum disorders found either in relatives of schizophrenia probands (i.e., with schizophreniform disorder, schizoaffective disorder, and schizotypal personality disorder) or in relatives of bipolar probands (i.e., with unipolar depression, major depressive episodes, cyclothymic and hyperthymic temperaments). In recent years, improved diagnostic tools have stimulated the search for quantitative phenotypes among patients and relatives. In particular, negative symptoms that represent deficiencies in normal behavior (such as flat affect and social withdrawal) and positive symptoms that represent behavioral excesses (e.g., delusions and hallucinations) have emerged as likely candidates for family studies mostly in schizophrenia but also in affective disorders. The Scale for the Assessment of Negative Symptoms (SANS) and the Scale for the Assessment of Positive Symptoms (SAPS) are commonly used to rate these symptoms (2). Negative symptoms appear to be more stable over time than positive symptoms (3) and seem to be the main source of familial aggregation in schizophrenia. Negative symptoms have been shown to be correlated between pairs concordant for schizophrenia (4), and they appear to be correlated with a positive family history of schizophrenia (5, 6). More precisely, we have demonstrated the existence of a subform of schizophrenia characterized by highly anhedonic schizophrenia probands with both a three times higher familial risk for schizophrenia spectrum disorders and a high level of anhedonia among their first-degree relatives (6). Using the SAPS and the SANS, Tsuang (1) showed that negative symptoms ratings are higher for the relatives of schizophrenia probands, whereas positive symptoms were similar among the relatives of schizophrenia patients and depressive control subjects. These findings suggest that negative symptoms could reflect familial liability to schizophrenia, whereas positive symptoms could reflect a clinical endophenotype common both to affective disorders and to schizophrenia. Indeed, family studies suggest shared liability between schizophrenia and affective disorders. Three data sets (7–9) have shown a higher than comparison rate of psychotic affective disorders in the relatives of schizophrenia probands, and Potash et al. (10) showed a familial aggregation of psychotic symptoms in affected relatives of bipolar I patients. Genetic linkage studies have also revealed an overlap between bipolar disorders and schizophrenia in four chromosomal regions (10p12–13, 13q32, 18p11.2, 22q11–13) (11). Thus, there may be a shared phenotype common to bipolar disorder and schizophrenia, and this common phenotype may be part of a positive symptom profile. Brzustowicz et al. (12) provided the first evidence of the value of using positive symptoms in linkage studies in multiplex schizophrenia families. Positive linkage with chromosome 6p markers was obtained only when using scores for positive symptoms as the phenotype among both schizophrenia patients and their nonaffected relatives, while negative linkage results were obtained with negative symptom scores or with a classical nosographical approach.
Clinical, familial, and genetic linkage studies have renewed interest in the hypothesis that vulnerability genes might contribute to a psychotic dimension, such as delusional proneness, common to both schizophrenia and bipolar disorder. Several issues remain unresolved:
| 1. | The evidence for overlapping liability between affective disorders and schizophrenia has been investigated, but not that between bipolar disorders per se and schizophrenia. | ||||
| 2. | The relatives included in the majority of family studies had diagnoses of affective disorders or schizophrenia spectrum disorders. Therefore, the familial aggregation observed may simply be the consequence of the ongoing disorder and consequently cannot be considered an endophenotype. | ||||
| 3. | The measurements used to assess positive symptoms have been either an evaluation of symptoms (7, 8, 10) or a quantitative assessment with instruments such as the SAPS. These measures were created and validated to be used among affected patients but not among nonaffected subjects. | ||||
In order to demonstrate the presence of an underlying psychotic dimension common to relatives of bipolar and/or schizophrenic patients, we used the Peters et al. Delusions Inventory (13), an instrument created to investigate delusional proneness in a normal population, a group of schizophrenia subjects, bipolar subjects, and their respective first-degree relatives.
The aim of our study was to assess the familiality of delusional proneness and to determine whether psychosis proneness is familial, i.e., more prevalent among nonschizophrenic first-degree relatives of schizophrenia probands with high levels of positive symptoms and/or among first-degree relatives of bipolar probands with psychotic features during episodes.
Method
Subjects
Probands suffering from bipolar disorder or schizophrenia were recruited from consecutive admissions to university-affiliated hospitals (Pitié-Salpêtrière and Albert Chenevier Hospitals, Paris). They were included in the study just before discharge. The patients had to meet DSM-IV criteria for either bipolar disorder or schizophrenia. To confirm the diagnosis among probands, the patients were directly interviewed by an experienced psychiatrist (F.S., A.S., or F.B.) with the French version of the Diagnostic Interview for Genetic Studies (14). After this interview, the bipolar probands were classified as subjects with or without psychotic symptoms defined by the presence of hallucinations and delusions, both mood-congruent and incongruent during manic or depressive episodes. The schizophrenia probands were classified into schizophrenia subtypes, i.e., paranoid, disorganized, catatonic, undifferentiated, or residual type according to DSM-IV criteria. Subtypes were attributed according to clinical case notes and narratives by psychiatrists (A.M. and A.S.) who were themselves blind to the results of the self-rating questionnaires completed by the probands or relatives regarding the existence of delusions or hallucinations.
First-degree relatives of the patients were contacted and asked to participate in the study. The relatives were also interviewed with the Diagnostic Interview for Genetic Studies to exclude the diagnosis of schizophrenia among the relatives of the schizophrenia probands and of bipolar disorder among the relatives of the bipolar probands. The information was supplemented, if required, with medical case notes for the probands and relatives.
To measure delusional ideation in the different groups, we used the French translation of a self-rating questionnaire, the Peters et al. Delusions Inventory (15). This self-rating questionnaire was created to assess lifetime delusional ideation in a normal population. This questionnaire assesses on a lifetime basis a wide range of delusions of attenuated severity with a dimensional approach. The internal consistency, concurrent validity, and criterion validity of the French version of the Peters et al. Delusions Inventory were established previously (15).
The Peters et al. Delusions Inventory is composed of 21 items derived from items used in the Present State Examination (16). The total score is obtained by summing the number of positive answers (maximum score=21). In addition, three questions assess auditory hallucinations (verbal hallucinations, voices conversing, and imperative hallucinations), and a question asks whether the subject has ever experienced phenomena described in the questionnaire when under the influence of drugs.
To be included in the study, the subjects (the patients and relatives) had to be normothymic as evaluated by a Montgomery-Åsberg Depression Rating Scale score ≤5 (17) and a Bech-Rafaelsen Mania Scale score ≤5 (18).
The research ethics board of Salpêtrière Hospital reviewed and approved the study. After complete description of the study to the subjects, written informed consent was obtained.
Data Analysis
Differences between groups were tested by using a two-tailed t test or analysis of variance (ANOVA) for continuous variables and a chi-square test for discrete variables. Scores on the Peters et al. Delusions Inventory were found to be nonnormally distributed, and therefore, nonparametric tests were used for group comparisons: the Mann-Whitney U test (for two groups) or the Kruskall-Wallis H test (for more than two groups). Spearman’s correlations were used to examine relationships between scores on the Peters et al. Delusions Inventory in the probands and in their respective first-degree relatives (to determine whether the score for the probands can predict the scores for their respective first-degree relatives). In a second stage, to study the intrafamilial correlation for delusional ideation, we used the intraclass correlation method with the Peters et al. Delusions Inventory score as a continuous variable. We tested for the existence of an intrafamilial correlation for delusional ideation with the intraclass correlation method (19).
Results
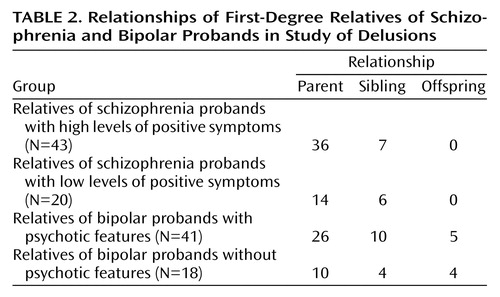
The group studied was composed of 32 schizophrenia probands (19 men and 13 women, mean age=32.2 years, SD=9.6), 61 bipolar patients (26 men and 35 women, mean age=41.2 years, SD=12.3), 63 first-degree relatives of the schizophrenia probands (25 men and 38 women, mean age=51.9 years, SD=14.5), and 59 first-degree relatives of the bipolar probands (33 men and 26 women, mean age=51.3 years, SD=16.5) (Table 1 and Table 2). The sex ratio did not differ between the groups (χ2=5.59, df=3, p=0.13). The schizophrenia probands were younger at the time of the interviews than the bipolar probands (t=3.56, df=91, p=0.0006), who were in turn younger at the interviews than the two groups of first-degree relatives (F=10.47, df=1, 2, p<0.0001, ANOVA). The two groups of first-degree relatives did not differ in age at the interviews (t=0.22, df=113, p=0.82). All of the subjects (probands and relatives) were euthymic at the time of the study, with scores ≤5 on the Montgomery-Åsberg Depression Rating Scale and the Bech-Rafaelsen Mania Scale. Thirty of the 61 bipolar probands did not have psychotic features during affective episodes, and 31 displayed psychotic features (Table 1). The schizophrenia probands were classified as paranoid (N=11), disorganized (N=14), undifferentiated (N=7), catatonic (N=0), and residual (N=0), according to DSM-IV subtype criteria.
Ratings for each item on the Peters et al. Delusions Inventory by the four groups of subjects are given in Table 3. The mean total Peters et al. Delusions Inventory score was higher for the schizophrenia probands than for the bipolar probands (mean=9.3, SD=4.6, versus mean=7.1, SD=3.9, respectively) (Mann-Whitney U=719, df=1, p=0.03). The Peters et al. Delusions Inventory scores for the bipolar subjects were higher than the scores for the two groups of first-degree relatives (Kruskall-Wallis H=26.79, df=2, p<0.0001). There was no significant difference between the scores on the Peters et al. Delusions Inventory for the first-degree relatives of the schizophrenia probands (mean=4.0, SD=2.9) and the first-degree relatives of the bipolar probands (mean=4.2, SD=3.4) (Mann-Whitney U=1812, df=1, p=0.81) (Table 3).
By subdividing the bipolar probands with (N=31) from those without (N=30) at least one thymic episode with psychotic features, we found that the bipolar subjects with psychotic features had a higher mean score on the Peters et al. Delusions Inventory than the bipolar probands without psychotic features (mean=8.5, SD=4.0, versus mean=5.7, SD=3.3) (Mann-Whitney U=274, df=1, p=0.006). Second, we demonstrated that the first-degree relatives of the bipolar subjects with psychotic features (N=41) had a higher mean score on the Peters et al. Delusions Inventory than the first-degree relatives of the bipolar subjects without psychotic features (N=18) (mean=4.8, SD=3.7, versus mean=2.7, SD=2.3) (Mann-Whitney U=245, df=1, p=0.04). These two subgroups of first-degree relatives of bipolar probands did not differ in terms of sex ratio (χ2=1.21, df=1, p=0.27), age at interview (t=1.08, df=51, p=0.28), or generation (χ2=0.97, df=2, p=0.61) (Table 1 and Table 2).
Similarly, we subdivided the schizophrenia subjects according to their DSM-IV subtype (i.e., paranoid [N=11], disorganized [N=14], catatonic [N=0], undifferentiated [N=7], and residual [N=0]) and considered the paranoid and undifferentiated types as those displaying strong positive symptoms and the disorganized type as those with few or weak positive symptoms. The validity of this dichotomy was confirmed by the fact that the schizophrenia subjects with severe positive symptoms had a higher mean score on the Peters et al. Delusions Inventory than the schizophrenia subjects with weak positive symptoms (mean=10.7, SD=4.2, versus mean=7.5, SD=4.6) (Mann-Whitney U=179, df=1, p=0.04). Moreover, the comparison of the mean scores on the Peters et al. Delusions Inventory between their respective first-degree relatives revealed that the first-degree relatives of paranoid and undifferentiated schizophrenia probands (N=43) had higher mean scores on the Peters et al. Delusions Inventory than the first-degree relatives of the schizophrenia subjects with other schizophrenia subtypes (N=20) (mean=4.6, SD=3.2, versus mean=2.8, SD=1.8) (Mann-Whitney U=566, df=1, p=0.04). The two subgroups of first-degree relatives of schizophrenia probands did not differ in terms of sex ratio (χ2=0.34, df=1, p=0.55), age at interview (t=1.76, df=61, p=0.08), or generation (χ2=1.57, df=1, p=0.21) (Table 1 and Table 2).
Furthermore, we also found a significant correlation between the scores on the Peters et al. Delusions Inventory for the probands and the first-degree relatives (rs=0.24, z=2.17, p=0.03).
In order to confirm the familial nature of delusional ideation, we calculated an intrafamilial correlation of delusional ideation using the score on the Peters et al. Delusions Inventory for all the sibling pairs of the relatives within the unaffected sibships. We excluded all probands from the analysis because their scores on the Peters et al. Delusions Inventory could be modified by the disease itself and/or by medication. We performed the analysis only in sibling pairs because siblings share more common environmental factors than siblings and parents. This analysis was carried out on 16 unaffected sibling pairs. Within sibling pairs of relatives, scores on the Peters et al. Delusions Inventory showed a clear intrafamilial resemblance (rs=0.46, F=5.27, df=1, 15, p=0.02, ANOVA).
Discussion
This study demonstrated familial aggregation of delusional proneness assessed with a dimensional self-report questionnaire (the Peters et al. Delusions Inventory) in a group of schizophrenia and bipolar patients and their respective first-degree relatives. We showed that delusional proneness scores are higher, specifically, among nonschizophrenic first-degree relatives of schizophrenia probands with productive symptom profiles and among first-degree relatives of bipolar probands with psychotic features during episodes. The extent of delusional proneness in bipolar or schizophrenia probands predicts that symptom in their respective first-degree relatives. In addition, we found an intrafamilial correlation of the scores for delusional proneness among nonaffected siblings of schizophrenia and bipolar probands. This indicates that delusional proneness reflects a clinical endophenotype common to subgroups with bipolar disorder and schizophrenia.
Bipolar probands who display psychotic features during manic or depressive episodes had unaffected relatives with high delusional proneness. This confirms and extends previous studies among bipolar families showing that probands with psychotic symptoms have more first-degree relatives with psychotic affective disorders (10). These findings suggest that psychotic bipolar disorder may constitute a subtype of value for genetic investigations.
We report similar findings for first-degree relatives of delusional schizophrenia probands who also have high scores for delusional proneness. This confirms the previous report that relatives of schizophrenia patients are at higher than normal risk for schizophrenia spectrum disorders (8, 20) and have elevated ratings for negative symptoms (1, 21). However, our study is the first to our knowledge to investigate the familiality of psychosis proneness among relatives after subdividing schizophrenia probands into groups with positive or negative symptom profiles. Discrepancies between studies assessing subclinical profiles among relatives of schizophrenia patients may be due to differences in assessment procedures and to inclusion criteria for relatives and schizophrenia probands. In the literature, positive symptoms have been measured in relatives with clinical interviews assessing symptoms, a self-report questionnaire for schizotypy (22–26), and clinical scales such as the SAPS (1). These instruments are not suitable for nonclinical populations, are not based on a lifetime assessment, and measure a large range of symptoms that do not belong to a particular clinical dimension. Here, using the Peters et al. Delusions Inventory in two groups of patients and their first-degree relatives, we demonstrated that proneness to delusional symptoms is a valuable endophenotype common to both schizophrenia and bipolar disorders. As expected, the total score on the Peters et al. Delusions Inventory was significantly higher for schizophrenia patients and (to a lesser degree) for bipolar subjects than for the two groups of first-degree relatives. This confirms that the Peters et al. Delusions Inventory is a good instrument for identifying the delusional components of a psychiatric disorder. The total scores on the Peters et al. Delusions Inventory for the two groups of first-degree relatives were similar to those reported by Verdoux et al. (15) for subjects with no identified psychiatric disorders tested with the French validation of the Peters et al. Delusions Inventory.
To further explore the familiality of delusional proneness, we showed first that scores on the Peters et al. Delusions Inventory are highly correlated between unaffected siblings of both bipolar and schizophrenia probands and second that the Peters et al. Delusions Inventory score for the affected probands predicts the scores of their relatives. The familiality of schizophrenia symptoms was previously investigated by Loftus et al. (27) in schizophrenia sibling pairs who showed the resemblance of first-rank symptoms, particularly thought insertion, thought broadcasting, thought withdrawal, and delusions of control. A quantitative measure of delusional proneness instead of a categorical approach has already proved fruitful in linkage studies of schizophrenia. Brzustowicz et al. (12) found that a schizophrenia susceptibility locus on chromosome 6 was related to severity of positive psychotic symptoms (evaluated with the SAPS), whereas in the same families, using a categorical disease definition, they failed to produce significant evidence for linkage.
The elevated Peters et al. Delusions Inventory scores for first-degree relatives of both productive schizophrenia patients and psychotic bipolar probands suggest that delusional proneness is an endophenotype common to schizophrenia and bipolar disorders or common only to a subgroup of bipolar and schizophrenia probands with productive symptom profiles. Our results suggest that there may be a continuum of vulnerability across affective disorders and nonaffective psychotic disorders and that psychosis proneness may be one of the markers of this shared liability. Commonalties between schizophrenia and bipolar disorder have already been demonstrated in biological, cognitive, and brain imaging studies. For example, abnormalities in glutamatergic neurotransmission have been shown in both disorders (28, 29). Both schizophrenia and remitted bipolar patients have deficits in attention tasks such as the Continuous Performance Test and in working memory and executive functions, such as those assessed by the Wisconsin Card Sorting Test (30–32). Other similarities between psychotic bipolar subjects and schizophrenia patients have been shown by brain imaging studies, in particular, abnormally low volumes of similar regions: the left hippocampus (33) and the left posterior amygdala-hippocampal complex (34). Functional imaging demonstrated high dopamine D2 receptor densities only in psychotic bipolar patients (but not in nonpsychotic bipolar patients), similar to the densities in schizophrenia patients (35).
We found evidence of familial aggregation of delusional proneness in both patients with schizophrenia and patients with bipolar disorder. These findings indicate that positive symptoms are not restricted to nosographical entities but may represent a continuum between these two diagnostic entities. It is noteworthy that several studies (36–38) have not shown an excess of schizophrenia relatives in the families of probands with bipolar disorder or an excess of relatives with bipolar disorder in the families of probands with schizophrenia. Nevertheless, partial overlap between bipolar disorder and schizophrenia is also supported by a latent class analysis of clinical characteristics of mood disorders and schizophrenia that identified six classes of nosological constructs with different familial vulnerability (39). This approach is not consistent either with the continuum model of psychosis of Crow (40) or with the dichotomous structure first suggested by Kraepelin, who wrote later in life, “It is becoming increasingly clear that we cannot distinguish satisfactorily between these two illnesses, and this brings home the suspicion that our formulation of the problem may be incorrect” (41). Although the small group we studied here makes it impossible to draw firm conclusions, the data provide preliminary information about the possible existence of a common inherited predisposition to both schizophrenia and bipolar disease, namely delusional proneness. Future studies should also look for delusional proneness in the relatives of those with nonaffective psychoses (schizoaffective, schizophreniform, delusional, and atypical psychoses) as well as bipolar II and major depression with psychotic features. This dimension could be valuable when used in the future as a quantitative phenotype in linkage and association studies in both schizophrenia and bipolar disorders.
 |
 |
 |
Received July 15, 2002; revision received Nov. 25, 2002; accepted Dec. 2, 2002. From the Service de Psychiatrie Adulte, Hôpital Albert Chenevier et Henri Mondor (Assistance Publique-Hôpitaux de Paris); the Institut National de la Sauté et de la Recherche Médicale, Unité 513, Neurobiologie et Psychiatrie, Hôpital Henri Mondor; and the Psychiatric and Neurodevelopmental Genetics Unit, Massachusetts General Hospital, Harvard Medical School, Charlestown, Mass. Address reprint requests to Dr. Schürhoff, Service de Psychiatrie Adulte du Pr. Rouillon, Hôpital Albert Chenevier, 40 rue Mesly, 94000 Créteil, France; [email protected] (e-mail). Supported by grants from the Assistance Publique-Hôpitaux de Paris (PHRC AOM98152), the Ministère de la Recherche (Cognitique), the Fondation pour la Recherche Médicale (to Drs. Szöke and Méary), and the Institut National de la Santé et de la Recherche Médicale. The authors thank Prof. Helene Verdoux for providing the Peters et al. Delusions Inventory.
1. Tsuang M: Genotypes, phenotypes and the brain: a search for connections in schizophrenia. Br J Psychiatry 1993; 163:299-307Crossref, Medline, Google Scholar
2. Andreasen NC, Olsen S: Negative v positive schizophrenia: definition and validation. Arch Gen Psychiatry 1982; 39:789-794Crossref, Medline, Google Scholar
3. Addington J, Addington D: Positive and negative symptoms of schizophrenia: their course and relationship over time. Schizophr Res 1991; 5:51-59Crossref, Medline, Google Scholar
4. Dworkin RH, Lenzenweger MF: Symptoms and the genetics of schizophrenia: implications for diagnosis. Am J Psychiatry 1984; 141:1541-1546Link, Google Scholar
5. Sautter FJ, McDermott DE, Garver DL: Familial and social determinants of outcome, in 1987 Annual Meeting New Research Program and Abstracts. Washington, DC, American Psychiatric Association, 1987Google Scholar
6. Schürhoff F, Szöke A, Bellivier F, Turcas C, Villemur M, Tignol J, Rouillon F, Leboyer M: Anhedonia in schizophrenia: a distinct clinical subtype? Schizophr Res (in press)Google Scholar
7. Kendler KS, Gruenberg AM, Tsuang MT: A DSM-III family study of the nonschizophrenic psychotic disorders. Am J Psychiatry 1986; 143:1098-1105Link, Google Scholar
8. Kendler KS, McGuire M, Gruenberg AM, O’Hare A, Spellman M, Walsh D: The Roscommon Family Study, I: methods, diagnosis of probands, and risk of schizophrenia in relatives. Arch Gen Psychiatry 1993; 50:527-540Crossref, Medline, Google Scholar
9. Erlenmeyer-Kimling L, Adamo UH, Rock D, Roberts SA, Basset AS, Squires-Wheelers E, Cornblatt BA, Endicott J, Pape S, Gottesman II: The New York High-Risk Project: prevalence and comorbidity of axis I disorders in offspring of schizophrenic parents at 25-year follow-up. Arch Gen Psychiatry 1997; 54:1096-1102Crossref, Medline, Google Scholar
10. Potash JB, Willour VL, Chiu Y-F, Simpson SG, MacKinnon DF, Pearlson GD, DePaulo JR Jr, McInnis MG: The familial aggregation of psychotic symptoms in bipolar disorder pedigrees. Am J Psychiatry 2001; 158:1258-1264Link, Google Scholar
11. Berettini WH: Susceptibility loci for bipolar disorder: overlap with inherited vulnerability to schizophrenia. Biol Psychiatry 2000; 47:245-251Crossref, Medline, Google Scholar
12. Brzustowicz LM, Honer WG, Chow EW, Hogan J, Hodgkinson K, Basset AS: Use of a quantitative trait to map a locus associated with severity of positive symptoms in familial schizophrenia to chromosome 6p. Am J Hum Genet 1997; 61:1388-1396Crossref, Medline, Google Scholar
13. Peters ER, Joseph SA, Garety PA: Measurement of delusional ideation in the normal population: introducing the PDI (Peters et al Delusions Inventory). Schizophr Bull 1999; 25:553-576Crossref, Medline, Google Scholar
14. Nurnberger JI Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T (NIMH Genetics Initiative): Diagnostic Interview for Genetic Studies: rationale, unique features, and training. Arch Gen Psychiatry 1994; 51:849-859Crossref, Medline, Google Scholar
15. Verdoux H, Van Os J, Maurice-Tison S, Gay B, Salamon R, Bourgeois M: Is early adulthood a critical development stage for psychosis proneness? a survey of delusional ideation in normal subjects. Schizophr Res 1998; 29:247-254Crossref, Medline, Google Scholar
16. Wing JK, Cooper JE, Sartorius N: The Measurement and Classification of Psychiatric Symptoms: An Instructional Manual for the PSE and CATEGO Programs. New York, Cambridge University Press, 1974Google Scholar
17. Montgomery SA, Åsberg M: A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134:382-389Crossref, Medline, Google Scholar
18. Bech P, Rafaelsen OJ, Kramp P, Bolwig TG: The Mania Rating Scale: scale construction and inter-observer agreement. Neuropharmacology 1978; 17:430-431Crossref, Medline, Google Scholar
19. Fischer RA: Statistical Methods for Research Workers. Edinburgh, Oliver and Boyd, 1925Google Scholar
20. Baron M, Gruen R, Rainer JD, Kane J, Asnis L, Lord S: A family study of schizophrenic and normal control probands: implications for the spectrum concept of schizophrenia. Am J Psychiatry 1985; 142:447-455Link, Google Scholar
21. Fanous A, Gardner C, Walsh D, Kendler KS: Relationship between positive and negative symptoms of schizophrenia and schizotypal symptoms in nonpsychotic relatives. Arch Gen Psychiatry 2001; 58:669-673Crossref, Medline, Google Scholar
22. Lenzenweger MF, Loranger AW: Detection of familial schizophrenia using a psychometric measure of schizotypy. Arch Gen Psychiatry 1989; 46:902-907Crossref, Medline, Google Scholar
23. Lenzenweger MF, Loranger AW: Psychosis proneness and clinical psychopathology: examination of the correlates of schizotypy. J Abnorm Psychol 1989; 98:3-8Crossref, Medline, Google Scholar
24. Clementz BA, Grove WM, Katsanis J, Iacono WG: Psychometric detection of schizotypy: perceptual aberration and physical anhedonia in relatives of schizophrenics. J Abnorm Psychol 1991; 100:607-612Crossref, Medline, Google Scholar
25. Grove WM, Lebow BS, Clementz BA, Cerri A, Medus C, Iacono WG: Familial prevalence and coaggregation of schizotypy indicators: a multitrait family study. J Abnorm Psychol 1991; 100:115-121Crossref, Medline, Google Scholar
26. Katsanis J, Iacono WG, Beiser M: Anhedonia and perceptual aberration in first-episode psychotic patients and their relatives. J Abnorm Psychol 1990; 99:202-206Crossref, Medline, Google Scholar
27. Loftus J, DeLisi LE, Crow TJ: Factor structure and familiality of first-rank symptoms in sibling pairs with schizophrenia and schizoaffective disorder. Br J Psychiatry 2000; 177:15-19Crossref, Medline, Google Scholar
28. Grimwood S, Slater P, Deakin JF, Hutson PH: NR2B-containing NMDA receptors are up-regulated in temporal cortex in schizophrenia. Neuroreport 1999; 25:461-465Crossref, Google Scholar
29. McCullumsmith RE, Meador-Woodruff JH: Striatal excitatory amino acid transporter transcript expression in schizophrenia, bipolar disorder, and major depressive disorder. Neuropsychopharmacology 2002; 26:368-375Crossref, Medline, Google Scholar
30. Addington J, Addington D: Attentional vulnerability indicators in schizophrenia and bipolar disorder. Schizophr Res 1997; 23:197-204Crossref, Medline, Google Scholar
31. Rund BR, Orbeck AL, Landro NI: Vigilance deficits in schizophrenics and affectedly disturbed patients. Acta Psychiatr Scand 1992; 86:207-212Crossref, Medline, Google Scholar
32. Sereno AB, Holtzman PS: Spatial selective attention in schizophrenic, affective disorder and normal subjects. Schizophr Res 1996; 20:33-50Crossref, Medline, Google Scholar
33. Velakoulis D, Pantelis C, McGorry PD, Dudgeon P, Brewer W, Cook M, Desmond P, Bridle N, Tierney P, Murrie V, Singh B, Copolov D: Hippocampal volume in first-episode psychosis and chronic schizophrenia: a high-resolution magnetic resonance imaging study. Arch Gen Psychiatry 1999; 56:133-141Crossref, Medline, Google Scholar
34. Hirayasu Y, Shenton ME, Salisbury DF, Dickey CC, Fischer IA, Mazzoni P, Kisler T, Arakaki H, Kwon JS, Anderson JE, Yurgelun-Todd D, Tohen M, McCarley R: Lower left temporal lobe MRI volumes in patients with first-episode schizophrenia compared with psychotic patients with first-episode affective disorder and normal subjects. Am J Psychiatry 1998; 155:1384-1391Link, Google Scholar
35. Pearlson GD, Wong DF, Tune LE, Ross CA, Chase GA, Links JM, Dannals RF, Wilson AA, Ravert HT, Wagner HNJ: In vivo D2 dopamine receptor density in psychotic and nonpsychotic patients with bipolar disorder. Arch Gen Psychiatry 1995; 52:471-477Crossref, Medline, Google Scholar
36. Gershon ES, Hamovit J, Guroff JJ, Dibble E, Leckman JF, Sceery W, Targum SD, Nurnberger JI Jr, Goldin LR, Bunney WE Jr: A family study of schizoaffective, bipolar I, bipolar II, unipolar and normal control probands. Arch Gen Psychiatry 1982; 39:1157-1167Crossref, Medline, Google Scholar
37. Gershon ES, DeLisi LE, Hamovit J, Nurnberger JI Jr, Maxwell ME, Schreiber J, Dauphinais D, Dingman CW, Guroff JJ: A controlled family study of chronic psychoses: schizophrenia and schizoaffective disorder. Arch Gen Psychiatry 1988; 45:328-336Crossref, Medline, Google Scholar
38. Maier W, Lichtermann D, Minges J, Hallmayer J, Heun R, Benkert O, Levinson DF: Continuity and discontinuity of affective disorders and schizophrenia: results of a controlled family study. Arch Gen Psychiatry 1993; 50:871-883Crossref, Medline, Google Scholar
39. Kendler KS, Karkowski LM, Walsh D: The structure of psychosis: latent class analysis of probands from the Roscommon Family Study. Arch Gen Psychiatry 1998; 55:492-499Crossref, Medline, Google Scholar
40. Crow TJ: The continuum of psychosis and its genetic origins: the sixty-fifth Maudsley Lecture. Br J Psychiatry 1990; 156:788-797Crossref, Medline, Google Scholar
41. Kraepelin E: Der Erscheinungsformen der Irreseins. Zeitschrift fur Gesammte Neurologia und Psychiatrie 1920; 62:1-29Crossref, Google Scholar



