Impact of Antidepressant Discontinuation After Acute Bipolar Depression Remission on Rates of Depressive Relapse at 1-Year Follow-Up
Abstract
OBJECTIVE: While guidelines for treating patients with bipolar depression recommend discontinuing antidepressants within 6 months after remission, few studies have assessed the implications of this strategy on the risk for depressive relapse. This study examined the effect of antidepressant discontinuation or continuation on depressive relapse risk among bipolar subjects successfully treated for an acute depressive episode. METHOD: Eighty-four subjects with bipolar disorder who achieved remission from a depressive episode with the addition of an antidepressant to an ongoing mood stabilizer regimen were followed prospectively for 1 year. The risk of depressive relapse among 43 subjects who stopped antidepressant treatment within 6 months after remission (“discontinuation group”) was compared with the risk among 41 subjects who continued taking antidepressants beyond 6 months (“continuation group”). RESULTS: A Cox proportional hazards regression analysis indicated that shorter antidepressant exposure time following successful treatment was associated with a significantly shorter time to depressive relapse. Furthermore, patients who discontinued antidepressant treatment within the first 6 months after remission experienced a significantly shorter period of euthymia before depressive relapse over the length of 1-year follow-up. One year after successful antidepressant response, 70% of the antidepressant discontinuation group experienced a depressive relapse compared with 36% of the continuation group. By the 1-year follow-up evaluation, 15 (18%) of the 84 subjects had experienced a manic relapse; only six of these subjects were taking an antidepressant at the time of manic relapse. CONCLUSIONS: The risk of depressive relapse in patients with bipolar illness was significantly associated with discontinuing antidepressants soon after remission. The risk of manic relapse was not significantly associated with continuing use of antidepressant medication and, overall, was substantially less than the risk of depressive relapse. Maintenance of antidepressant treatment in combination with a mood stabilizer may be warranted in some patients with bipolar disorder.
Treatment guidelines for bipolar depression recommend that antidepressants be discontinued within the first 3–6 months after remission of depressive symptoms (1–3). The rationale for this approach is influenced by a concern that continued antidepressant treatment might induce switches into mania or cycle acceleration. However, the possibility that antidepressant discontinuation could itself contribute to a depressive relapse has been understudied.
Few studies have directly compared the impact of antidepressant discontinuation versus continuation on the risk for depressive relapse after remission of an episode of bipolar depression (4, 5). One study demonstrated that tricyclic antidepressant monotherapy significantly decreased the risk of depressive relapse compared with placebo monotherapy in 44 bipolar subjects who had been recently hospitalized and successfully treated for bipolar depression (4). However, the risk of switches into mania was higher in those receiving antidepressant monotherapy (67%) compared with placebo (33%). Antidepressant monotherapy is not currently considered to be a first-line treatment for bipolar depression (3). Concerns, however, that antidepressant treatment, even in conjunction with a mood stabilizer, might induce switches or cycle acceleration have led to a reluctance to continue these medications (3).
A recent retrospective study of 44 bipolar subjects (39 with bipolar I disorder, five with bipolar II disorder) treated for an acute depressive episode with the addition of an antidepressant to an ongoing adequate mood stabilizer regimen indicated that termination (compared with continuation) of antidepressant treatment within the first year of remission significantly increased the risk of depressive relapse within that year (6). Moreover, continuation of the antidepressant was not associated with a higher risk of relapse into mania. The current study was undertaken to 1) replicate and extend these findings with a different, larger clinical patient group using detailed prospectively collected data and 2) explicitly test the recommendation to discontinue antidepressants within 6 months of depressive symptom remission. Thus, two distinct treatment strategies are compared: antidepressant discontinuation within 6 months of improvement versus antidepressant continuation beyond 6 months after improvement.
Method
Subjects
Subjects were drawn from a group of adults with bipolar disorder or schizoaffective disorder who were enrolled in the Stanley Bipolar Treatment Network, as previously described by Leverich et al. (7) and Post et al. (8). All subjects in the Stanley Bipolar Treatment Network were recruited from network sites located in Bethesda, Md.; Cincinnati; Dallas; Los Angeles; Munich; and Utrecht, the Netherlands. At the time of the analysis, 1,078 subjects were enrolled. Subjects received their bipolar disorder diagnosis following assessment with the Structured Clinical Interview for DSM-IV (9) and psychiatric interviews conducted by a highly trained clinician. Subjects provided information about their illness history (e.g., age at onset of symptoms), the presence of comorbid disorders, their current and past level of functioning, and family history of psychiatric disorders. As part of the network, subjects engaged in routine prospective clinical assessments every 2–4 weeks. During these visits, a number of clinical instruments were completed, including the Clinical Global Impression (CGI) for Bipolar Disorder scale (10). Further, the subject’s tolerance and responsiveness to medications were carefully monitored, and adjustments were made and recorded as clinically indicated. Upon entrance into the network, subjects provided written informed consent to participate in a longitudinal study of the course and treatment of their illness. In this naturalistic follow-up study, the protocol did not dictate a specific medication regimen. Subjects also had opportunities to participate in acute clinical trials sponsored by the network if they relapsed into episodes of depression or mania, and separate written informed consent was obtained if they chose to participate in such clinical trials. The informed consent document for the clinical trial fully informed subjects of the risks associated with antidepressant therapy, including the potential for mania induction and the potential consequences of stopping antidepressants. In addition, the attending psychiatrist reviewed the potential risks and benefits of adjunctive antidepressant therapy with each study subject before making the decision to begin treatment. Subjects remained on their medication regimen unless they had a manic episode or if their depressive symptoms were either worsening or not remitting within specified time periods. If a medication successfully treated the depressive episode, subjects were able to continue taking the medication for 1 year. However, no subjects were randomly assigned to continue versus discontinue their antidepressant medication.
Inclusion Criteria
All subjects in the Stanley Bipolar Treatment Network who were treated with an antidepressant for an index episode of depression while in the network were considered for the current study. Three additional criteria needed to be met for inclusion in this study. First, all patients had to be experiencing a depressive episode defined as a depression severity score of ≥3 (mildly ill or greater) on the CGI for Bipolar Disorder scale accompanied by a psychiatrist’s decision to begin treatment with an antidepressant. Second, all subjects treated with an antidepressant were required to have been treated for at least 60 days (i.e., long enough to have been given an adequate medication trial). Third, subjects were included only if they had achieved a successful antidepressant response (“remission”), operationalized as ≥42 days of improvement (i.e., at least 6 consecutive weeks of depression severity scores of 1 [normal, not ill] or 2 [minimally ill] on the CGI for Bipolar Disorder scale—our definition of euthymia).
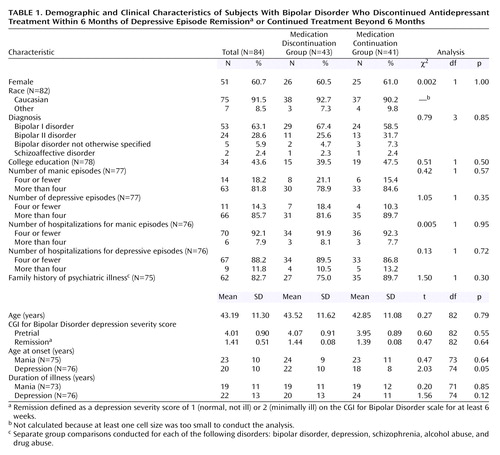
Of the 1,078 subjects enrolled in the network, 549 were treated with an antidepressant for at least 1 day while in the network. However, only 189 of these subjects were treated for an index episode of depression with an antidepressant for at least 60 days. Of these 189 subjects, 84 (44%) had a successful antidepressant response. Information on the 84 subjects who were thus included in the present study is provided in Table 1.
Subjects were divided into two groups on the basis of length of time they continued to receive an antidepressant after remission. The “medication discontinuation group” consisted of 43 subjects who discontinued medication within 6 months of meeting study criteria for a successful antidepressant response (mean=74 days, SD=8). The “medication continuation group” consisted of 41 patients who continued taking the antidepressants for more than 6 months after depressive episode remission (mean=484 days, SD=45). All subjects were given standard antidepressant doses used for unipolar depression. Table 2 indicates the average dose of each antidepressant taken by each group at the point of remission. Subjects were receiving a range of mood stabilizers that included valproic acid (52%, N=44), lithium (40%, N=34), carbamazepine (18%, N=15), and putative mood stabilizers including gabapentin (14%, N=12), topiramate (10%, N=8), and lamotrigine (1%, N=1). Twenty-eight subjects (33%) were receiving more than one mood stabilizer at the time the antidepressant trials were initiated.
At the point of remission, 19% of the subjects (N=16) were taking atypical antipsychotics (olanzapine: 10% [N=8]; clozapine: 5% [N=4]; risperidone: 2% [N=2]; quetiapine: 2% [N=2]), and 14% of the subjects (N=12) were taking an anxiolytic (benzodiazepines: 13% [N=11]; zolpidem tartrate: 1% [N=1]). Sixteen subjects (19%) participated in psychotherapy. No significant differences in the rates of these interventions were seen between those who discontinued versus those who continued antidepressant treatment.
Assessment of Relapse
To determine depressive relapse, all subjects in this study were followed for up to 1 year after meeting study criteria for a positive treatment response. Thus, time at risk for relapse was defined as the time from positive treatment response (≥6 weeks of euthymia) through the 1-year follow-up period. Some subjects were followed for shorter lengths of time because they dropped out of the Stanley Bipolar Treatment Network before the study’s conclusion.
Depressive relapse
Relapse into a depressive episode was operationalized by using depression severity scores from the CGI for Bipolar Disorder scale. The CGI for Bipolar Disorder scale has been used extensively in the past 5 years in research protocols to rate the clinical outcome of patients with bipolar disorder. Validity and high interrater reliability have been demonstrated (10). A patient was determined to have relapsed if the depression severity score was ≥4 (moderately ill) at any follow-up visit. Time to relapse was calculated by subtracting the date of successful antidepressant response (i.e., 42nd day of having a depression severity score of 1 or 2) from the relapse date and converting that number to weeks. Subjects in the medication discontinuation and continuation groups were then compared on the number of weeks that elapsed between their remission date and their depressive relapse date. In many cases, subjects (in either group) did not experience a depressive relapse during the follow-up period. These subjects were included in subsequent analyses through the last date information about them was available.
Manic relapse
Relapse into an episode of mania was operationalized by using the mania severity scores from the CGI for Bipolar Disorder scale. A patient was determined to have relapsed into mania if the mania severity score was ≥4 (moderately ill) at any visit. Time to relapse was calculated by subtracting the date of successful antidepressant response from the relapse date and converting that number to weeks. Subjects who did not experience a manic relapse during the follow-up period were included in subsequent analyses through the last date information about them was available.
Statistical Analyses
All statistical analyses were completed by using SPSS for Windows version 10.1.3 (SPSS, Inc., Chicago, 2001). Because our primary hypotheses compared groups dichotomized on the variable “time taking an antidepressant after reaching euthymia,” a number of analyses were completed to ensure that the medication discontinuation and continuation groups differed only along this dimension. Potential demographic or clinical differences between groups were first carefully examined to discern whether they could account for our findings. Fisher’s exact p was used for all chi-square analyses.
Cox proportional hazards regression analyses (hereafter called Cox regression analyses) were conducted to 1) test the relationship between antidepressant exposure and time to depressive and manic relapse in separate analyses; 2) test the effects of the two antidepressant treatment strategies (discontinuation versus continuation) on the primary dependent variables: time to depressive relapse or last known status (i.e., last date of available data) and time to manic relapse or last known status; and 3) examine the relapse rates for each treatment strategy after 1 follow-up year. Cox regression analysis estimates the probability of a given outcome at each time point that an event occurs (i.e., a person relapses) on the basis of available data at that point in time. Thus, subjects who dropped out of the study before experiencing a relapse are used to calculate the survival rate at each time point only until they drop out; once they drop out, the overall sample is recalculated with their data omitted. The benefits of this approach are that it allows us to directly test the efficacy of the two different treatment approaches and it takes maximum advantage of each subject’s available data, even though subjects were tracked for different lengths of time.
Results
As shown in Table 1, there were no significant differences between the groups in age, gender, race, diagnosis, pretrial depression severity, or on a number of illness-related variables, including family history, the number of prior manic or depressive episodes (all χ2<1.5, df=1, p>0.30), or the age at onset of manic symptoms. Patients in the medication discontinuation group reported a significantly later onset of depressive symptoms than those in the medication continuation group, although this effect was not significant after Hochberg’s correction for multiple comparisons. The age at onset of depressive symptoms was assessed to determine whether it was related to the primary dependent variables, time to depressive relapse and time to manic relapse. Neither of these analyses was significant (all p>0.08).
The medication discontinuation and continuation groups had approximately the same distribution of antidepressant medications (Table 2). The medication discontinuation group responded on average by 33 days, and the continuation group responded by 44 days (t=0.13, df=82, p=0.90). There were also no differences between groups in the frequency of antipsychotic or anxiolytic use (all χ2<0.21, df=1, all p>0.70) or in their participation in psychotherapy (χ2<0.01, df=1, p>0.90). The CGI for Bipolar Disorder depression severity scores at the point of remission between the discontinuation and continuation groups were also similar (1.44 and 1.39, respectively; t=0.48, df=82, p=0.60).
Depressive Episode Relapse
A Cox regression analysis, with time receiving antidepressant medication following remission used to predict time to relapse, indicated that shorter periods of antidepressant exposure predicted earlier relapse (χ2=9.63, df=1, p=0.002). The results of a survival analysis indicated that there was a significantly greater risk for depressive relapse among those who discontinued antidepressant treatment within 6 months of remission (mean=29 weeks [SD=3]) than among those who continued treatment beyond 6 months (mean=41 weeks [SD=3]) (χ2=8.2, df=1, p=0.004) (odds ratio=2.69, 95% CI=1.35–5.36; Wald χ2=7.91, df=1, p=0.005). After 1 year of follow-up after remission, 70% of the medication discontinuation group had relapsed into an episode of depression compared with 36% of the medication continuation group (Figure 1).
To further evaluate the optimal length of time that antidepressants may offer protection against relapse, the medication continuation group was stratified into subgroups on the basis of length of antidepressant treatment. Relapse rates were calculated for a designated time point of 1 year after successful treatment response was obtained and compared with the relapse rate of the medication discontinuation group. Results showed that 70% of those who discontinued antidepressant treatment within 6 months, 53% of those who discontinued antidepressant treatment between 6 and 12 months, and 24% of those who continued antidepressant treatment for at least 12 months had relapsed by 1 year after successful antidepressant treatment response (Table 3 and Figure 2). When the medication discontinuation group was compared with those who continued antidepressant treatment for at least 12 months after remission, the risk of relapse for the medication discontinuation group was four times greater (odds ratio=4.12, 95% CI=1.54–11.03; Wald χ2=7.90, df=1, p=0.005).
Medication discontinuers and continuers were combined, and analyses were conducted to determine whether there were any differences between those who did and did not experience a depressive episode relapse. These analyses considered the number and type of concomitant mood stabilizers, and a number of clinical variables including diagnosis (bipolar I versus bipolar II), number of prior manic and depressive episodes, age at onset of bipolar illness, and illness duration. A chi-square analysis revealed no effect of the number or type of mood stabilizer on relapse into depression (all χ2<0.80, df=1, all p>0.40). There were also no differences in the rates of depressive relapse between patients with bipolar I and bipolar II diagnoses (χ2<1.1, df=1, p=0.33). Subjects who relapsed at any time during the follow-up period were more likely to have had a family history of depression (77% versus 50%, respectively) (χ2=5.37, df=1, p=0.03) and to have first received medication for mania at a later age (mean=37 [SD=10.34] versus 30 [SD=9.89] years, respectively) (t=2.68, df=65, p=0.01). However, after Hochberg’s correction for multiple comparisons, neither effect remained significant.
Regression and survival analyses were rerun with the bipolar disorder not otherwise specified and schizoaffective patients omitted. In all cases, the results were consistent with results using the whole sample.
Manic Episode Relapse
The majority of the sample (82%) did not experience a manic episode over the 1-year follow-up period. Of the 15 (18%) subjects that did experience a manic relapse, six were taking an antidepressant at the time of relapse, and nine were not. Results of a Cox regression analysis in which time receiving antidepressant was used to predict relapse into mania indicated that shorter antidepressant exposure time was associated with a greater risk of developing mania over the follow-up period (χ2=8.92, df=1, p=0.003). Subjects who discontinued antidepressant treatment within the first 6 months after attaining euthymia experienced a significantly shorter time before experiencing a manic episode (mean=42 weeks, SD=3) compared with those who continued receiving antidepressants 6 months after remission (mean=49 weeks, SD=1) (χ2=4.21, df=1, p=0.04). One year after successful antidepressant response, 29% of the medication discontinuation group had relapsed into an episode of mania compared with 22% of those who continued treatment for 6–12 months and 5% of those receiving antidepressant treatment beyond 12 months. (These numbers are derived from the Cox regression analysis and are based on the number of subjects available over the course of follow-up.) Thus, in this sample, early antidepressant discontinuation as opposed to continuation was also associated with a significantly greater risk of a relapse into mania albeit smaller than the risk for relapse into depression. In the group who discontinued antidepressant treatment, almost all of the relapses into mania occurred within the first 6 months after successful antidepressant response. Between 6 and 12 months after successful antidepressant response, the number of additional relapses into mania was low, suggesting that risk of manic relapse with antidepressant medication discontinuation is highest within the initial 6 months after discontinuation.
Exploration of Possible Biases
One possibility of concern was that patients in the medication discontinuation group were in this group because relapse into a manic episode while receiving treatment led to early antidepressant discontinuation. If this were true, there would be a close temporal correspondence between the manic relapse date and the antidepressant medication end date among the medication discontinuers. Of the 15 subjects who relapsed into a manic episode during the first year of follow-up, 30% (N=3 of 10) of the medication discontinuation group and 40% (N=2 of 5) of the medication continuation group stopped their medication within 1 week of a manic relapse. However, for the remaining 10 subjects who relapsed into an episode of mania, there was no correspondence between manic relapse and antidepressant discontinuation. That is, manic relapses occurred several weeks after or before the antidepressant was discontinued. Given the similarity between the medication discontinuation and continuation groups in the temporal association between antidepressant discontinuation and manic relapse, this explanation (that patients in the medication discontinuation group were more vulnerable to relapse) seems unlikely.
Another possibility was that patients in the discontinuation group not only discontinued antidepressants but discontinued all medication, including antimanic agents, thus accounting for the increased rate of manic relapse. Evaluation of medication blood levels at the time of relapse was not possible because only one subject had such an evaluation within 2 weeks of the switch into mania. However, life chart data regarding medication use revealed that of the 10 subjects in the discontinuation group who relapsed into mania, only two (20%) had discontinued all medications, including their mood stabilizers, before their manic relapse. There were no reported changes in mood stabilizer dosages around the time of relapse for the other subjects.
Overall Relapse Rates
When relapse into either mania or depression was assessed 1 year after successful treatment of an acute depressive episode, the number of patients in both antidepressant groups combined who relapsed into mania (N=15; 18%) was smaller than the number who relapsed into depression (N=36; 43%), suggesting that the risk of a manic episode relapse is lower than the risk of a depressive episode relapse 1 year after treatment for bipolar depression.
Discussion
This study has several important limitations. First, while the data were collected prospectively, the study was neither blinded nor randomized. Patients either continued or discontinued antidepressant treatment at clinician and patient discretion, and a number of important variables such as length of antidepressant use and rate of taper schedules in those who discontinued were thus not able to be controlled. However, the data were collected a priori of this study idea or design, and thus rater bias was unlikely to contribute to the current findings. Second, only about half (N=84) of the 189 subjects exposed to antidepressants for at least 60 days in the Stanley Bipolar Treatment Network met the inclusion criteria of having a positive response to antidepressant treatment, operationalized as ≥6 weeks of sustained euthymia. Since subjects who did not have a positive response to an antidepressant or who could not tolerate 60 days of antidepressant exposure because of either side effects or an acute switch into mania would have been excluded from this study, our results may not apply to all bipolar depressed subjects but rather more specifically only to those bipolar subjects who respond positively to antidepressants and do not have a sensitivity to manic switching within the initial weeks of the exposure. Third, only a few subjects were exposed to tricyclic antidepressants, so our data about switch rates into mania in association with this class of drugs cannot be adequately compared with prior reports in the literature.
Notwithstanding these limitations, our current and previous (6) studies suggest that discontinuing antidepressants in those bipolar subjects who achieve successful remission of a depressive episode with antidepressant treatment puts them at significant risk for depressive relapse.
Risk of Depressive Relapse
Our results indicate that for patients who have a positive initial treatment response to adjunctive antidepressant therapy, a longer period of antidepressant exposure is associated with a longer time before depressive relapse. In fact, 70% of those who discontinued antidepressants within the first 6 months after remission and 53% of those who discontinued within 6–12 months after remission had relapsed during the 1-year follow-up period, whereas only 24% of those subjects who remained on antidepressant regimens throughout the first year after remission experienced a relapse. Since no group differences existed in response time or quality of response to initial antidepressant treatment, it is unlikely that differences in time to relapse are due to differences in the initial or overall medication responsiveness. Furthermore, the demographic and treatment similarities between the two groups provide a reasonable measure of confidence that any differences found on the primary dependent variables are due to the length of time treated with an antidepressant. Since we operationalized remission as ≥6 weeks of minimal or no symptoms, our results suggest that discontinuing antidepressant therapy within 7 months of depressive remission significantly increases (by fourfold) the chance that a patient will relapse compared with remaining on an antidepressant regimen throughout and past the first year of acute treatment. Our current results are consistent with a prior retrospective study (6) and suggest that maintaining antidepressant medication for at least 6 and perhaps 12 or more months after resolution of an acute episode of bipolar depression may contribute to continued mood stability.
These findings indicate that the current recommendations for persons with bipolar disorder to discontinue treatment with an antidepressant within 3–6 months after acute remission from a depressive episode (1–3) should be reevaluated. These recommendations are based on only a few controlled (4, 5, 11–14) and uncontrolled (15) maintenance studies, and clinician concern about the possible risk of inducing mania or cycle acceleration with continued antidepressant exposure (8, 11, 16–23).
To our knowledge, only four randomized studies exist that directly assess the longitudinal impact of antidepressant medication exposure on risk for recurrence of bipolar depression in subjects with bipolar disorder concurrently treated with a mood stabilizer (5, 11–13). While the studies concluded that a mood stabilizer (lithium) in conjunction with an antidepressant (tricyclic) confer no greater protection against depressive relapse than a mood stabilizer alone, these studies are not directly comparable to the present study. In three (11–13) of the four studies, euthymic patients were followed who had not necessarily been recently treated for either an acute depressive or manic episode and thus were not necessarily at a point in their illness course when they were most vulnerable for risk of relapse. In the current study, all subjects were given an antidepressant in addition to a mood stabilizer to treat an acute depressive episode and were then followed after remission. Our study would suggest that if an antidepressant is needed in addition to a mood stabilizer to successfully treat a depressive episode, then the continued combination strategy may be more effective at preventing depressive relapse than the continuation of the mood stabilizer(s) alone.
In the one study that did randomly assign patients to a treatment condition soon after inpatient treatment for an acute bipolar episode, depressive relapse rates over a 2-year follow-up period were similar in the group receiving a combination of lithium and imipramine (22%) compared with those receiving lithium monotherapy (29%) (5). However, patients could enter that study after either an index episode of mania or depression, and the relationship of treatment effectiveness to the pole of the index episode was not fully reported. In a reanalysis of these data that allowed for relapse rates to be assessed differentially as a function of the initial index episode, Shapiro et al. (24) found that in patients whose index episode was one of depression, combination treatment with lithium and an antidepressant resulted in less than half the risk of experiencing a relapse over the follow-up period compared with those receiving lithium monotherapy. While these results did not quite reach significance (30 subjects were receiving lithium monotherapy and only 18 were receiving the combination), the authors concluded that the combination treatment was the most effective treatment for those patients with bipolar disorder with an index episode of depression. The survival curves presented in their study are strikingly similar to our own.
Risk of Manic Relapse
One of the principal concerns clinicians have regarding continued use of antidepressants in patients with bipolar disorder is the risk of inducing a manic episode (4, 8, 17, 23, 25, 26) or cycle acceleration and rapid cycling (14, 15, 27). Prior studies, including one of our own (23), have assessed acute switch rates into mania (usually defined as a switch within 6–8 weeks after exposure to antidepressants) and have determined that a proportion of bipolar subjects appear vulnerable to the acute switch. The current study cannot add information to the field regarding acute switch rates into mania with antidepressant exposure, since our study group involved only the subgroup of subjects who were able to remain on an antidepressant regimen for 6 weeks without switching into mania. In this subgroup of patients who recover from depression and do not switch to mania within those first weeks of treatment, the risk of experiencing a depressive relapse over the next year is greater than that of experiencing a manic relapse.
Eighteen percent of our study group switched into a manic episode at some point over the 1-year follow-up period, with only 7% switching while receiving an antidepressant. Exposure to continued antidepressants per se was not associated with either a shorter time in remission before a manic relapse or an increased risk for relapse overall. Thus, our data do not support the clinical concern that continued antidepressant exposure in bipolar subjects who respond acutely to an antidepressant (and do not switch acutely into a mania) will enhance the likelihood of a person later experiencing a switch into mania.
Paradoxically, it was the subjects who discontinued antidepressants who switched into mania in a shorter follow-up time compared with those who continued receiving antidepressants. The reason why antidepressant discontinuation would be associated with greater risk for a manic relapse is unclear, but several possibilities exist. One possibility is that those who discontinued were discontinued because they were already beginning to experience hypomania/mania. However, our data did not support this concern.
As mentioned earlier, another possibility is that patients who discontinued antidepressants also discontinued their mood stabilizers and switched into a manic episode because of the latter. Although blood levels of mood stabilizers were not available for most patients at the time of the mood switch, a review of patient medications at the time of antidepressant discontinuation and at the time of manic relapse did not reveal a major change in mood stabilizer usage for the majority of those who relapsed into mania.
A third possibility is that discontinuing antidepressants may temporarily increase the risk for relapse into mania among some bipolar subjects. The phenomenon of increased risk for relapse after the discontinuation of a drug has been reported with withdrawal of mood stabilizers in subjects with bipolar disorder (28–31) and removal of antidepressants in subjects with unipolar depressive disorder (32). The phenomenon of apparent antidepressant discontinuation-related hypomania or mania has also been observed to occur in some bipolar patients within 2–4 weeks of an antidepressant taper/discontinuation (33–35). While often attributed to the natural return of the illness, a drug withdrawal-induced effect cannot be excluded. In this regard, a meta-analysis of subjects with unipolar depression revealed that risk for relapse while not taking versus taking antidepressants was greatest (over threefold) within the first 3 months of discontinuation and became less with time (1.5-fold by 4 or 5 years of follow-up) (32). In the current study, the greatest risk of a manic switch was in the first 6 months after antidepressant discontinuation (26%), with very few additional cases at the 12-month follow-up period (3%). The explanatory mechanism for a medication withdrawal sensitivity is unclear but has been suggested to involve the following: a mobilization of the monoaminergic systems due to withdrawal-induced cholinergic overdrive (36), norepinephrine and dopamine receptor sensitization (33, 36), or acute enhancement of serotonin uptake with antidepressant-treated desensitization of serotonin and norepinephrine receptors (37).
Acute Manic Switching
Our study suggests that subjects who respond to antidepressant exposure and do not switch into an episode of mania in the first 6 weeks are not at greater risk of switching into mania with continued antidepressant exposure over the year. Our study does not address acute switch rates, and a review of the literature on risk for antidepressant-induced mania with acute exposure is beyond the scope of this paper (18, 38–48). However, the natural switch rate into mania within 8 weeks following a depression can be high (49, 50) and can lead to the incorrect conclusion that antidepressants precipitated the switch when it was actually due to the illness itself (23). Data from one study found a switch rate of 41% from depression to mania in bipolar inpatients taking no medications during hospitalization (51).
Four large, randomized, controlled treatment trials of bipolar I depression have enhanced our understanding of the natural versus drug-induced switch rate from bipolar depression into mania over time and the effect of different classes of antidepressants on the likelihood of switching (8, 42, 44, 52). In a 7-week acute treatment trial of two doses of lamotrigine for the treatment of bipolar depression, Calabrese et al. (52) demonstrated clinical efficacy at both doses, with no greater acute switch rate with active drug (5.4%) than with placebo (4.6%). The trial operationalized switching as an investigator-reported adverse event in which the patient developed hypomania, mania, or a mixed state. In the other three randomized, controlled studies, there was no placebo group and therefore a natural/spontaneous switch rate could not be ascertained. However, data from these studies suggest that the risk of acute switching has been overestimated and shed light on the differential impact of classes of antidepressants on risk for switch and on the ability of a concurrent mood stabilizer to prevent such a switch.
Our subject group contained only two patients receiving tricyclic antidepressants and two receiving MAOIs, and a high proportion were receiving SSRIs or bupropion. This could contribute to our low switch rate over time in those who continued taking antidepressants. Several studies exist in the literature examining whether one group of antidepressants is more likely than another to induce an acute switch (42, 44, 48, 49, 52–54). In the aforementioned studies that included a tricyclic antidepressant and a nontricyclic antidepressant comparator, switches in patients taking tricyclic antidepressants occurred more commonly than switches while taking the newer nontricyclic antidepressants, supporting prior impressions that tricyclic antidepressants may pose a higher risk for switching than the newer antidepressant classes (38, 55). A review of clinical trials (18) also found a higher switch rate for tricyclic antidepressants (11.2%) compared with SSRIs (3.7%) or placebo (4.2%) (SSRI versus tricyclic antidepressant p<0.01). If the 4.6% placebo switch rate from the Calabrese study (52) is used as a historical control of a natural switch rate, it appears that MAOIs may double and tricyclic antidepressants may triple the natural switch rate (44).
Whether the concomitant use of mood stabilizers can block an episode of antidepressant-induced mania is a question that requires further study. Four prior studies suggest a lack of complete protection by a mood stabilizer against the mania-inducing properties of antidepressants (8, 11, 23, 42), and four have suggested a protective prophylactic effect (5, 39, 56, 57). In the current study, all subjects were taking mood stabilizers, and the last levels obtained within 2–6 months before manic relapse were within therapeutic plasma concentrations.
It is possible that not only are some antidepressants less likely to induce a switch than others but that mood stabilizers are variably effective at preventing the potential for switch, depending on the class of antidepressant agent implicated in the switch. For example, two possible interpretations of one study (42) could be that either SSRIs induce less switching than tricyclic antidepressants or that a mood stabilizer may protect better against SSRI-induced rather than a tricyclic-antidepressant-induced switch. Which of these two conclusions is correct awaits further study.
Conclusions
For bipolar subjects successfully treated with an antidepressant for acute depressive episodes, the risk of relapse into depression may be increased by the common clinical practice of discontinuing antidepressants soon after remission. Continuing antidepressants for at least 1 year after successful treatment of a depressive episode may protect against relapse into depression and did not appear in our study to increase risk for manic relapse. Concerns regarding a risk of switching into mania may actually interfere with establishing an optimal treatment paradigm to prevent relapse of bipolar depression (38, 40, 55, 58). Guidelines more similar to those for the maintenance treatment of unipolar depression may ultimately be in order for those bipolar depressed subjects who respond well to acute adjunctive antidepressant treatment (59, 60).
Recent treatment studies have suggested that adjunctive antidepressant therapy for acute bipolar depression may be no more effective than either lithium with high serum levels (42) or two mood stabilizers (61). Our study did not address the ideal or optimal treatment for bipolar depression. Rather, it suggests that when a person with bipolar depression is successfully treated with an antidepressant as adjunctive treatment to a mood stabilizer that alone did not treat the depression, removal of the antidepressant may increase the likelihood of a relapse. A controlled, randomized study is needed to definitively answer the questions this study raises and to further assess the impact of continued antidepressant exposure on cycling.
If the findings from our study are replicated, current guidelines regarding the duration of antidepressant treatment after resolution of an acute episode of bipolar depression may need to be reconsidered. Maintenance treatment involving the continued use of medications that treat both “poles” of bipolar disorder may ultimately be most effective at decreasing illness morbidity.
Acknowledgments
The Stanley Bipolar Treatment Network consists of the following sites: the Department of Psychiatry and Biobehavioral Sciences, University of California, Los Angeles; the Department of Psychiatry, VA Greater Los Angeles Healthcare System, West Los Angeles Healthcare Center, Los Angeles; the Department of Psychiatry, University of Texas Southwestern Medical Center, Dallas; the NIMH Biological Psychiatry Branch, Bethesda, Md.; the Biological Psychiatry Program, Department of Psychiatry, University of Cincinnati College of Medicine, Cincinnati; University Medical Center Utrecht, Altrech Institute for Mental Health Care, Utrecht, the Netherlands; Zentrum für Innovative Therapie Bipolarer Störungen am Universitätsklinikum Freiburg, Freiburg, Germany; and the NIMH Mood and Anxiety Disorders Program, Bethesda, Md.
 |
 |
 |
Received March 15, 2002; revision received Nov. 27, 2002; accepted Dec. 17, 2002. From the Stanley Bipolar Treatment Network. Address reprint requests to Dr. Altshuler, 300 UCLA Medical Plaza, Suite 1544, Box 957057, Los Angeles, CA 90095-7057; [email protected] (e-mail). Supported by the Stanley Medical Research Institute. For the portion of data gathered from the double-blind study, the following companies supplied free study drug and placebo but no other financial support: Glaxo Wellcome (now GlaxoSmithKline), Pfizer, and Wyeth-Ayerst. The authors thank Dr. John Bartko for consultation on the statistical design for this study, the work of the research assistants at each of the Stanley Bipolar Treatment Network sites, and the contribution of the Bethesda Data Coordinating Center.
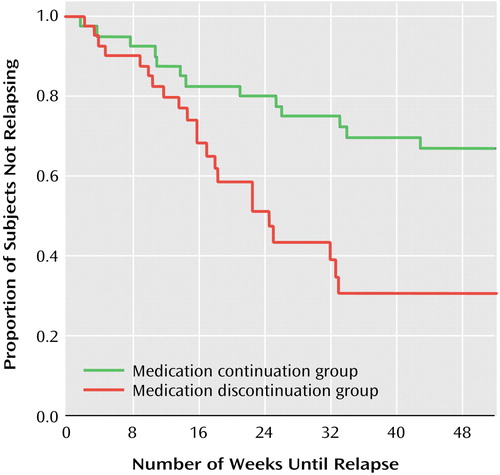
Figure 1. Time to Relapse for Subjects With Bipolar Disorder Who Discontinued Antidepressant Treatment Within 6 Months of Depressive Episode Remission or Continued Treatment Beyond 6 Monthsa
aRemission defined as a depression severity score of 1 (normal, not ill) or 2 (minimally ill) on the CGI for Bipolar Disorder scale for at least 6 weeks (score of 1 or 2 considered “euthymia”). Relapse defined as a depression severity score ≥4 (moderately ill) on the CGI for Bipolar Disorder scale. Time 0=42nd day of euthymia. Results of Cox regression analyses, in which effects that are constant over time are assumed and plots represent estimates, are presented in text. For the sake of clarity, 1-year Kaplan-Meier plots are illustrated in the figure because they more accurately reflect the raw data. The Kaplan-Meier results were highly consistent with those of the Cox regression analyses (log rank=8.5, p=0.004).
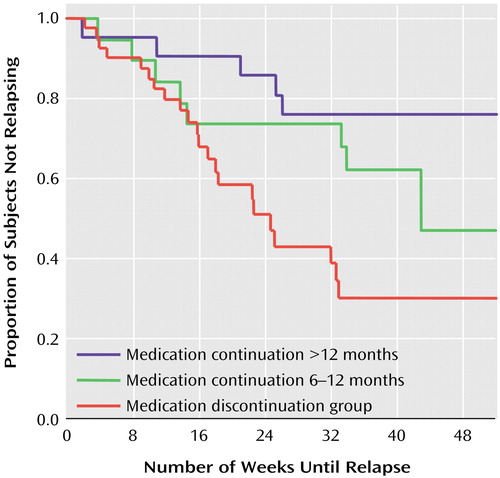
Figure 2. Time to Relapse Among Subjects With Bipolar Disorder Who Discontinued Antidepressant Treatment Within 6 Months of Depressive Episode Remission or Continued Treatment for 6–12 Months or Beyond 12 Monthsa
aRemission defined as a depression severity score of 1 (normal, not ill) or 2 (minimally ill) on the CGI for Bipolar Disorder scale for at least 6 weeks. Relapse defined as a depression severity score ≥4 (moderately ill) on the CGI for Bipolar Disorder scale. Time 0=42nd day of euthymia. Results of Cox regression analyses, in which effects that are constant over time are assumed and plots represent estimates, are presented in text. For the sake of clarity, 1-year Kaplan-Meier plots are illustrated in the figure because they more accurately reflect the raw data. The Kaplan-Meier results were highly consistent with those of the Cox regression analyses (log rank=10.09, p=0.006).
1. Frances A, Kahn D, Carpenter S, Docherty J, Donovan S: The expert consensus guidelines for treating depression in bipolar disorder. J Clin Psychiatry 1998; 59(suppl 4): S73-S79Google Scholar
2. Yatham L, Kusumakar V, Parikh S, Haslam D, Matte R, Sharma V, Kennedy S: Bipolar depression: treatment options. Can J Psychiatry 1997; 42(suppl 2): S87-S91Google Scholar
3. Sachs GS, Printz DJ, Kahn DA, Carpenter D, Docherty JP: The Expert Consensus Guidelines Series: Medication Treatment of Bipolar Disorder. New York, McGraw-Hill, April 2000, pp 1-102Google Scholar
4. Prien RF, Klett CJ, Caffey EM: Lithium carbonate and imipramine in prevention of affective episodes. Arch Gen Psychiatry 1973; 29:420-425Crossref, Medline, Google Scholar
5. Prien RF, Kupfer DJ, Mansky PA, Small JG, Tuason VB, Voss CB, Johnson WE: Drug therapy in the prevention of recurrences in unipolar and bipolar affective disorders. Arch Gen Psychiatry 1984; 41:1096-1104Crossref, Medline, Google Scholar
6. Altshuler L, Kiriakos L, Calcagno J, Goodman R, Gitlin M, Frye M, Mintz J: The impact of antidepressant discontinuation versus antidepressant continuation on one-year risk for relapse of bipolar depression: a retrospective chart review. J Clin Psychiatry 2001; 62:612-616Crossref, Medline, Google Scholar
7. Leverich GS, Nolen WA, Rush AJ, McElroy SL, Keck PE Jr, Denicoff KD, Suppes T, Altshuler LL, Kupka R, Kramlinger KG, Post RM: The Stanley Foundation Bipolar Treatment Outcome Network, I: longitudinal methodology. J Affect Disord 2001; 67:33-44Crossref, Medline, Google Scholar
8. Post RM, Altshuler LL, Frye MA, Suppes T, Rush AJ, Keck PE Jr, McElroy SL, Denicoff KD, Leverich GS, Kupka R, Nolen WA: Rate of switch in bipolar patients prospectively treated with second-generation antidepressants as augmentation to mood stabilizers. Bipolar Disord 2001; 3:259-265Crossref, Medline, Google Scholar
9. Spitzer RL, Williams JBW, Gibbon M, First MB: Structured Clinical Interview for DSM-IV (SCID). New York, New York State Psychiatric Institute, Biometrics Research, 1995Google Scholar
10. Spearing M, Post R, Leverich G, Brandt D, Nolen W: Modification of the Clinical Global Impressions (CGI) Scale for use in bipolar illness (BP): the CGI for bipolar disorder. Psychiatry Res 1997; 73:159-171Crossref, Medline, Google Scholar
11. Quitkin FM, Kane J, Rifkin A, Lorenzi-Ramos JR, Nayak DV: Prophylactic lithium carbonate with and without imipramine for bipolar I patients. Arch Gen Psychiatry 1981; 38:902-907Crossref, Medline, Google Scholar
12. Kane JM, Quitkin FM, Rifkin A, Ramos-Lorenzi JR, Nayak DD, Howard A: Lithium carbonate and imipramine in the prophylaxis of unipolar and bipolar II illness: a prospective, placebo-controlled comparison. Arch Gen Psychiatry 1982; 39:1065-1069Crossref, Medline, Google Scholar
13. Johnstone E, Owens D, Lambert M, Crow T, Frith C, Done D: Combination tricyclic antidepressant and lithium maintenance medication in unipolar and bipolar depressed patients. J Affect Disord 1990; 20:225-233Crossref, Medline, Google Scholar
14. Wehr TA, Goodwin FK: Rapid cycling in manic-depressives induced by tricyclic antidepressants. Arch Gen Psychiatry 1979; 36:555-559Crossref, Medline, Google Scholar
15. Kukopulos A, Reginaldi D, Laddomada P, Floris G, Serra G, Tondo L: Course of the manic-depressive cycle and changes caused by treatment. Pharmakopsychiatr Neuropsychopharmakol 1980; 13:156-167Medline, Google Scholar
16. Wehr TA, Goodwin SK: Do antidepressants cause mania? Psychopharmacol Bull 1987; 23:61-65Medline, Google Scholar
17. Wehr T, Goodwin F: Can antidepressants cause mania and worsen the course of affective illness? Am J Psychiatry 1987; 144:1403-1411Link, Google Scholar
18. Peet M: Induction of mania with selective serotonin re-uptake inhibitors and tricyclic antidepressants. Br J Psychiatry 1994; 164:549-550Crossref, Medline, Google Scholar
19. Lerer B, Birmacr B, Ebstein RP, Belmaker RH: 48-hour depressive cycling induced by antidepressants. Br J Psychiatry 1980; 137:181-185Crossref, Medline, Google Scholar
20. Wehr TA, Sack DA, Rosenthal NE, Cowdry RW: Rapid cycling affective disorder: contributing factors and treatment responses in 51 patients. Am J Psychiatry 1988; 145:179-184Link, Google Scholar
21. Tondo L, Laddomada P, Serra G, Minnai G, Kukopulos A: Rapid cyclers and antidepressants. Int Pharmacopsychiatry 1981; 16:119-123Crossref, Medline, Google Scholar
22. Kukopulos A, Caliari B, Tundo A, Minnai G, Florrs G, Reginaldi D, Tondo I: Rapid cyclers, temperament and antidepressants. Compr Psychiatry 1983; 24:249-258Crossref, Medline, Google Scholar
23. Altshuler LL, Post RM, Leverich GS, Mikalauskas K, Rosoff A, Ackerman L: Antidepressant-induced mania and cycle acceleration: a controversy revisited. Am J Psychiatry 1995; 152:1130-1138Link, Google Scholar
24. Shapiro DR, Quitkin FM, Fleiss JL: Response to maintenance therapy in bipolar illness: effect of index episode. Arch Gen Psychiatry 1989; 46:401-405Crossref, Medline, Google Scholar
25. Stoll AL, Mayer PV, Kolbrener M, Goldstein E, Suplit B, Lucier J, Cohen BM, Tohen M: Antidepressant-associated mania: a controlled comparison with spontaneous mania. Am J Psychiatry 1994; 151:1642-1645Link, Google Scholar
26. Nasrallah HA, Lyskowski J, Schroeder D: TCA-induced mania: differences between switchers and nonswitchers. Biol Psychiatry 1982; 17:271-274Medline, Google Scholar
27. Wehr TA, Goodwin SK: Tricyclics modulate frequency of mood cycles. Chronobiologia 1979; 6:377-385Medline, Google Scholar
28. Baldessarini RJ, Tondo L, Viguera AC: Discontinuing lithium maintenance treatment in bipolar disorders: risks and implications. Bipolar Disord 1999; 1:17-24Crossref, Medline, Google Scholar
29. Faedda GL, Baldessarini RJ, Suppes T, Tondo L, Becker I, Lipschitz D: Pediatric-onset bipolar disorder: a neglected clinical and public health problem. Harv Rev Psychiatry 1995; 3:171-195Crossref, Medline, Google Scholar
30. Suppes T, Baldessarini RJ, Faedda GL, Tohen M: Risk of recurrence following discontinuation of lithium treatment in bipolar disorder. Arch Gen Psychiatry 1991; 48:1082-1088Crossref, Medline, Google Scholar
31. Koukopoulos A, Reginaldi D, Minnai G, Serra G, Pani L, Johnson FN: The long term prophylaxis of affective disorders, in Depression and Mania: From Neurobiology to Treatment, vol 49. Edited by Gessa G, Fratta W, Pani L, Serra G. New York, Raven Press, 1995, pp 127-147Google Scholar
32. Viguera AC, Baldessarini RJ, Friedberg J: Discontinuing antidepressant treatment in major depression. Harv Rev Psychiatry 1998; 5:293-306Crossref, Medline, Google Scholar
33. Shriver AE, Sachs GS, Baldassano CF: Mania and hypomania following antidepressant discontinuation, in 1998 Annual Meeting New Research Program and Abstracts. Washington, DC, American Psychiatric Association, 1998, number 161, p 111Google Scholar
34. Goldstein TR, Frye MA, Denicoff KD, Smith-Jackson E, Leverich GS, Bryan AL, Ali SO, Post RM: Antidepressant discontinuation-related mania: critical prospective observation and theoretical implications in bipolar disorder. J Clin Psychiatry 1999; 60:563-567Crossref, Medline, Google Scholar
35. Mirin SM, Schatzberg AF, Creasey DE: Hypomania and mania after withdrawal of tricyclic antidepressants. Am J Psychiatry 1981; 138:87-89Link, Google Scholar
36. Dilsaver SC, Greden JF: Antidepressant withdrawal-induced activation (hypomania and mania): mechanism and theoretical significance. Brain Res Brain Res Rev 1984; 7:29-48Crossref, Google Scholar
37. Zajecka J, Tracy KA, Mitchell S: Discontinuation symptoms after treatment with serotonin reuptake inhibitors: a literature review. J Clin Psychiatry 1997; 58:291-297Crossref, Medline, Google Scholar
38. Calabrese JR, Rapport DJ, Kimmel SE, Shelton MD: Controlled trials in bipolar I depression: focus on switch rates and efficacy. Eur Psychopharmacol 1999; 9(suppl 4): S109-S112Google Scholar
39. Rouillon F, Lejoyeux M, Filteau MJ: Unwanted effects of long term treatment, in Long Term Treatment of Depression. Edited by Montgomery SA, Rouillon FA. New York, John Wiley & Sons, 1992, pp. 81-111Google Scholar
40. Nolen WA, Bloemkolk D: Treatment of bipolar depression, a review of the literature and a suggestion for an algorithm. Neuropsychobiology 2000; 42 (suppl 1):11-17Google Scholar
41. Ghaemi SN, Lenox MS, Baldessarini RJ: Effectiveness and safety of long-term antidepressant treatment in bipolar disorder. J Clin Psychiatry 2001; 62:565-569Crossref, Medline, Google Scholar
42. Nemeroff CB, Evans DL, Gyulai L, Sachs GS, Bowden CL, Gergel IP, Oakes R, Pitts CD: Double-blind, placebo-controlled comparison of imipramine and paroxetine in the treatment of bipolar depression. Am J Psychiatry 2001; 158:906-912Link, Google Scholar
43. Barak Y, Kimhi R, Weizman R: Is selectivity for serotonin uptake associated with a reduced emergence of manic episodes in depressed patients? Int Clin Psychopharmacol 2000; 15:53-56Crossref, Medline, Google Scholar
44. Silverstone T: Moclobemide vs imipramine in bipolar depression: a multicentre double-blind clinical trial. Acta Psychiatr Scand 2001; 104:104-109Crossref, Medline, Google Scholar
45. Henry C, Sorbara F, Lacoste J, Gindre C, Leboyer M: Antidepressant-induced mania in bipolar patients: identification of risk factors. J Clin Psychiatry 2001; 62:249-255Crossref, Medline, Google Scholar
46. Goren JL, Levin GM: Mania with bupropion: a dose-related phenomenon? Ann Pharmacother 2000; 34:619-621Crossref, Medline, Google Scholar
47. Joffe RT, MacQueen GM, Marriott M, Robb J, Begin H, Young LT: Induction of mania and cycle acceleration in bipolar disorder: effect of different classes of antidepressant. Acta Psychiatr Scand 2002; 105:427-430Crossref, Medline, Google Scholar
48. Himmelhoch JM, Thase ME, Mallinger AG, Houck P: Tranylcypromine versus imipramine in anergic bipolar depression. Am J Psychiatry 1991; 148:910-916Link, Google Scholar
49. Angst J: Switch from depression to mania—a record study of decades between 1920-1982. Psychopathology 1985; 18:140-154Crossref, Medline, Google Scholar
50. Boerlin HL, Gitlin MJ, Zoellner LA, Hammen CL: Bipolar depression and antidepressant-induced mania: a naturalistic study. J Clin Psychiatry 1998; 59:374-379Crossref, Medline, Google Scholar
51. Lewis JL, Winokur G: The induction of mania: a natural history study with controls. Arch Gen Psychiatry 1982; 39:303-306Crossref, Medline, Google Scholar
52. Calabrese JR, Bowden CL, Sachs GS, Ascher JA, Monaghan E, Rudd GD (Lamictal 602 Study Group): A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. J Clin Psychiatry 1999; 60:79-88Crossref, Medline, Google Scholar
53. Sachs GS, Lafer B, Stoll AL, Banov M, Thibault AB, Tohen M, Rosenbaum JF: A double-blind trial of bupropion vs desipramine for bipolar depression. J Clin Psychiatry 1994; 55:391-393Medline, Google Scholar
54. Cohn JB, Collins G, Ashbrook E, Wernicke JF: A comparison of fluoxetine, imipramine and placebo in patients with bipolar depressive disorder. Int Clin Psychopharmacol 1989; 4:313-322Crossref, Medline, Google Scholar
55. Sachs GS, Koslow CL, Ghaemi SN: The treatment of bipolar depression. Bipolar Disord 2000; 2:256-260Crossref, Medline, Google Scholar
56. Bottlender R, Rudolf D, Strauss A, Möller HJ: Mood-stabilizers reduce the risk of developing antidepressant-induced maniform states in acute treatment of bipolar I depressed patients. J Affect Disord 2001; 63:79-83Crossref, Medline, Google Scholar
57. Jann MW, Bitar AH, Rao A: Lithium prophylaxis of tricyclic-antidepressant-induced mania in bipolar patients. Am J Psychiatry 1982; 139:683-684Link, Google Scholar
58. Möller HJ, Grunze H: Have some guidelines for the treatment of acute bipolar depression gone too far in the restriction of antidepressants? Eur Arch Psychiatry Clin Neurosci 2000; 250:57-58Crossref, Medline, Google Scholar
59. American Psychiatric Association: Practice guideline for the treatment of patients with major depressive disorder (revision). Am J Psychiatry 2000; 157(April suppl)Google Scholar
60. Kupfer DJ. Long-term treatment of depression. J Clin Psychiatry 1991; 52:(suppl 5):28-34Google Scholar
61. Young LT, Joffe RT, Robb JC, MacQueen GM, Marriott M, Patelis-Siotis I: Double-blind comparison of addition of a second mood stabilizer versus an antidepressant to an initial mood stabilizer for treatment of patients with bipolar depression. Am J Psychiatry 2000; 157:124-126Link, Google Scholar



