The Impact of Medical Comorbidity on Acute Treatment in Major Depressive Disorder
Abstract
OBJECTIVE: The authors investigated the impact of medical comorbidity on the acute phase of antidepressant treatment in subjects with major depressive disorder. METHOD: A total of 384 outpatients meeting DSM-III-R criteria for major depressive disorder enrolled in 8-week open treatment with fluoxetine, 20 mg/day. The authors used the Cumulative Illness Rating Scale to measure the burden of medical comorbidity and the 17-item Hamilton Rating Scale for Depression to assess changes in depressive symptoms. The outcome measures were response to treatment (≥50% reduction in score) and clinical remission (score ≤7 at the end of the trial). RESULTS: Compared to responders to fluoxetine, nonresponders had significantly higher Cumulative Illness Rating Scale scores and a greater number of Cumulative Illness Rating Scale categories were endorsed. Compared to subjects who achieved remission with antidepressant treatment, those who did not achieve remission had significantly higher Cumulative Illness Rating Scale scores and a greater number of Cumulative Illness Rating Scale categories were endorsed (i.e., more organs were affected by medical illness). The final Hamilton depression scale score was directly correlated with the total Cumulative Illness Rating Scale score and the number of Cumulative Illness Rating Scale categories endorsed. CONCLUSIONS: The total burden of medical illness and the number of organ systems affected by medical illness had a significantly negative predictive value for clinical outcome in the acute phase of treatment in major depressive disorder.
For decades, the diagnosis and treatment of depressed patients with comorbid medical illness have been controversial (1, 2). Early investigators of depression in persons with medical comorbidity judged the depression to be “reactive,” i.e., a psychological consequence of having an illness and to have a less severe clinical course (3).
More recently, several studies aimed at assessing the medical and social impact of major depressive disorder associated with comorbid medical illness. Most researchers have focused on the association of major depressive disorder with specific medical problems: coronary artery disease (4), congestive heart failure (5), myocardial infarct (6, 7), stroke (8), diabetes (9, 10), cancer (11), autoimmune diseases, Parkinson’s disease, and dementia (12).
When analyzing the overall impact of medical illness on patients with major depressive disorder, the presence of comorbid medical illness was found to be associated with a higher prevalence of major depressive disorder (13–15). Other investigators found that comorbid medical illness is a risk factor for major depressive disorder (16–18). However, in other studies, the severity of baseline medical comorbidity in patients with major depressive disorder did not correlate with severity of depression (19).
Does the presence of comorbid medical illness have an impact on the outcome of antidepressant treatment? To this important clinical question, the answers to date have been mixed. The presence of medical comorbid disease has been associated with lower recovery rates (20) and greater chronicity of depression (21, 22). However, other researchers have reported that patients with chronic physical illness respond to antidepressants as well as those without such illness (23, 24).
In the current study, we investigated further the role of comorbid medical illness on severity of depression and antidepressant treatment outcome in subjects with major depressive disorder. We hypothesized that subjects with major depressive disorder and medical comorbidity would experience more severe symptoms of depression and lower rates of response and remission compared with subjects with major depressive disorder with no medical comorbidity.
Method
This study was conducted at the Depression Clinical and Research Program at Massachusetts General Hospital between 1992 and 1999 (25). A total of 380 subjects between the ages of 18 and 65 were recruited through advertisements and clinical referrals in the first phase of open-label fluoxetine treatment in a clinical study. The primary goal of the first phase was to establish prospectively nonresponse to fluoxetine. In a second phase of the study, nonresponders to fluoxetine were randomly assigned to several augmentation strategies; the results were reported elsewhere (25).
Subjects were enrolled from the general population and not from a medical setting. All subjects met criteria for major depressive disorder, diagnosed by the physician-administered Structured Clinical Interview for DSM-III-R—Patient Version (26). The subjects were required to have a score of ≥16 on the modified 17-item Hamilton Rating Scale for Depression (27) at the screening visit. Written consent was obtained from all study participants.
The exclusion criteria for this study included women of childbearing potential who were not using medically accepted means of contraception (i.e., an IUD or barrier device but not birth control pills) and women who were lactating or pregnant, had a serious suicidal risk, or a serious medical illness that was not stabilized, such as hospitalization for treatment of that illness that was likely within the next 2 weeks. Criteria also included having a seizure disorder, a history of organic mental disorders, substance use disorders, including alcohol, that were active within the last year, schizophrenia, delusional disorder, psychotic disorders not otherwise specified, bipolar disorder, mood congruent or incongruent psychotic features, or antisocial personality disorder. Subjects were also excluded if they had a history of multiple adverse drug reactions or an allergy to fluoxetine, concurrent use of psychotropic drugs, hypothyroidism, or depression that had failed in the past to respond to 60–80 mg/day of fluoxetine, to a combination of fluoxetine and lithium, or to a combination of fluoxetine and desipramine. Subjects were also excluded who failed to respond to treatment during the current episode of major depressive disorder to at least one adequate antidepressant trial (e.g., 6 weeks or more of treatment with imipramine, ≥150 mg/day, or a monoamine oxidase inhibitor, ≥60 mg/day), or had a Hamilton depression scale score <16 at the screen visit or at visit 1.
At the screening visit, the study physicians generated for each subject (meeting DSM-III-R criteria for major depression) a list of all existing and past medical illnesses, detailed by organ systems, and a list of current and past treatments. Each subject underwent physical examinations and screening laboratory tests. The results of these procedures and any subsequent medical workups were also incorporated in the list of medical illnesses. A trained physician (D.V.I.) reviewed the charts of all patients enrolled in the trial blind to treatment outcome and assigned a score on the Cumulative Illness Rating Scale, ranging from 0 to 4 for each of 13 organ systems, mental health excluded.
The Cumulative Illness Rating Scale (28, 29) is a comprehensive recording of all comorbid diseases of a patient. It classifies comorbidities by 14 organ systems affected and rates them according to their severity from 0 to 4. Within each category, when two diseases are present, the disease with the higher score is counted. A score of 0 represents “no problem,” 1=“current mild or past significant problem,” 2=“moderate disability requiring first-line treatment,” 3=“uncontrollable chronic problems or significant disability,” and 4=“end-organ failure requiring immediate treatment.” For this study, we never assigned a score of 4, since the presence of severe/emergent medical conditions was an exclusion criterion. We generated four ratings for each patient, according to the instructions of the Cumulative Illness Rating Scale: total score, number of categories endorsed, severity index (total score/number of categories endorsed), and number of categories at level 3.
The 17-item Hamilton depression scale was administered six times during the study (at screening, baseline, then every other week). We measured clinical improvement as percent of change in Hamilton depression scale score, response to treatment (defined as a ≥50% reduction in score from baseline to the end of trial), and clinical remission (defined as a score ≤7 for the last two visits of the trial).
We used multiple logistic regression to test whether any of the four Cumulative Illness Rating Scale scores measuring the burden and severity of baseline medical comorbidity could predict treatment response or remission after adjusting for patient age, gender, and baseline Hamilton depression scale score. Multiple linear regressions were used to test the correlation between baseline Cumulative Illness Rating Scale score and initial, final, and percent change in Hamilton depression scale score, after adjustment for the patient age and gender. In all of our analyses, we used the last observation carried forward.
Results
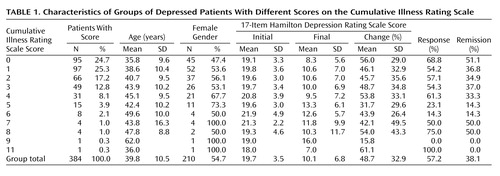
Out of 384 patients, 210 (54.7%) were female. The mean age was 39.8 years old (SD=10.5); 95 subjects (24.7%) had no comorbid medical illness (i.e., their Cumulative Illness Rating Scale score was 0). The mean total Cumulative Illness Rating Scale score was 1.90 (SD=1.86). The mean number of endorsed categories was 1.62 (SD=1.41). The mean Cumulative Illness Rating Scale severity index for this population was 1.14 (SD=0.32). Table 1 presents the clinical characteristics of our patient group and the distribution of those characteristics by burden of medical illnesses (Cumulative Illness Rating Scale score).
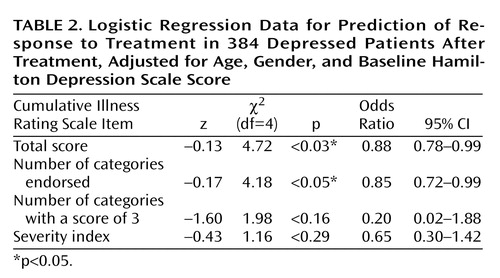
Both clinical response to fluoxetine treatment and clinical remission with treatment were significantly correlated with the total Cumulative Illness Rating Scale score and the number of organ systems affected by medical illness (number of categories endorsed). Compared to responders to fluoxetine, nonresponders had significantly higher Cumulative Illness Rating Scale scores, even when adjustments were made for age, gender, and baseline Hamilton depression scale score (2.17 versus 1.68) (logistic regression coefficient=–0.13; χ2=4.72, df=3, p=0.03). The odds ratio was 0.88, with a 95% confidence interval (CI) between 0.78 and 0.99 (i.e., there was a 12.5% decrease in the chance of achieving response for each additional point on the total burden of disease subscale of the Cumulative Illness Rating Scale). Nonresponders also had a higher number of Cumulative Illness Rating Scale categories endorsed, adjusted for age, gender, and baseline Hamilton depression scale score (1.82 versus 1.47) (logistic regression coefficient=–0.17; χ2=4.18, df=4, p<0.05). The odds ratio was 0.85, with a 95% CI between 0.72 and 0.99 (i.e., there was a 15.2% decrease in the chance of achieving response for each additional organ system affected by comorbid medical illness).
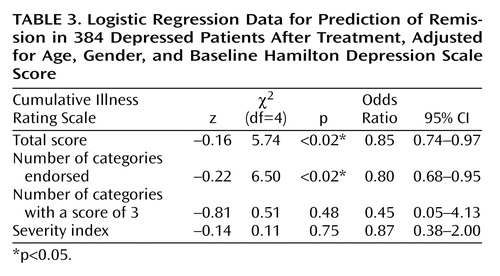
Similarly, after adjustment for age, gender, and baseline Hamilton depression scale score, subjects who achieved remission with antidepressant treatment had significantly lower Cumulative Illness Rating Scale scores than those not achieving remission (1.55 versus 2.10) (logistic regression coefficient=–0.16; χ2=5.74, df=4, p<0.02). The odds ratio was 0.85, with a 95% CI between 0.74 and 0.97 (i.e., there was a 15% decrease in the chance of remission for each additional point on the total Cumulative Illness Rating Scale score, measuring burden of disease). Subjects achieving remission also had a lower number of organ systems affected by medical illness (1.35 versus 1.79 categories endorsed) (logistic regression coefficient=–0.22; χ2=6.50, df=4, p<0.02). The odds ratio was 0.80, with a 95% CI between 0.68 and 0.95 (i.e., there was a 19.8% decrease in the chance of achieving remission for each additional organ system affected by comorbid medical illness).
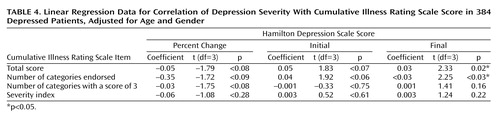
The initial severity of depression (Hamilton depression scale score) was not significantly correlated with the total Cumulative Illness Rating Scale score (linear regression coefficient=0.05; t=1.83, df=3, p<0.07), or the number of categories endorsed (linear regression coefficient=0.04; t=1.92, df=3, p<0.06). However, after we adjusted for age and gender, the final Hamilton depression scale score was directly correlated with the total Cumulative Illness Rating Scale score (linear regression coefficient=0.03; t=2.33, df=3, p=0.02) and the number of organ systems affected by any comorbid medical illness (number of categories endorsed) (linear regression coefficient=0.02; t=2.25, df=3, p<0.03).
As presented in Table 2, Table 3, and Table 4, neither of the two Cumulative Illness Rating Scale scores measuring the severity of medical comorbidity (severity index and number of categories scored as 3) correlated with any of the clinical outcome measures (remission, recovery, and percent improvement in baseline or final Hamilton depression scale scores). Our study group had low scores for severity of medical illness: the mean Cumulative Illness Rating Scale severity index was 1.14 (SD=0.32), and the mean number of Cumulative Illness Rating Scale categories scored as 3 was 0.014 (SD=0.116). The majority of our subjects had stable chronic illnesses (scored as 1 or 2 on the Cumulative Illness Rating Scale). Only six subjects had medical disorders scored as 3 on the Cumulative Illness Rating Scale (“uncontrollable chronic problems or significant disability”).
We used multiple logistic regression analysis to test whether medical illness in any of the 13 organ systems defined by the Cumulative Illness Rating Scale could predict treatment response or remission, after adjustment for age and gender. Only illnesses of the genitourinary system appeared significantly correlated with antidepressant treatment response (logistic regression coefficient=–0.68; χ2=5.34, df=13, p<0.03). No organ system was significantly correlated with remission status. Also, in multiple linear regression, no organ system was correlated with percent improvement in Hamilton depression scale scores (results not shown).
Discussion
In this large group of subjects with major depressive disorder, both response to fluoxetine treatment and clinical remission were significantly related to the burden of comorbid medical illness (Cumulative Illness Rating Scale scores) and the number of organ systems involved (number of categories endorsed). Also, subjects with a higher burden of medical comorbidity and greater number of organ systems involved had significantly higher Hamilton depression scale scores (more severe depression) at the end of treatment. This is an important result, and shows that medical comorbidity can have a significant negative impact on outcome of acute treatment of depression.
We did not find a correlation between the other measures of severity of medical comorbidity and clinical outcome (remission, recovery, or percent improvement of Hamilton depression scale scores). This is likely because of the low rates of severe medical illness in our study population.
Our findings suggest that the overall burden of medical disease, rather than just a few specific diseases, is correlated with lower rates of remission or recovery in the acute phase of antidepressant treatment in major depressive disorder. This finding is consistent with other reports in the literature (30). Keitner and colleagues (20) found that patients whose depression was compounded by medical or psychiatric illness had lower rates of recovery than patients with pure depression (28% versus 51% at 6 months).
Evans and co-workers (31, 32) presented the results of treatment with fluoxetine or placebo for a group of 62 elderly patients with major depressive disorder, 43 of whom were diagnosed with “serious physical illness” (i.e., cardiac or respiratory disease rated as moderate or severe). Although the primary analysis was a comparison between fluoxetine and placebo treatment, the tables presented results that subjects with major depressive disorder and serious physical illness had a significantly lower rate of response at 8 weeks than subjects without serious physical illness (39.5% versus 58.9%) (p<0.01). Restricting the comparison to the subjects treated with fluoxetine yielded no statistically significant difference because of the low numbers of subjects (19 subjects with serious physical illness versus 10 subjects without serious physical illness).
More recently, in a group of 671 elderly subjects with major depressive disorder who were treated with multiple antidepressants, Oslin et al. (33) found that medical comorbidity, as measured with the medical illness checklist (34), predicted more severe symptoms of depression at the 3-month follow-up. The total burden of medical illness was significantly correlated (odds ratio=0.86, 95% CI=0.79–0.94; p=0.01), with lower rates of remission from depression at the 3-month follow-up.
However, other studies are not consistent with our findings. In a large (N=671) group of depressed older patients, Small and collaborators (23) found no difference in the rates of response to antidepressants between subjects with and without chronic physical illness. However, patients were only followed for 6 weeks in their study, which may explain the lack of separation in response rates between subjects with and without chronic physical illness. Papakostas et al. (24) also reported that Cumulative Illness Rating Scale scores at baseline did not significantly predict treatment response in patients with treatment-resistant depression who were treated with nortriptyline. But for patients with treatment-resistant depression, the additional impact of medical comorbidity on treatment response may be less discernible than in our group without treatment-resistant depression.
Our study was not designed to answer questions regarding the mechanism by which medical illness affects clinical response in major depressive disorder. There are, however, several hypotheses. Ciechanowski and collaborators (34) postulated that the relationship between medical illness and depressive symptoms may be mediated by factors such as self-care, nutrition, and adherence to treatment. Pharmacokinetic or pharmacodynamic properties of antidepressants may be changed in the context of comorbid medical illness or concurrent medications (35).
Another hypothesis involves the role of cytokines, nonantibody proteins released by cells on contact with antigens. Cytokine levels are higher in a variety of infectious and noninfectious illnesses that involve activation of the immune system. Examples of chronic, noninfectious diseases associated with increased levels of cytokines include coronary artery disease (36, 37), hypertension (38), other vascular atherosclerosis (39), chronic obstructive pulmonary disease (40), diabetes (41), and arthritis and autoimmune diseases (42). Further observations have shown that administration of cytokines such as interleukin 2, tumor necrosis factor, or interferon alpha may induce depressive symptoms (42, 43). The increased production of cytokines has further impact on cortisol production and on the hypothalamic-pituitary-adrenal axis. Also, antidepressants have been shown to reduce the immune response and suppress cytokine production (42, 44). However, the association between cytokine production and response rates in major depressive disorder is still unproven.
Our study has several limitations. First, since our subjects had low rates of severe medical illness, we cannot generalize our results to populations of severely medically ill subjects. Second, since our results compare response rates of a fixed dose of fluoxetine (20 mg/day), we cannot exclude the possibility that subjects with medical comorbid illness would have a greater rate of response at higher doses of fluoxetine. Third, although the Cumulative Illness Rating Scale has been used before for the measurement of the burden of medical illness in depressed subjects (45, 46), it is unclear if it is the best instrument for this purpose. The Cumulative Illness Rating Scale has been largely used in clinical series to rate medical comorbidity, and it has good interrater and test-retest reliability (47). However, since we do not understand fully the impact of medical illness on depression outcome, we do not know if comorbid diseases should be given equal weight (as in the Cumulative Illness Rating Scale) or if certain diseases and organ systems should be given more weight in calculating the burden of medical disease. Further studies are needed to elucidate the complex relationship between the burden of medical illness and the outcome of treatment in major depression.
In conclusion, in this large group of patients with major depression treated with fluoxetine, the total burden of comorbid medical illness and the number of organ systems affected by medical illness had a significant negative prognostic value on clinical response and remission in the acute phase of antidepressant treatment with fluoxetine.
 |
 |
 |
 |
Received Dec. 27, 2002; revision received April 30, 2003; accepted May 2, 2003. From the Depression Clinical and Research Program, Psychiatry Department, Massachusetts General Hospital and Harvard Medical School. Address reprint requests to Dr. Iosifescu, Massachusetts General Hospital, 50 Staniford St., Suite 401, Boston, MA 02114; [email protected] (e-mail). Supported in part by a NIMH grant MH-48483 (to Dr. Fava) and by a Kaplen Fellowship Award for Depression from Harvard Medical School (to Dr. Iosifescu).
1. Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen H-U, Kendler KS: Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: results from the National Comorbidity Survey. Arch Gen Psychiatry 1994; 51:8–19Crossref, Medline, Google Scholar
2. Gillespie L: The clinical differentiation of types of depression. Guy’s Hospital Reports 1929; 79:1109–1114Google Scholar
3. Klerman GL: Depression in the medically ill. Psychiatr Clin North Am 1981; 4:301–317Crossref, Medline, Google Scholar
4. Steffens DC, O’Connor CM, Jiang WJ, Pieper CF, Kuchibhatla MN, Arias RM, Look A, Davenport C, Gonzalez MB, Krishnan KR: The effect of major depression on functional status in patients with coronary artery disease. J Am Geriatr Soc 1999; 47:319–322Crossref, Medline, Google Scholar
5. Koenig HG: Depression in hospitalized older patients with congestive heart failure. Gen Hosp Psychiatry 1998; 20:29–43Crossref, Medline, Google Scholar
6. Lesperance F, Frasure-Smith N, Talajic M: Major depression before and after myocardial infarction: its nature and consequences. Psychosom Med 1996; 58:99–110Crossref, Medline, Google Scholar
7. Frasure-Smith N, Lesperance F, Gravel G, Masson A, Juneau M, Talajic M, Bourassa MG: Social support, depression, and mortality during the first year after myocardial infarction. Circulation 2000; 101:1919–1924Crossref, Medline, Google Scholar
8. Robinson RG, Starr LB, Price TR: A two year longitudinal study of mood disorders following stroke: prevalence and duration at six months follow-up. Br J Psychiatry 1984; 144:256–262Crossref, Medline, Google Scholar
9. Grandinetti A, Kaholokula JK, Crabbe KM, Kenui CK, Chen R, Chang HK: Relationship between depressive symptoms and diabetes among native Hawaiians. Psychoneuroendocrinology 2000; 25:239–246Crossref, Medline, Google Scholar
10. Gavard JA, Lustman PJ, Clouse RE: Prevalence of depression in adults with diabetes: an epidemiological evaluation. Diabetes Care 1993; 16:1167–1178Crossref, Medline, Google Scholar
11. Holland JC, Romano SJ, Heiligenstein JH, Tepner RG, Wilson MG: A controlled trial of fluoxetine and desipramine in depressed women with advanced cancer. Psychooncology 1998; 7:291–300Crossref, Medline, Google Scholar
12. Reichman WE, Coyne AC: Depressive symptoms in Alzheimer’s disease and multi-infarct dementia. J Geriatr Psychiatry Neurol 1995; 8:96–99Crossref, Medline, Google Scholar
13. Wells KB, Golding JM, Burnam MA: Psychiatric disorder in a sample of the general population with and without chronic medical conditions. Am J Psychiatry 1988; 145:976–981Link, Google Scholar
14. Crum RM, Cooper-Patrick L, Ford DE: Depressive symptoms among general medical patients: prevalence and one-year outcome. Psychosom Med 1994; 56:109–117Crossref, Medline, Google Scholar
15. Patten SB: Long-term medical conditions and major depression in the Canadian population. Can J Psychiatry 1999; 44:151–157Crossref, Medline, Google Scholar
16. Koenig HG, Meador KG, Cohen HJ, Blazer DG: Depression in elderly hospitalized patients with medical illness. Arch Intern Med 1988; 148:1929–1936Crossref, Medline, Google Scholar
17. Koenig HG, Meador KG, Shelp F, Goli V, Cohen HJ, Blazer DG: Major depressive disorder in hospitalized medically ill patients: an examination of young and elderly male veterans. J Am Geriatr Soc 1991; 39:881–890Crossref, Medline, Google Scholar
18. Ganzini L, Smith DM, Fenn DS, Lee MA: Depression and mortality in medically ill older adults. J Am Geriatr Soc 1997; 45:307–312Crossref, Medline, Google Scholar
19. Coulehan JL, Schulberg HC, Block MR, Madonia MJ, Rodriguez E: Treating depressed primary care patients improves their physical, mental, and social functioning. Arch Intern Med 1997; 157:1113–1120Crossref, Medline, Google Scholar
20. Keitner GI, Ryan CE, Miller IW, Kohn R, Epstein NB: 12-month outcome of patients with major depression and comorbid psychiatric or medical illness (compound depression). Am J Psychiatry 1991; 148:345–350Link, Google Scholar
21. Akiskal H: Factors associated with incomplete recovery in primary depressive illness. J Clin Psychiatry 1982; 43:266–271Medline, Google Scholar
22. Swindle RW, Cronkite RC, Moos RH: Risk factors for sustained nonremission of depressive symptoms: a 4-year follow-up. J Nerv Ment Dis 1998; 186:462–469Crossref, Medline, Google Scholar
23. Small GW, Birkett M, Meyers BS, Koran LM, Bystritsky A, Nemeroff CB (Fluoxetine Collaborative Study Group): Impact of physical illness on quality of life and antidepressant response in geriatric major depression. J Am Geriatr Soc 1996; 44:1220–1225Crossref, Medline, Google Scholar
24. Papakostas GI, Petersen T, Iosifescu DV, Roffi PA, Alpert JE, Rosenbaum JF, Fava M, Nierenberg AA: Axis III disorders in treatment-resistant major depressive disorder. Psychiatry Res 2003; 118:183–188Crossref, Medline, Google Scholar
25. Fava M, Alpert J, Nierenberg A, Lagomasino I, Sonawalla S, Tedlow J, Worthington J, Baer L, Rosenbaum JF: Double-blind study of high-dose fluoxetine versus lithium or desipramine augmentation of fluoxetine in partial responders and nonresponders to fluoxetine. J Clin Psychopharmacol 2002; 22:379–387Crossref, Medline, Google Scholar
26. Spitzer RL, Williams JBW, Gibbon M, First MB: Structured Clinical Interview for DSM-III-R—Patient Version (SCID-P). New York, New York State Psychiatric Institute, Biometrics Research, 1989Google Scholar
27. Hamilton M: Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol 1967; 6:278–296Crossref, Medline, Google Scholar
28. Linn BS, Linn MW, Gurel L: Cumulative Illness Rating Scale. J Am Geriatr Soc 1968; 16:622–626Crossref, Medline, Google Scholar
29. Miller MD, Paradis CF, Houck PR, Mazumdar S, Stack JA, Rifai AH, Mulsant B, Reynolds CF III: Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res 1992; 41:237–248Crossref, Medline, Google Scholar
30. Popkin MK, Callies AL, Mackenzie TB: The outcome of antidepressant use in the medically ill. Arch Gen Psychiatry 1985, 42:1160–1163Google Scholar
31. Evans M, Hammond M, Wilson K, Lye M, Copeland J: Placebo-controlled treatment trial of depression in elderly physically ill patients. Int J Geriatr Psychiatry 1997; 12:817–824Crossref, Medline, Google Scholar
32. Evans M, Hammond M, Wilson K, Lye M, Copeland J: Treatment of depression in the elderly: effect of physical illness on response. Int J Geriatr Psychiatry 1997; 12:1189–1194Crossref, Medline, Google Scholar
33. Oslin DW, Datto CJ, Kallan MJ, Katz IR, Edell WS, TenHave T: Association between medical comorbidity and treatment outcomes in late-life depression. J Am Geriatr Soc 2002; 50:823–828Crossref, Medline, Google Scholar
34. Ciechanowski PS, Katon WJ, Russo JE: Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch Intern Med 2000, 27; 160:3278–3285Google Scholar
35. DeVane CL: Metabolism and pharmacokinetics of selective serotonin reuptake inhibitors. Cell Mol Neurobiol 1999; 19:443–466Crossref, Medline, Google Scholar
36. Gottsater A, Forsblad J, Matzsch T, Persson K, Ljungcrantz I, Ohlsson K, Lindgarde F: Interleukin-1 receptor antagonist is detectable in human carotid artery plaques and is related to triglyceride levels and Chlamydia pneumoniae IgA antibodies. J Intern Med 2002; 251:61–68Crossref, Medline, Google Scholar
37. Stenvinkel P, Heimburger O, Jogestrand T: Elevated interleukin-6 predicts progressive carotid artery atherosclerosis in dialysis patients: association with Chlamydia pneumoniae seropositivity. Am J Kidney Dis 2002; 39:274–282Crossref, Medline, Google Scholar
38. Cottone S, Mule G, Amato F, Riccobene R, Vadala A, Lorito MC, Raspanti F, Cerasola G: Amplified biochemical activation of endothelial function in hypertension associated with moderate to severe renal failure. J Nephrol 2002; 15:643–648Medline, Google Scholar
39. van der Meer IM, de Maat MP, Bots ML, Breteler MM, Meijer J, Kiliaan AJ, Hofman A, Witteman JC: Inflammatory mediators and cell adhesion molecules as indicators of severity of atherosclerosis: the Rotterdam Study. Arterioscler Thromb Vasc Biol 2002; 22:838–842Crossref, Medline, Google Scholar
40. Reid PT, Sallenave JM: Cytokines in the pathogenesis of chronic obstructive pulmonary disease. Curr Pharm Des 2003; 9:25–38Crossref, Medline, Google Scholar
41. Woodman RJ, Watts GF, Puddey IB, Burke V, Mori TA, Hodgson JM, Beilin LJ: Leukocyte count and vascular function in Type 2 diabetic subjects with treated hypertension. Atherosclerosis 2002; 163:175–181Crossref, Medline, Google Scholar
42. Yirmiya R, Pollak Y, Morag M, Reichenberg A, Barak O, Avitsur R, Shavit Y, Ovadia H, Weidenfeld J, Morag A, Newman ME, Pollmacher T: Illness, cytokines, and depression. Ann NY Acad Sci 2000; 917:478–487Crossref, Medline, Google Scholar
43. Meyers CA: Mood and cognitive disorders in cancer patients receiving cytokine therapy, in Cytokines, Stress and Depression. Edited by Danzer R, Wollman EE, Yirmiya R. New York, Kluwer Academic/Plenum, 1999, pp 75–81Google Scholar
44. Danzer R, Wollman E, Vitkovic L, Yirmiya R: Cytokines and depression: fortuitous or causative association. Mol Psychiatry 1999, 4:328–332Google Scholar
45. Lyness JM, Caine ED, Conwell Y, King DA, Cox C: Depressive symptoms, medical illness, and functional status in depressed psychiatric inpatients. Am J Psychiatry 1993; 150:910–915Link, Google Scholar
46. Alexopoulos GS, Meyers BS, Robert YC, Kalayam B, Kakuma T, Gabrielle M, Sirey JA, Hull J: Executive dysfunction and long-term outcomes of geriatric depression. Arch Gen Psychiatry 2000; 57:285–290Crossref, Medline, Google Scholar
47. Extermann M: Measurement and impact of comorbidity in older cancer patients. Crit Rev Oncol Hematol 2000; 35:181–200Crossref, Medline, Google Scholar



