Schizophrenia: Reproductive Hormones and the Brain
Abstract
OBJECTIVE: Onset of schizophrenia occurs during the reproductive period in more than 80% of those affected. The author reviews neuroendocrine and physiologic events that occur in the basal forebrain at the initiation of and throughout the reproductive period and proposes their possible relationship to the onset of schizophrenia. METHOD: The neuroendocrine changes that occur in specific areas of the anterior basal forebrain during the reproductive period are reviewed and analyzed in relation to reported anatomic, molecular, and biochemical pathologies of schizophrenia. RESULTS: The reproductive period is associated with development of regular pulsatile release in the brain and bloodstream of gonadotropic releasing hormones from the hypothalamus, luteinizing and follicle stimulating hormones from the pituitary, and gonadal hormones from the ovaries and testes. In addition to being concentrated in the hypothalamus, brain receptors for gonadotropic and gonadal hormones are concentrated in specific subcortical forebrain nuclei of the limbic system that project to the thalamus and to cortical and subcortical structures that subserve perception, cognition, and behavior. CONCLUSIONS: There is a flood of estrogen and testosterone to the brain and body during puberty and throughout the reproductive period. To avoid hyperexcitability and seizures, the surge of these excitatory hormones must be counterbalanced by appropriate inhibitory factors. Excessive focal inhibition may be induced by increased release of or increased receptors for one or more inhibitory transmitters, e.g., dopamine, serotonin, and γ-aminobutyric acid in the anterior basal forebrain. Further investigation of the physiology and pathology of this brain region, where abnormal electrical activity was recorded from individuals with schizophrenia many years ago and where dopamine D2 and dopamine D3 receptors targeted by the most effective antipsychotic agents are maximally expressed, could lead to greater understanding of the critical pathophysiology for development of schizophrenia.
I emerged from irrational thinking ultimately, without medicine other than the natural hormonal changes of aging. (1)
—John Nash, Nobel laureate who developed schizophrenia at age 32
Activation of the gonadotropic axis with pubertal maturation represents one of the most dramatic endocrine processes of the human life cycle (2).
The etiology of schizophrenia remains unknown in a majority of cases (Figure 1). An important clue to the genesis of this disorder is the distribution of ages at onset, which closely parallels the ages at onset and decline of the reproductive period (3) (Figure 2). Between childhood and adulthood, pulsatile secretion of luteinizing hormone (LH), the hormone that stimulates gonadal growth and gonadal hormone secretion, increases more than 30-fold in boys and 100-fold in girls (2). These hormonal changes are associated with the cascade of somatic, temperamental, and behavioral changes characteristic of puberty and of adult sexual and social behavior—and with the onset of schizophrenia in 0.5%–1% of the population. During the onset and duration of the reproductive period, circulation of the generally neuroexcitatory reproductive hormones to the brain and to the body requires compensatory changes in neurophysiology to augment the action of specific brain inhibitory systems or to diminish the action of endocrine excitatory systems. Failure to establish such an equilibrium is likely to be pertinent to the precipitation of schizophrenia in susceptible individuals.
A second clue is that, although whole brain weight is within normal limits in a majority of individuals with a diagnosis of schizophrenia, 25%–35% of those so diagnosed have larger than normal lateral ventricles and more than 50% have a larger than normal third ventricle and a lower than normal volume and number of neurons or molecular markers in one or more thalamic nuclei (3–7). A significantly lower than normal volume of the cerebral gyri or cortex, generally in the frontal or temporal lobes, has been reported in 10%–15% of schizophrenic patients (4). Statistically significant differences in the number of dendritic spines or intracellular or synaptic proteins are reported in subgroups of patients (8). Since skull size is normal in the majority of patients with schizophrenia (8), a lower than normal volume of brain tissue must in a majority of cases involve subcortical structures or, if cortical structures are involved, must occur after age 4–5 years, when head size reaches an adult level.
A third clue is derived from the EEG. In contrast to patients with epilepsy or other disorders involving cortical structures of the brain, the majority of (unmedicated) patients with acute or long-term schizophrenia have scalp EEG results generally within normal limits (9). However, earlier studies in which electrodes were placed in subcortical structures of patients with schizophrenia revealed episodic spike activity in the septal area or the amygdala during acute psychotic periods (10–13). Spike activity recorded from these individuals usually occurred singly, unlike the prolonged runs of rapid rhythmic spikes recorded during epileptic seizures, and did not generally propagate to or appear in cortical sites, in contrast to interictal EEG spikes in a majority of epilepsies.
Treatment and Pathophysiology
The first effective modern treatment for schizophrenia was induction of epileptic seizures by pharmacologic convulsants or by electrical stimulation at the scalp (14). Thus, it is not surprising that current effective treatment uses a variety of antipsychotics that antagonize cerebral receptors for neurotransmitters that are generally considered inhibitory, e.g., dopamine, serotonin (5-HT), norepinephrine, and γ-aminobutyric acid (GABA) (15–19). Pharmacologic blockade of receptors for each of these neurotransmitters can induce epileptic seizures in both animals and humans, depending on the dose of and individual susceptibility to the blocking agent. In contrast, schizophrenia-like psychoses may be precipitated in susceptible individuals by pharmacologic agents that enhance inhibitory factors, e.g., the excitatory amino acid antagonists phencyclidine, MK801, and monoamine agonists such as the amphetamines and lysergic acid diethylamide (LSD) (20, 21).
The most effective agent against treatment-resistant symptoms of schizophrenia is the atypical antipsychotic clozapine (22, 23). Unlike earlier antipsychotics, which have a high affinity for dopamine D2 receptors, clozapine has greater affinity for the serotonin (5-HT1A, 5-HT2, 5-HT3), muscarinic, histamine, dopamine D3, and dopamine D4 receptors and blocks N-methyl-d-aspartic acid antagonists (18, 19). Although all neuroleptics can cause seizures in susceptible individuals or when given in high doses, clozapine is the most proconvulsive of these agents (24). Clozapine blocks dopamine D3 receptors in the nucleus accumbens more than in the caudate-putamen, and, in contrast to other antipsychotics, induces c-fos expression in the accumbens shell but not in the caudate-putamen (25). Clozapine does not produce the extrapyramidal side effects associated with the earlier antipsychotics and with high doses of the newer atypical antipsychotics (16–18). Higher levels of dopamine in the nucleus accumbens and the islands of Calleja (26) and expression of dopamine D3 receptors in the nucleus accumbens, the ventral striatal gateway from amygdala and hippocampus to thalamus and frontal lobes have been reported in brains from some schizophrenic patients (27). In one recent report, a higher level of dopamine D3 receptors was reported from peripheral blood lymphocytes of unmedicated schizophrenic patients as well as those receiving neuroleptics (28). Polymorphisms of the dopamine D3 receptor have been associated with schizophrenia (29). Clozapine has greater affinity for 5-HT2A and dopamine D4than for dopamine D3 receptors. However, in contrast to agents that block dopamine D2 and dopamine D3 receptors, 5-HT2A and dopamine D4 antagonists are not effective against symptoms of schizophrenia (30). Unlike neuroleptics that principally block the dopamine D2 receptor and cause a sustained and substantial increase in serum prolactin and Fos in the caudate-putamen, clozapine induces only a transient smaller increase in serum prolactin and elicits expression of Fos in the paraventricular nucleus of thalamus (31) and expression of Fos and c-fos mRNA in the islands of Calleja in the basal forebrain (32). The possible significance of these phenomena will be discussed in the next section.
Schizophrenia and the Reproductive Circuits of the Brain
The onset of schizophrenia at the beginning of or during the reproductive period suggests a relationship between this disorder and the dramatic changes in the brain and the body that take place during adolescence and throughout the fertility period. During adolescence, these changes include activation and amplification of the pulsed release of gonadotropin-releasing (luteinizing) hormone, a 30-fold increase in release of LH in boys and a 100-fold increase in girls, and a rapid increase in circulating estrogens and androgens from the gonads (2). In addition to being present in the hypothalamus, androgen and estrogen receptors in the brain are concentrated in the medial amygdala, bed nucleus of stria terminalis, and lateral septum in the basal forebrain of all mammals including humans (Figure 3[33]). Steroid-induced changes in the limbic-hypothalamic circuit during the reproductive cycle have been reported by several investigators. Woolley and McEwen (34) reported a change in the numbers of dendritic spines on pyramidal neurons in the highly epileptogenic CA1 area of the hippocampus of the rat in response to estrogen. The increase in circulating androgens and estrogens associated with sexual maturation leads to other significant changes in brain axons and receptors as well as the well-known physical, physiologic, psychologic, and behavioral changes associated with the flood of hormones to brain and body during this period (35, 36).
Between the ages of 9 and 13 years, the pulsatile release of gonadotropin-releasing hormone is associated with regular episodic bursts of neuronal discharge in the arcuate nucleus of the hypothalamus reflected in the episodic release of LH to the bloodstream from the anterior pituitary (2). Episodic neuronal discharges also occur in the amygdala, hippocampus, bed nucleus of stria terminalis, and septal and other limbic nuclei during coition, ovulation, and other critical events of the reproductive cycle (37–41). The physiologic occurrence of these sharply localized high-frequency discharges in limbic forebrain structures that concentrate and express receptors for reproductive hormones indicates the importance of brief synchronous bursts of rapid neuronal activity and of the limitation of such bursts to circumscribed areas for delivery of vital messages in the neuroendocrine network.
The amygdala and hippocampus, specific regions of which project to these basal forebrain structures and to the hypothalamus, are normally endowed with the lowest threshold for rapid neuronal seizure-like discharge of any brain region. This fact requires that excitatory circuits (principally glutamatergic and cholinergic axon terminals that project from the excitatory neurons of the amygdala and hippocampus to the nucleus accumbens, septum, and other forebrain sites) be protected by inhibitory networks to prevent propagation of episodic physiologic rapid discharges outside areas of physiologic utility, leading to seizures. Inhibitory activity is provided by the high concentration of dopamine D3, 5-HT, and GABA-ergic terminals and receptors in this region. The action of these inhibitory systems must be increased to balance the effect of the entry of the excitatory reproductive hormones into specific brain areas at puberty and throughout the reproductive period. However, excessive inhibitory response to these physiologic events via dopamine, 5-HT, or GABA can cause psychosis in susceptible individuals. Indeed, the psychotic response to potentiators of inhibition such as amphetamine and ketamine does not occur before puberty (20, 42). Very few published studies have examined the changes in innervation of the brain that occur during and after the onset of puberty and the reproductive period (B. McEwen, personal communication, 2001). However, Benes et al. (43) reported a progressive increase in myelination in the subiculum and presubiculum of the hippocampus during normal human maturation, and Fink et al. (44) described a marked increase in 5 HT2A receptors in the forebrain coincident with proestrus in the rat. The importance of gonadal hormones in modulating the dopamine system is dramatically illustrated by the permanent loss of 30% of dopamine neurons from the substantia nigra after oophorectomy in the monkey (45).
In the brain of both male and female subjects, estrogen α and β receptors and progesterone, androgen, and prolactin receptors are widely distributed in several extrahypothalamic basal forebrain sites, including the amygdala, lateral septum, bed nucleus of stria terminalis, nucleus of diagonal band, basal nucleus of Meynert, periaqueductal grey matter, and islands of Calleja (46–50) (Figure 3). In addition to receptors for LH and for estrogen and testosterone, gonadotropic-releasing hormone mRNA is present outside the hypothalamus in a number of basal forebrain and limbic structures, including the central and dorsal nuclei of amygdala, ventral striatum, ventral pallidum, putamen, basal nucleus of Meynert, bed nucleus of stria terminalis, and septal and dorsal preoptic regions in both rat and human (51).
The islands of Calleja are multiple discrete clusters of small granular neurons in the ventral striatum surrounding larger pallidal-like neurons and a vascular core. Islands of Calleja complexes are present in all species and attain maximum development and dispersion in humans (52). Surrounded by a dense plexus of cholinergic, dopaminergic, and peptidergic axons, the islands of Calleja complexes are embedded in the ventral and medial border of the ventral striatum (nucleus accumbens in primates, as olfactory tubercle has largely disappeared in primate species). The large island of Calleja (major) is located in the medial border of the accumbens adjacent to the vertical limb of the diagonal band (Figure 3). Unlike the neurons in the neostriatum and ventral striatum, some of the larger neurons of the islands of Calleja contain gonadotropin-releasing hormone (luteinizing hormone-releasing hormone), concentrate estradiol, and express estrogen and testosterone receptors (53–56). The ventral group of islands of Calleja is dispersed along the pial border of the basal forebrain in close relation to blood vessels, suggesting possible humoral inputs, including serum endocrine factors (53, 54). Axons from the large medial island of Calleja, in parallel to the ventral striatum, project to the dorsomedial nucleus of thalamus and could thus influence this relay nucleus to the frontal lobes. The islands of Calleja are surrounded by muscarinic and 5-HT terminals and contain the highest concentration of dopamine D3 receptors in brain (55, 56). In addition to antagonizing dopamine D3 and 5-HT receptors, clozapine is a potent muscarinic antagonist and elicits Fos immunoreactivity in the islands of Calleja (57).
Fallon and associates (53, 54) proposed that the anterior basal forebrain network containing gonadotropic hormones and androgen and estrogen receptors constitutes an extrahypothalamic hormone regulatory system closely related to the sensory, motor, and affective aspects of reproductive behavior. A threefold increase in the pituitary hormone prolactin during sleep, abnormal growth hormone response to luteinizing hormone-releasing hormone and thyrotropin-releasing hormone, and a lower level of total gonadotropins and testosterone were reported in unmedicated male schizophrenic patients, relative to age-matched comparison subjects (58, 59). Other investigators have attributed the later onset of schizophrenia in women and the postmenopausal onset of the disorder to the protective effect of estrogen (3, 60, 61). Estrogen is excitatory in the brain, and changes in estrogen level during the menstrual cycle correlate with exacerbation or remission of psychosis or seizures in susceptible girls and women (61, 62). Aromatase, which converts testosterone to estrogen, is widely distributed in the brain and other organs and may be of significance in schizophrenia (63).
The extension of the sharply localized episodic and rapid neuronal discharges associated with endocrine release beyond regions of physiologic utility, which could lead to seizures, is opposed by widely distributed inhibitory pathways and transmitters. These include dopamine, norepinephrine, 5-HT, GABA, specific peptides, and the multiple receptors for these transmitters. Dopamine D2 receptors are maximally expressed in the neostriatum (caudate-putamen) where excitatory (glutamate) axons from the neocortex and thalamus converge. Dopamine D3 receptors predominate in the ventral striatum (nucleus accumbens in humans), where axons from the amygdala and hippocampus, the regions that physiologically demonstrate the lowest threshold for epileptic discharge in the brain, project. The accumbens projects to the ventral pallidum and then to the hypothalamus and to the dorsomedial thalamus and orbital and medial frontal lobes (64, 65). Maximum expression of dopamine D3 receptors in the islands of Calleja and nucleus accumbens is consistent with the required limitation of neuronal discharges from the physiologically seizure-prone amygdala and hippocampus from spreading beyond their projection sites in the hypothalamus, accumbens, septal, and related basal forebrain nuclei. The therapeutic as well as the epileptogenic propensity of clozapine may relate to preferential block of dopamine D3 and 5-HT2A receptors that are strongly expressed in these basal forebrain sites, which are rich in reproductive hormones and endocrine receptors.
The concentration of excitatory (muscarinic) and inhibitory (dopaminergic) plexuses enclosing islands of forebrain neurons that express receptors for reproductive hormones is consistent with a balance of inhibitory and excitatory activity that regulates the effect of the reproductive hormones on neuronal excitability in this region. Pharmacotherapeutic evidence for disturbed cerebral monoamine regulation in schizophrenia draws attention to this forebrain area. The surge of excitatory reproductive hormones to this area during the reproductive period must be counterbalanced by enhanced inhibitory activity to prevent seizures, but overcompensation may induce psychosis. Maximum occurrence of schizophrenia during the fertility period and the peculiar olfactory, tactile, and sexual hallucinations and delusions frequently experienced by individuals with this disorder may indicate abnormal augmentation of one or more inhibitory factors induced by the physiologic flood of excitatory gonadal steroids to the anterior basal forebrain areas where receptors for the excitatory reproductive hormones, olfactory centers, and projections to the thalamus and frontal lobes converge.
Other research has provided additional evidence of altered excitability of neurons in the basal forebrain in schizophrenia. Many years ago several groups of investigators reported episodic spike activity recorded from electrodes implanted in the septal region or amygdala of patients with active symptoms of schizophrenia (10–13). Similar single-spike activity is often observed in the EEGs of individuals with epilepsy recorded during periods between seizures. Multiple spikes resembling focal seizures were also recorded by EEG from the septal region of normal men during orgasm or during orgasmic sensations induced by electrical or cholinergic stimulation of this region (10, 11). Rats with stimulating electrodes placed in the septal accumbens area will press a lever hundreds of times per minute for electric stimulation to the point of producing seizures (66). Stimulation of septal-accumbens area is associated with positive reward in rats and orgasm in humans (10, 11). The dense dopaminergic innervation and high concentration of dopamine D3 receptors in the basal forebrain are consistent with an extrahypothalamic regulatory system that limits propagation of physiologic excitatory discharges critical to sexual and reproductive function from extending beyond areas of physiologic utility. The special value of clozapine for treatment of some otherwise treatment-resistant cases of schizophrenia could relate to the relatively greater affinity of this drug for these receptors, which are physiologically strongly expressed in the basal forebrain area and may be excessively expressed in schizophrenia (26–28) (as, for example, are several excitatory systems in epilepsy).
Conclusions
Development of schizophrenia during the reproductive period in a majority of those affected suggests that this disorder is related to a disturbance in the balance between one or more inhibitory and excitatory factors in response to the flood of reproductive hormones to the brain and consequent compensatory remodeling of synapses in specific brain areas. Receptors for and neurons containing reproductive hormones are strongly expressed in the hypothalamus, and receptors for these hormones are strongly expressed in the extrahypothalamic nuclei of the basal forebrain that receive afferents from the amygdala and hippocampus and project via the thalamus to the cerebral cortex. The forebrain nuclei that express reproductive hormone receptors are regulated by glutamatergic and cholinergic excitation and by dopaminergic, serotonergic, and GABA-ergic inhibition. Although no single anatomic site or disturbance in physiology that is pathognomonic or essential for development of schizophrenia has been identified, recordings from the basal forebrain nuclei in schizophrenic patients demonstrated abnormal electrical activity in these areas and pathologic examination demonstrated a higher than normal level of dopamine receptors in this region in some individuals with this disorder. Amelioration of symptoms of schizophrenia with antagonists of inhibitory receptors or by convulsive therapy could indicate that the underlying pathophysiology is a loss of the physiologic equilibrium between brain excitatory and inhibitory systems in this critical brain area in genetically susceptible individuals. The maximum occurrence of schizophrenia during the reproductive period and the range of responses to different antipsychotic agents by different individuals suggest that this imbalance is associated with pathologic extension of physiologic inhibition by one or more inhibitory factors beyond the basal forebrain in response to the flood of excitatory hormones to the brain during the reproductive period. This hypothesis can be tested by studies of anatomic and neurochemical circuitry of this region in schizophrenia.
Received July 9, 2001; revision received Nov. 8, 2001; accepted Nov. 16, 2001. From the Departments of Neurology and Psychiatry, Oregon Health Sciences University. Address reprint requests to Dr. Stevens, Department of Psychiatry, Oregon Health Sciences University, 3181 S.W. Sam Jackson Park Rd., Portland, OR 97201-3098; [email protected] (e-mail). The author thanks Drs. Duane Denney, Lennart Heimer, Bruce McEwen, and Susan Smith for comments and suggestions on the manuscript.
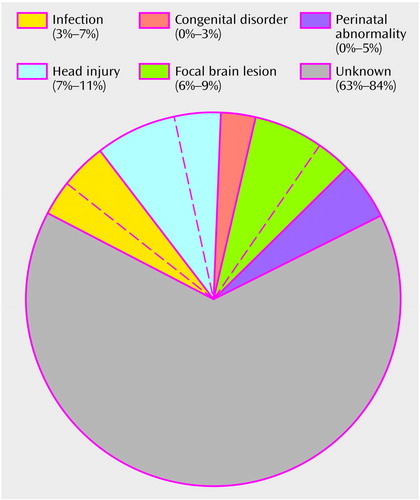
Figure 1. Events Preceding Onset of Schizophrenia
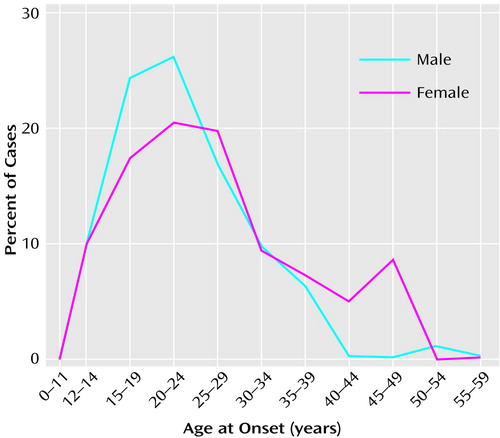
Figure 2. Age at First Sign of Mental Disorder in Patients With a Diagnosis of Schizophrenia (N=248)a
aAdapted, with the permission of Cambridge University Press, from Häfner et al. (3).
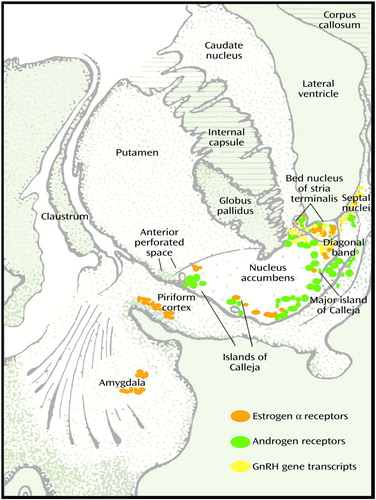
Figure 3. Cross-Section of Human Basal Forebraina
aAdapted from Riley (33).
1. Nasar S: A Beautiful Mind: The Life of Mathematical Genius and Nobel Laureate John Nash. New York, Simon & Schuster, 1998Google Scholar
2. Veldhuis JD: Neuroendocrine mechanisms mediating awakening of the human gonadotropic axis in puberty. Pediatr Nephrol 1996; 10:304-317Crossref, Medline, Google Scholar
3. Häfner H, Riecher-Rössler A, an der Heiden W, Maurer K, Fätkenheuer B, Löffler W: Generating and testing a causal explanation of the gender difference in age at first onset of schizophrenia. Psychol Med 1993; 23:925-940Crossref, Medline, Google Scholar
4. Shenton ME, Dickey CC, Frumin M, McCarley RW: A review of MRI findings in schizophrenia. Schizophr Res 2001; 49:1-52Crossref, Medline, Google Scholar
5. Andreasen NC: The role of the thalamus in schizophrenia. Can J Psychiatry 1997; 42:27-33Crossref, Medline, Google Scholar
6. Pakkenberg B: The volume of the medial dorsal thalamic nucleus in treated and in untreated schizophrenia. Schizophr Res 1992; 7:95-100Crossref, Medline, Google Scholar
7. Pearlson GD, Marsh L: Structural brain imaging in schizophrenia: a selective review. Biol Psychiatry 1999; 46:627-649Crossref, Medline, Google Scholar
8. Falkai P, Bogerts B: The neuropathology of schizophrenia, in Schizophrenia. Edited by Hirsch SR, Weinberger DR. Oxford, UK, Blackwell Science, 1995, pp 275-292Google Scholar
9. Stevens JR, Livermore A Jr: Telemetered EEG in schizophrenia: spectral analysis during abnormal behavior episodes. J Neurol Neurosurg Psychiatry 1982; 45:385-395Crossref, Medline, Google Scholar
10. Heath RG: Studies in Schizophrenia. Cambridge, Mass, Harvard University Press, 1954Google Scholar
11. Sem-Jacobson CW: Some observations during recording in psychotic patients. Electroencephalogr Clin Neurophysiol 1956; 8:531-532Google Scholar
12. Hanley J, Rickles WR, Crandall PH, Walter RD: Automatic recognition of EEG correlates of behavior in a chronic schizophrenic patient. Am J Psychiatry 1972; 128:1524-1528Link, Google Scholar
13. Kendrick JF, Gibbs F: Origin, spread and neurosurgical treatment of the psychomotor type of seizure discharge. J Neurosurg 1957; 14:270-283Crossref, Medline, Google Scholar
14. von Meduna LJ: Über experimentelle Campherepilepsie. Arch Psychiatr 1934; 102:333-339Crossref, Google Scholar
15. Carlsson A: The current status of the dopamine hypothesis of schizophrenia. Neuropsychopharmacology 1988; 1:179-186Crossref, Medline, Google Scholar
16. Meltzer HY: Treatment of schizophrenia and spectrum disorders: pharmacotherapy, psychosocial treatments and neurotransmitter interactions. Biol Psychiatry 1999; 46:1321-1327Crossref, Medline, Google Scholar
17. Kane J: Pharmacologic treatment of schizophrenia. Biol Psychiatry 2000; 46:1396-1408Crossref, Google Scholar
18. Ackenheil M: Clozapine-pharmacokinetic investigations and biochemical effects in man. Psychopharmacology (Berl) 1989; 99(suppl 1):32-37Google Scholar
19. Leysin JE: Serotonergic receptors in brain tissue: properties and identification of various 3H-ligand binding sites in vitro. J Physiol (Paris) 1981; 77:351-362Medline, Google Scholar
20. Javitt DC, Zukin SR: Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry 1991; 148:1301-1308Link, Google Scholar
21. Angrist B, Rotrosen J, Gershon S: Differential effects of amphetamine and neuroleptics on negative vs positive symptoms in schizophrenia. Psychopharmacology (Berl) 1980; 72:17-19Crossref, Medline, Google Scholar
22. Conley RR, Tamminga CA, Kelly DL, Richardson CM: Treatment-resistant schizophrenic patients respond to clozapine after olanzapine non-response. Biol Psychiatry 1999; 46:73-77Crossref, Medline, Google Scholar
23. Volavka J, Czobor P, Sheitman B, Lindenmeyer J-P, Citrome L, McEvoy JP, Cooper TB, Chakos M, Lieberman JA: Clozapine, olanzapine, risperidone, and haloperidol in the treatment of patients with chronic schizophrnenia and schizoaffective disorder. Am J Psychiatry 2002; 159:255-262Link, Google Scholar
24. Devinsky O, Honigfeld G, Patin J: Clozapine-related seizures. Neurology 1991; 41:369-371Crossref, Medline, Google Scholar
25. Guo N, Vincent SR, Fibiger HC: Phenotypic characteristics of neuroleptic-sensitive neurons in the forebrain: contrasting targets of haloperidol and clozapine. Neuropsychopharmacology 1998; 19:133-145Crossref, Medline, Google Scholar
26. Bird ED, Spokes ES, Iversen LL: Increased dopamine concentration in limbic areas of brains from patients dying with schizophrenia. Brain 1979; 102:347-360Crossref, Medline, Google Scholar
27. Gurevich EV, Bordelon Y, Shapiro RM, Arnold SE, Gur RE, Joyce JN: Mesolimbic dopamine D3 receptors and use of antipsychotics in patients with schizophrenia: a postmortem study. Arch Gen Psychiatry 1997; 54:225-232Crossref, Medline, Google Scholar
28. Ilani T, Ben-Shachar D, Strous RD, Mazor M, Sheinkman A, Kotler M, Fuchs S: A peripheral maker for schizophrenia: increased levels of D3 dopamine receptor mRNA in blood lymphocytes. Proc Natl Acad Sci USA 2001; 98:625-628Crossref, Medline, Google Scholar
29. Schwartz JC, Diaz J, Bordet R, Griffon N, Perachon S, Pilon C, Ridray S, Sokoloff P: Functional implications of multiple dopamine receptor subtypes: the D1/D3 receptor coexistence. Brain Res Brain Res Rev 1998; 26:236-242Crossref, Medline, Google Scholar
30. Truffinet P, Tamminga CA, Fabre LF, Meltzer HY, Rivière ME, Papillon-Downey C: Placebo-controlled study of the D4/5-HT2A antagonist fananserin in the treatment of schizophrenia. Am J Psychiatry 1999; 156:419-425Abstract, Google Scholar
31. Buber M, Deutch AY: Thalamic paraventricular nucleus neurons collateralize to innervate the prefrontal cortex and nucleus accumbens. Brain Res 1998; 787:304-310Crossref, Medline, Google Scholar
32. Wirtshafter D: D1 dopamine receptors mediate neuroleptic-induced Fos expression in the islands of Calleja. Synapse 1998; 28:154-159Crossref, Medline, Google Scholar
33. Riley HA: An Atlas of the Basal Ganglia, Brain Stem and Spinal Cord. Baltimore, Williams & Wilkins, 1943, p 304Google Scholar
34. Woolley C, McEwen BS: Estradiol regulates hippocampal dendritic spine density via an NMDA receptor dependent mechanism. Abstracts of the Society for Neuroscience 1993; 19:379Google Scholar
35. McEwen BS, Alves SE: Estrogen actions in the central nervous system. Endocr Rev 1999; 20:279-301Medline, Google Scholar
36. MacLean PD: Some psychiatric implications of physiological studies on frontotemporal portion of limbic system (visceral brain). Electroencephalogr Clin Neurophysiol 1952; 4:407-418Crossref, Medline, Google Scholar
37. O’Byrne KT, Thalabard JC, Grosser PM, Wilson RC, Williams CL, Chen MD, Ladendorf D, Hotchkiss J, Knobil E: Radiotelemetric monitoring of hypothalamic gonadotropin-releasing hormone pulse generator activity throughout the menstrual cycle of the rhesus monkey. Endocrinology 1991; 129:1207-1214Crossref, Medline, Google Scholar
38. Knobil E: Remembrance: the discovery of the hypothalamic gonadotropin-releasing hormone pulse generator and of its physiological significance. Endocrinology 1992; 131:1005-1006Medline, Google Scholar
39. Kawakami M, Terasawa E, Ibuki T: Changes in multiple unit activity of the brain during the estrous cycle. Neuroendocrinology 1970; 6:30-48Crossref, Medline, Google Scholar
40. Kawakami M, Uemura T, Hayashi R: Electrophysiological correlates of pulsatile gonadotropin release on rats. Neuroendocrinology 1982; 35:63-67Crossref, Medline, Google Scholar
41. Andrew DA, Dudek FE: Analysis of intracellularly recorded phasic bursting by mammalian neuroendocrine cells. J Neurophysiol 1984; 51:453-468Google Scholar
42. Stevens JR: All that spikes is not fits, in What Is Epilepsy? Edited by Trimble M, Reynolds T. Edinburgh, Churchill Livingstone, 1986, pp 97-115Google Scholar
43. Benes FM, Turtle M, Khan Y, Faroi P: Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Arch Gen Psychiatry 1994; 51:477-484Crossref, Medline, Google Scholar
44. Fink G, Sumner BEH, McQueen JK, Wilson H, Rosie R: Sex steroid control of mood, mental state and memory. Clin Exp Pharmacol Physiol 1998; 25:764-775Crossref, Medline, Google Scholar
45. Leranth C, Roth EH, Elsworth JD, Naftolin F, Horvath TL, Redmond DE Jr: Estrogen is essential for maintaining nigrostriatal dopamine neurons in primates: implications for Parkinson’s disease and memory. J Neurosci 2000; 20:8604-8609Medline, Google Scholar
46. Pfaff D, Keiner M: Atlas of estrogen binding cells in the central nervous system of female rat. J Comp Neurol 1973; 151:121-158Crossref, Medline, Google Scholar
47. Mufson EJ, Cai WJ, Jaffar S, Chen EY, Stebbins G, Senders T, Kordower JH: Estrogen receptor immunoreactivity within subregions of the rat forebrain: neuronal distribution and association with perikarya-containing choline acetyltransferase. Brain Res 1999; 849:253-274Crossref, Medline, Google Scholar
48. Greco B, Edwards DA, Michael RP, Clancy AN: Androgen receptors are colocalized in male rat hypothalamic and limbic neurons that express Fos immunoreactivity induced by mating. Neuroendocrinology 1998; 67:18-28Crossref, Medline, Google Scholar
49. Kuiper GG, Shugrue PJ, Merchenthaler I, Gustafssson JA: The estrogen receptor β subtype: a novel mediator of estrogen action in neuroendocrine systems. Front Neuroendocrinol 1998; 19:253-286Crossref, Medline, Google Scholar
50. Fernandez-Guasti A, Kruijver FPM, Fodor M, Swaab DF: Sex differences in the distribution of androgen receptors in the human hypothalamus. J Comp Neurol 2000; 425:422-435Crossref, Medline, Google Scholar
51. Rance NE, Young WS III, McMullen NT: Topography of neurons expressing luteinizing hormone-releasing hormone gene transcripts in the human hypothalamus and basal forebrain. J Comp Neurol 1994; 339-386Google Scholar
52. Meyer G, Gonzalez-Hernandez T, Carrillo-Padilla F, Ferres-Torres R: Aggregations of granule cells in the basal forebrain (islands of Calleja): Golgi and cytoarchitectonic study in different mammals, including man. J Comp Neurol 1989; 284:405-428Crossref, Medline, Google Scholar
53. Fallon JH, Loughlin SE, Ribak CE: The islands of Calleja complex of rat basal forebrain, III: histochemical evidence for a striatopallidal system. J Comp Neurol 1983; 218:91-120Crossref, Medline, Google Scholar
54. Fallon JH: The islands of Calleja complex of rat basal forebrain, II: connections of medium and large sized cells. Brain Res Bull 1983; 10:775-793Crossref, Medline, Google Scholar
55. Talbot K, Woolf NJ, Butcher LL: Feline islands of Calleja complex, II: cholinergic and cholinesterasic features. J Comp Neurol 1988; 275:580-603Crossref, Medline, Google Scholar
56. Suzuki M, Hurd YL, Sokoloff P, Schwartz JC, Sedvall G: D3 dopamine receptor mRNA is widely expressed in the human brain. Brain Res 1998; 779:58-74Crossref, Medline, Google Scholar
57. Bolden C, Cusack B, Rihelson E: Clozapine is a potent and selective muscarinic antagonist at the five cloned human muscarinic acetylcholine receptors expressed in CHO-K1 cells. Eur J Pharmacol 1991; 192:205-206Crossref, Medline, Google Scholar
58. Van Cauter P, Linkowski P, Kerkhofs M, Hubain P, L’Hermite-Baleriaux M, Leclercq R, Brasseur M, Capinschi G, Mendelewicz J: Circadian and sleep related endocrine rhythms in schizophrenia. Arch Gen Psychiatry 1991; 48:348-356Crossref, Medline, Google Scholar
59. Gil-Ad I, Dickerman Z, Weizman R, Weizman A, Tyano S, Laron Z: Abnormal growth hormone response to LRH and TRH in adolescent schizophrenic boys. Am J Psychiatry 1981; 138:357-360Link, Google Scholar
60. Kulkarni J, Riedel A, de Castella AR, Fitgerald PB, Rolfe TJ, Taffe J, Burger H: Estrogen—a potential treatment for schizophrenia. Schizophr Res 2001; 48:137-144Crossref, Medline, Google Scholar
61. Riecher-Rossler A, Hafner H, Dutsch-Strobel A, Oster M, Stumbaum M, van Gulick-Bailer M, Loffler W: Further evidence for a specific role of estradiol in schizophrenia? Biol Psychiatry 1994; 36:492-495Crossref, Medline, Google Scholar
62. Herzog AG: Psychoneuroendocrine aspects of temporal lobe epilepsy, part II: epilepsy and reproductive steroids. Psychosomatics 1999; 48:102-108Crossref, Google Scholar
63. Cohen RZ, Seeman MV, Gotowiec A, Kopala L: Earlier puberty as a predictor of later onset of schizophrenia in women. Am J Psychiatry 1999; 156:1059-1064Abstract, Google Scholar
64. Heimer L, DeOlmos JS, Alheid GF, Pearson J, Sakamoto N, Shinoda K, Marsteiner J, Switzer RC III: The human basal forebrain, part II, in Handbook of Chemical Neuroanatomy. Edited by Bloom FE, Bjorklund A, Hokfelt T. Amsterdam, Elsevier, 1999, pp 57-225Google Scholar
65. Stevens JR: Epilepsy, schizophrenia, and the extended amygdala. Ann NY Acad Sci 1999; 877:548-561Crossref, Medline, Google Scholar
66. Olds J, Milner P: Positive reinforcement produced by electrical stimulation of septal areas and other regions of the rat brain. J Comp Physiol Psychol 1954; 47:419-427Crossref, Medline, Google Scholar



