Relationship Between Distressing Cancer-Related Recollections and Hippocampal Volume in Cancer Survivors
Abstract
OBJECTIVE: Having cancer is extremely stressful, and distressing cancer-related recollections are frequently reported by cancer survivors. Smaller hippocampal volume has been observed in stress-related neuropsychiatric disorders, such as posttraumatic stress disorder (PTSD) and major depression. The aim of this study was to determine whether there is a similar association between distressing cancer-related recollections and hippocampal volume. METHOD: The subjects were 67 women who had had breast cancer surgery 3 or more years earlier and had no history of PTSD or major depression before the cancer. Each woman was evaluated with a semistructured interview to determine whether she had a history of distressing cancer-related recollections. Hippocampal volume was measured by three-dimensional magnetic resonance imaging, and memory function was assessed by the Wechsler Memory Scale—Revised. RESULTS: The volume of the left hippocampus was significantly smaller (5%) in the subjects with a history of distressing cancer-related recollections (N=28) than in those without any such history (N=39). There was no significant difference in right hippocampal volume or whole brain volume measured as a control. There were no significant differences in delayed memory or percentage retention. However, significantly worse immediate visual memory, but not verbal memory, was observed in the subjects with a history of distressing cancer-related recollections. CONCLUSIONS: Having distressing cancer-related recollections is associated with smaller left hippocampal volume in survivors of breast cancer.
Having cancer is extremely stressful, and distressing recollections related to cancer experiences are frequently observed in a clinical oncology setting. Previous studies focusing on distress caused by cancer (1, 2) have shown that 20%–37% of cancer survivors suffer from reexperiencing symptoms, including distressing recollections of cancer-related experiences. Intrusions have been reported by 24%–44% of cancer survivors (3, 4). Although distressing symptoms have been frequently reported by cancer survivors (5), little is known about the neuropsychobiological basis of intrusive and distressing recollections related to cancer experiences.
Hippocampal volume abnormalities have been reported in neuropsychiatric disorders associated with stress (6). Volumetric studies of patients with major depression (7–9), using high-resolution magnetic resonance imaging (MRI), have indicated 8%–19% smaller bilateral hippocampi than in matched comparison subjects. Posttraumatic stress disorder (PTSD) is another stress-related psychiatric disorder. One study of Vietnam combat veterans (10) showed that PTSD patients have a significantly smaller right hippocampus (8% smaller) than matched comparison subjects, and another study (11) showed a bilaterally smaller hippocampus (22% and 26% smaller, respectively). Patients who were physically and sexually abused during childhood have been found to have a significantly smaller left hippocampus (5%–12% smaller) (12, 13).
Animal studies have indicated that stress and glucocorticoids can induce atrophy of dendritic processes, cause neuronal loss, and inhibit neurogenesis (14–18) by decreasing the level of brain-derived neurotrophic factor and releasing excitatory amino acids (19, 20). Associations between cortisol levels and hippocampal atrophy in humans have been reported (21). While glucocorticoids have been postulated to induce neuronal damage, there is no direct evidence of hippocampal neuronal damage in major depression or PTSD (6, 7).
The purpose of this study was to investigate the relationship between hippocampal volume and a history of distressing cancer-related recollections among cancer survivors. A secondary purpose was to investigate the relationship between memory function and a history of distressing cancer-related recollections. We hypothesized that subjects with a history of such recollections would have smaller hippocampal volumes than those without any such history.
Method
Subjects
This study was approved by the institutional review board and the ethics committee of the National Cancer Center, Tokyo. The study was performed after patients’ written informed consent was obtained.
The subjects were recruited from February 1998 to April 1999 during follow-up visits to the Division of Breast Surgery, National Cancer Center Hospital East. From the records on breast cancer surgery we selected all patients who had survived more than 3 years since surgery so that the interview itself would not be distressing (22, 23). The inclusion criteria were 1) female gender, 2) age between 18 and 55 years, and 3) no double cancer or clear evidence of residual or recurrent cancer during regular medical checkups conducted by an oncologist (S.I.). The exclusion criteria were 1) left-handedness, 2) a history of PTSD or major depression before the cancer, 3) a history of any neurological disorder or traumatic brain injury accompanied by periods of unconsciousness, 4) a history of substance abuse or dependence, 5) a family history of early dementia among first- or second-degree relatives, 6) any physical symptoms that interfered with daily life, as assessed by performance status defined by the Eastern Cooperative Oncology Group (24), 7) psychotropic medication within the previous month, and 8) cognitive impairment, defined as having a score of less than 24 on the Mini-Mental State Examination (25, 26).
Of the 375 patients who had survived more than 3 years after surgery, 148 patients met the criteria, and 128 could be contacted at the clinic. Forty-six of them refused to participate in the study (31 were too busy to participate, seven were not interested, five refused the MRI examination, and three refused for unknown reasons). Two subjects who had used psychotropic medication within the previous month and three left-handed subjects were excluded. Seventy-seven subjects were interviewed with a semistructured interview, including the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID), clinician version (27), conducted by a trained psychiatrist (T.N.). After the interview, six subjects who had a history of major depression, two subjects who had a history of PTSD, and one subject who had a history of PTSD and major depression before the cancer were excluded from the analyses. One subject was excluded because of MRI acquisition error. The remaining 67 study participants were not currently being treated for breast cancer except for adjuvant tamoxifen treatment (N=8). There were no significant differences in age, days after surgery, or pathological stage between the 67 subjects who participated in the study and the 72 eligible subjects who did not.
Whether the subjects had a history of distressing cancer-related recollections, but not their frequency or intensity, was determined by semistructured interview. We defined distressing cancer-related recollections, on the basis of a modification of criterion B1 of the PTSD module in DSM-IV, as “recurrent and intrusive distressing recollections of the cancer-related event, including images, thoughts, or perceptions” with a duration of 1 month or more. We used sentence F42 from the criteria for PTSD in the administration booklet of the SCID (27) with minor modification: “Did you think about cancer-related events when you did not want to or did thoughts about the cancer-related events come to you suddenly when you didn’t want them to?” Typical responses were as follows: “All of a sudden, the distressing images of cancer recurrence and death popped into my head” or “When the scar on my breast smarts, I remember the distressing scene when I was informed that I had cancer.” A history of intrusive recollections before the cancer was determined by semistructured interview. Two psychiatrists (T.N. and A.K.) both assessed the same nine subjects, and the kappa value for a history of distressing cancer-related recollections after cancer was 1.0.
Of the 67 study participants, 28 (42%) met the criteria for a history of distressing cancer-related recollections. Characteristics of the groups with and without a history of such recollections are presented in Table 1; analyses indicated no significant differences. There were also no differences in menopausal status, clinical stage of the cancer, lymph node metastasis, surgical procedure, tamoxifen therapy, or adjuvant chemotherapy. None of the subjects met the criteria for current PTSD or major depression at the time of the investigation.
Memory Measures
As surrogate markers of hippocampal function we used the delayed memory index and the percentage retention (delayed memory score/immediate memory score×100) from the Wechsler Memory Scale—Revised (WMS-R) (28, 29). We also obtained the WMS-R indexes of attention/concentration, immediate visual memory, and immediate verbal memory for each participant.
MRI Acquisition and Volumetric Measurements
The images were generated on a 1.5-T MRI unit (Signa scanner, GE Medical Systems, Milwaukee) with three-dimensional spoiled gradient-recalled acquisition: 1.5-mm contiguous sections perpendicular to the anterior-posterior commissure plane, field of view=230 mm, matrix=256×256 pixels, TR=25 msec, TE=5 msec, flip angle=45°. The images were analyzed by ANALYZE-AVW (Biomedical Imaging Resource, Mayo Foundation, Rochester, Minn.).
The hippocampal borders were traced manually with a pen-tablet pointing device. The hippocampus was defined as shown in Figure 1. A sagittal view was also used to confirm the anterior end of the hippocampal head. Both the left and right hippocampi of each subject were measured twice in random order by a skilled rater (M.W.) who was blind to the subjects’ characteristics. The intraclass correlation coefficients (ICCs) for intrarater reliability derived from 67 subjects and interrater reliability derived from 30 subjects were 0.97 and 0.96, respectively.
The volume of the whole brain was measured for purposes of comparison. The semiautomatic procedure for measuring whole brain volume that we used is similar to that reported by Mori et al. (30). All of the whole brains were measured in randomized order by a skilled rater (M.I.) who was blinded to the subjects’ characteristics. The ICCs for the intrarater reliability derived from 30 subjects and interrater reliability derived from 30 subjects were 0.99 and 0.98, respectively.
Data Analyses
In previous studies (7–13), comparison groups differed from patients by 5% to 26% in hippocampal volume. On the basis of our pilot study, we estimated that the difference and standard deviation would be 7% and 10%, respectively. For a two-tailed alpha of 0.05 and a beta of 0.20, 32 subjects in each group were needed. However, the groups with and without distressing cancer-related recollections in this study contained 28 and 37 subjects, respectively.
In accordance with the method of analysis in previous reports (7, 9, 10, 13), we did not use the ratio of the hippocampal volume to the whole brain volume or the hippocampal volume adjusted for whole brain volume to analyze differences in hippocampal volume. Hippocampal volume and whole brain volume were analyzed by one-way analysis of variance (ANOVA) and analysis of covariance (ANCOVA) using age, height, years of education, alcohol consumption, and cumulative duration of major depression to control for differences between the two groups. Repeated measures ANCOVA with side as the repeated measures (within-group) factor and age, height, years of education, alcohol consumption, and cumulative duration of major depression as covariates was used to compare left and right hippocampal volumes in the subjects with and without a history of distressing cancer-related recollections.
In addition, ANCOVA with whole brain volume as one of the covariates was performed to facilitate comparisons with the findings of prior studies that have demonstrated significant differences in hippocampal volume after covariance for whole brain volume.
The comparisons of the subjects with and without a history of distressing cancer-related recollections also included analysis of the delayed memory index, percentage retention, attention/concentration index, immediate visual index, and immediate verbal memory index by ANOVA and by ANCOVA with years of education, alcohol consumption, and cumulative duration of major depression as covariates. The alpha level of significance in most statistical analyses was p<0.05. In the analyses of memory function, the p value was set at 0.01, because multiple comparisons were made. All analyses were performed by using SPSS version 10 (SPSS, Inc., Chicago).
Results
Hippocampal Volume
The volume of the left hippocampus in the subjects with a history of distressing cancer-related recollections was 5% smaller than in those without distressing recollections, and the difference was statistically significant by ANOVA (Figure 2). The 1.5% smaller volume of the right hippocampus was not significantly different, and there was no significant difference in whole brain volume measured as a control (Figure 2). ANCOVA continued to show a significant difference in left hippocampal volume between subjects with and without a history of distressing cancer-related recollections (F=4.04, df=1, 60, p=0.05), but the difference in the volume of the right hippocampus was not significant (F=0.05, df=1, 60, p=0.83). The repeated measures ANCOVA showed a significant interaction between side and distressing cancer-related recollections (F=4.28, df=1, 60, p=0.04) but not a significant main effect of side (F=3.48, df=1, 60, p=0.07). There was not a significant correlation between left hippocampal volume and number of days after surgery in the subjects with a history of distressing cancer-related recollections, according to Pearson’s correlation test (r=0.13, N=67, p=0.52).
To examine the possibility that a smaller hippocampus was already present in the subjects with a history of distressing cancer-related recollections before their cancers, we analyzed the difference in the number of subjects with a history of distressing cancer-related recollections between those with a small hippocampus and those with a large hippocampus. First we used the median hippocampal volume on the left (2,612 mm3) to divide the subjects into those with low and high left-side hipppocampal volumes. Seventeen of the subjects with a small left hippocampus (N=33) had a history of distressing cancer-related recollections, as opposed to 11 of the subjects with a large left hippocampus (N=34); this difference was not significant (χ2=2.53, df=1, p=0.11). The median value for the right side (2,694 mm3) was then used as the dividing line to separate subjects with low and high right-side hippocampal volumes. Fifteen of the subjects with a small right hippocampus (N=33) had a history of distressing cancer-related recollections, versus 13 of the subjects with a large right hippocampus (N=34); this difference also was not significant (χ2=0.36, df=1, p=0.55).
An additional ANCOVA with whole brain volume as one of the covariates showed no significant difference between groups in hippocampal volume on the left side (F=2.14, df=1, 59, p=0.15) or right side (F=0.17, df=1, 59, p=0.68). The adjusted mean left hippocampal volumes in the subjects with and without a history of distressing cancer-related recollections were 2,561 mm3 (SD=363) and 2,648 mm3 (SD=305), respectively, and the difference was 3.3%. The adjusted mean right hippocampal volumes in the subjects with and without a history of distressing cancer-related recollections were 2,709 mm3 (SD=376) and 2,683 mm3 (SD=316), respectively, and the difference was –1.0%.
Memory Functioning
The memory measures for the subjects with and without a history of distressing cancer-related recollections are shown in Table 2. There was no significant difference between the two groups in the delayed memory index, attention/concentration index, or immediate verbal memory index. However, the subjects with a history of distressing cancer-related recollections had a significantly lower immediate visual memory index. The ANCOVA showed no significant difference in the delayed memory index (F=2.82, df=1, 62, p=0.10) or the immediate verbal memory index (F=0.93, df=1, 62, p=0.34). However, there continued to be a significant difference in the immediate visual memory index between the subjects with and without a history of distressing cancer-related recollections (F=9.72, df=1, 62, p=0.003). The memory scale scores showed no significant difference between groups in delayed memory or in the percentage of retention for the visual or verbal memory score. In addition, there was no significant difference in attention/concentration score or immediate verbal memory score. However, there was a significant difference in the immediate visual memory score.
Among the 67 subjects as a whole, there was no significant correlation between left or right hippocampal volume and the delayed memory index (left: r=0.14, p=0.27; right: r=0.03, p=0.82) or percentage retention (left: r=–0.02, p=0.83; right: r=–0.06, p=0.65). There was no significant correlation between the attention/concentration and immediate visual memory indexes (r=0.01, p=0.92). There also was no significant correlation between left hippocampal volume and the immediate visual memory index (r=0.21, p=0.10) or immediate verbal memory index (r=0.08, p=0.54). The right hippocampal volume was not significantly correlated with either the immediate visual memory index (r=0.12, p=0.33) or the immediate verbal memory index (r=0.02, p=0.89).
Discussion
To our knowledge, this is the first study to demonstrate a significant association between hippocampal volume and a history of distressing cancer-related recollections in cancer survivors. Cancer survivors with a history of distressing recollections had a significantly smaller left hippocampal volume than cancer survivors without a history of such recollections, but there was no significant difference in the volume of the right hippocampus or the whole brain volume measured as a control. No significant differences in demographic, medical, or psychological factors between subjects with and without a history of distressing cancer-related recollections were found in this study. Even after adjustment for the covariates age, height, years of education, alcohol consumption, and duration of major depression, which were potential confounding factors in regard to hippocampal volume, there continued to be a significant difference in left hippocampal volume. These results suggest a significant association between a history of distressing cancer-related recollections and left hippocampal volume.
The difference in left hippocampal volume between the subjects with and without a history of distressing cancer-related recollections was 5%. Although we know of no other studies showing an association between a history of distressing cancer-related recollections and hippocampal volume, it might be possible to compare this finding to the results of studies of stress-related neuropsychiatric disorders, such as PTSD and major depression. Studies have indicated differences in hippocampal volume between patients and comparison subjects of 5%–26% for PTSD (10–13) and 8%–19% for major depression (8, 9). Although the 5% difference in our study was small, it was at or near the lower ends of these ranges.
Since only left hippocampal volume, and not right hippocampal volume, was different between subjects with and without a history of distressing cancer-related recollections in this study, there was laterality in these results. Since studies of major depression (7–9) have shown a smaller hippocampus on both sides in patients than in healthy comparison subjects, there may be no laterality in the difference in hippocampal volume in major depression. A study of sexually abused female subjects with PTSD (12) showed a smaller hippocampus only in the left hemisphere, and in another study (13) abused male and female subjects with PTSD were found to have a significantly smaller hippocampus on the left side alone. In contrast, male patients with combat-related PTSD were found to have a significantly smaller hippocampus on the right side (10) or on both the right and left sides (11). Based on the results of these studies, Bremner et al. (13) suggested that left hippocampal atrophy predominates in victims of trauma that occurred in childhood and that right hippocampal atrophy predominates in those who have experienced trauma in adulthood. On the other hand, the results of the present study suggest a possible sex difference in the effect on hippocampal volume. All of our subjects were female, and they experienced cancer in adulthood, not in childhood. Although distressing cancer-related recollections may not be the same as the symptoms in PTSD, the results of the present study favor the existence of a sex difference in the laterality of hippocampal volume differences.
When the difference developed remains a question. There are three possibilities: the difference existed before the cancer experience, a reduction in volume occurred during the cancer experience, or a reduction in volume developed as a result of distress after the cancer experience, for example, as a result of distressing cancer-related recollections. Since there was no significant difference in the proportion of subjects with a history of distressing cancer-related recollections between those having a larger left hippocampus and those having a smaller left hippocampus, the smaller hippocampus in the subjects with a history of distressing cancer-related recollections may not predispose to these recollections. Since there was no correlation between the number of days after surgery and hippocampal volume in this study, the difference in hippocampal volume may not have occurred as a result of distress after cancer in the form of distressing cancer-related recollections. However, there was no evidence in this study that hippocampal volume decreased during the cancer experience. A prospective longitudinal study is needed to provide such evidence.
There were no significant differences between the subjects with and without a history of distressing cancer-related recollections in delayed memory and percentage retention, which have typically been used as surrogate markers of hippocampal function. Nor was there a significant correlation between hippocampal volume and delayed memory in this study. Thus, having a smaller hippocampus may not be of any consequence functionally. More study is needed to answer the question of whether the difference in hippocampal volume is reflected in other hippocampal functions. Unexpectedly, the subjects in this study who had a history of distressing cancer-related recollections had a significantly lower (8% lower) WMS-R immediate visual memory index than those who did not. The difference in immediate visual memory cannot be explained by the attention/concentration function, because there was no significant difference in the attention/concentration index between subjects with and without a history of distressing cancer-related recollections, and the attention/concentration index was not significantly correlated with the immediate visual memory index. The immediate visual memory index was not significantly correlated with hippocampal volume, and since all of the subjects were right-handed, it would seem more logical for the smaller left hippocampal volume to have been associated with verbal rather than visual memory impairment. This lower visual memory index may not be simply a consequence of smaller left hippocampal volume, and it may have other causes. The memory components reported to be impaired, for example, visual and verbal memory, in studies that demonstrated hippocampal atrophy among patients with PTSD or major depression have been inconsistent (8, 10, 11, 13). More study is needed to answer the question of whether the worse memory performance in subjects with a history of distressing cancer-related recollections is a consequence of their smaller left hippocampal volume.
The additional analysis by ANCOVA with whole brain volume as one of the covariates showed no significant difference in hippocampal volume between subjects with and without a history of distressing cancer-related recollections. However, it is unclear whether absolute or relative regional brain volume is more important in volumetric studies. The result of the ANCOVA with whole brain volume as one of the covariates might not have shown any significant difference because of the problems, such as an increase in variability, that have been reported to interfere with detection of true differences (31, 32). Given the increase in variability, a study with more subjects is needed to confirm our results.
The present study had the following limitations First, because of its cross-sectional design, it could not confirm any causality between hippocampal volume and a history of distressing cancer-related recollections. Moreover, since the histories of distressing cancer-related recollections, PTSD, and major depression were identified retrospectively, the influence of recall bias should be taken into consideration. Second, the results were obtained from only one institution. Thus, the subjects are not representative of all cancer survivors. Third, it was impossible to determine whether small hippocampal volume occurred as a result of distressing cancer-related recollections, because we did not examine the frequency, duration, or severity of the recollections. Fourth, it is possible that distressing responses other than cancer-related recollections are related to hippocampal volume, because we did not measure the total distress caused by the cancer experience. Fifth, although it would have been a useful adjunct to this study, we did not evaluate early life stressors in these subjects. Finally, the study was limited by the lack of a healthy comparison group. Thus, it was impossible to determine whether the hippocampal volume of the subjects with a history of distressing cancer-related recollections was smaller than normal or the volume of the subjects without a history of distressing cancer-related recollections was larger than normal.
In conclusion, the findings in this study suggest an association between distressing cancer-related recollections and morphological differences in the hippocampus of cancer survivors. The presence of a smaller hippocampal volume was consistent with findings from previous studies of stress-related psychiatric disorders, such as PTSD and depression, although the laterality differed from that in some of the other studies. A prospective longitudinal study of cancer survivors is needed to elucidate the source of this difference and clarify the causal relationship between distressing cancer-related recollections and hippocampal alteration.
 |
 |
Received Nov. 3, 2001; revision received July 3, 2002; accepted July 11, 2002. From the Psycho-Oncology Division, National Cancer Center Research Institute East; and the Psychiatry Division, the Division of Breast Surgery, and the Department of Radiology, National Cancer Center Hospital East, Kashiwanoha, Kashiwa, Chiba, Japan. Address reprint requests to Dr. Uchitomi, Psycho-Oncology Division, National Cancer Center Research Institute East, 6-5-1 Kashiwanoha, Kashiwa, Chiba 277-8577, Japan; [email protected] (e-mail). Supported in part by three grants from the Japanese Ministry of Health and Welfare (one each from Grant-in-Aid for Cancer Research, Second-Term Comprehensive 10-Year Strategy for Cancer Control, and Research on Brain Science) and by a grant from the Japanese Ministry of Education, Culture, Science, and Technology (from the Target-Oriented Brain Science Program). Drs. Nakano, Inagaki, Matsuoka, and Sugahara are recipients of Research Resident Fellowships and Dr. Wenner is a Foreign Research Fellow, Foundation for the Promotion of Cancer Research in Japan. The authors thank Morihiro Sugishita, Ph.D., Hiroshi Matsuda, M.D., Ph.D., Takeshi Uema, M.D., Nobumasa Kato, M.D., Ph.D., and Etsuro Mori, M.D., Ph.D., for their advice and Ms. Yuko Kojima for research assistance.
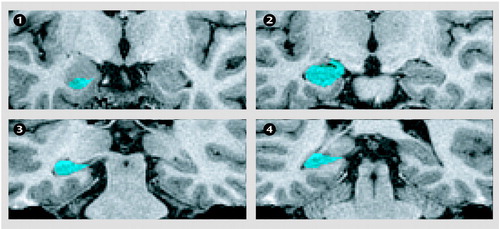
Figure 1. Boundaries of the Hippocampus Used in a Study of the Relationship of MRI-Derived Hippocampal Volume to History of Distressing Cancer-Related Recollectionsa
aSelected images from a breast cancer survivor illustrating the boundaries of the hippocampal formation from anterior limits (panel 1) through posterior limits (panel 4). The blue objects indicate the hippocampal formation. It contains the Ammon’s horn, dentate gyrus, fimbria, and subiculum. The anterior end slice was defined as the final slice on which the white alveus surrounding the remaining knuckles of the hippocampal head remains visible (panel 1). The posterior end slice of the hippocampal tail was defined as the slice in which the crus of the fornix is the longest on a coronal section (panel 4).
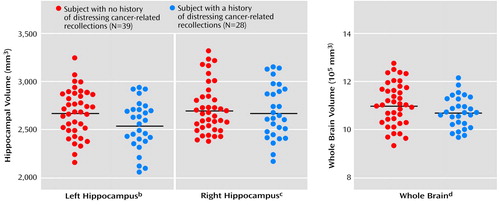
Figure 2. Hippocampal Volumes of Women With a History of Breast Cancer Surgery Who Did or Did Not Have a History of Distressing Cancer-Related Recollectionsa
aHorizontal bars indicate mean volumes.
bSignificant difference between groups according to ANOVA (F=4.78, df=1, 65, p=0.03). The mean volumes in the subjects without and with distressing cancer-related recollections were 2,667 mm3 (SD=242) and 2,535 mm3 (SD=249), respectively.
cNonsignificant difference between groups according to ANOVA (F=0.39, df=1, 65, p=0.54). The mean volumes in the subjects without and with distressing cancer-related recollections were 2,711 mm3 (SD=259) and 2,670 mm3 (SD=278), respectively.
dNonsignificant difference between groups according to ANOVA (F=2.86, df=1, 65, p=0.10). The mean volumes in the subjects without and with distressing cancer-related recollections were 1,102,814 mm3 (SD=884,921) and 1,069,555 mm3 (SD=664,678), respectively.
1. Green BL, Rowland JH, Krupnick JL, Epstein SA, Stockton P, Stern NM, Spertus IL, Steakley C: Prevalence of posttraumatic stress disorder in women with breast cancer. Psychosomatics 1998; 39:102-111Crossref, Medline, Google Scholar
2. Alter CL, Pelcovitz D, Axelrod A, Goldenberg B, Harris H, Meyers B, Grobois B, Mandel F, Septimus A, Kaplan S: Identification of PTSD in cancer survivors. Psychosomatics 1996; 37:137-143Crossref, Medline, Google Scholar
3. Tjemsland L, Soreide JA, Malt UF: Posttraumatic distress symptoms in operable breast cancer III: status one year after surgery. Breast Cancer Res Treat 1998; 47:141-151Crossref, Medline, Google Scholar
4. McBride CM, Clipp E, Peterson BL, Lipkus IM, Demark-Wahnefried W: Psychological impact of diagnosis and risk reduction among cancer survivors. Psychooncology 2000; 9:418-427Crossref, Medline, Google Scholar
5. Smith MY, Redd WH, Peyser C, Vogl D: Post-traumatic stress disorder in cancer: a review. Psychooncology 1999; 8:521-537Crossref, Medline, Google Scholar
6. Sapolsky RM: Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry 2000; 57:925-935Crossref, Medline, Google Scholar
7. Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW: Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci USA 1996; 93:3908-3913Crossref, Medline, Google Scholar
8. Sheline YI, Sanghavi M, Mintun MA, Gado MH: Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci 1999; 19:5034-5043Crossref, Medline, Google Scholar
9. Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS: Hippocampal volume reduction in major depression. Am J Psychiatry 2000; 157:115-117Link, Google Scholar
10. Bremner JD, Randall P, Scott TM, Bronen RA, Seibyl JP, Southwick SM, Delaney RC, McCarthy G, Charney DS, Innis RB: MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry 1995; 152:973-981Link, Google Scholar
11. Gurvits TV, Shenton ME, Hokama H, Ohta H, Lasko NB, Gilbertson MW, Orr SP, Kikinis R, Jolesz FA, McCarley RW, Pitman RK: Magnetic resonance imaging study of hippocampal volume in chronic, combat-related posttraumatic stress disorder. Biol Psychiatry 1996; 40:1091-1099Crossref, Medline, Google Scholar
12. Stein MB, Koverola C, Hanna C, Torchia MG, McClarty B: Hippocampal volume in women victimized by childhood sexual abuse. Psychol Med 1997; 27:951-959Crossref, Medline, Google Scholar
13. Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, Capelli S, McCarthy G, Innis RB, Charney DS: Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse—a preliminary report. Biol Psychiatry 1997; 41:23-32Crossref, Medline, Google Scholar
14. Uno H, Tarara R, Else JG, Suleman MA, Sapolsky RM: Hippocampal damage associated with prolonged and fatal stress in primates. J Neurosci 1989; 9:1705-1711Crossref, Medline, Google Scholar
15. Sapolsky RM, Uno H, Rebert CS, Finch CE: Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J Neurosci 1990; 10:2897-2902Crossref, Medline, Google Scholar
16. Watanabe Y, Gould E, McEwen BS: Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res 1992; 588:341-345Crossref, Medline, Google Scholar
17. Cameron HA, McEwen BS, Gould E: Regulation of adult neurogenesis by excitatory input and NMDA receptor activation in the dentate gyrus. J Neurosci 1995; 15:4687-4692Crossref, Medline, Google Scholar
18. Duman RS, Malberg J, Nakagawa S, D’Sa C: Neuronal plasticity and survival in mood disorders. Biol Psychiatry 2000; 48:732-739Crossref, Medline, Google Scholar
19. Smith MA, Makino S, Kvetnansky R, Post RM: Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci 1995; 15(3, part 1):1768-1777Google Scholar
20. Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E: Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci 1997; 17:2492-2498Crossref, Medline, Google Scholar
21. Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NP, Thakur M, McEwen BS, Hauger RL, Meaney MJ: Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci 1998; 1:69-73Crossref, Medline, Google Scholar
22. Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB: Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry 1995; 52:1048-1060Crossref, Medline, Google Scholar
23. Newman E, Walker EA, Gefland A: Assessing the ethical costs and benefits of trauma-focused research. Gen Hosp Psychiatry 1999; 21:187-196Crossref, Medline, Google Scholar
24. Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP: Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982; 5:649-655Crossref, Medline, Google Scholar
25. Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189-198Crossref, Medline, Google Scholar
26. Mori E, Mitani Y, Yamadori A: Usefulness of a Japanese version of the Mini-Mental State in neurological patients. Shinkeishinrigaku 1985; 1:82-90Google Scholar
27. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders, Administration Booklet. Washington, DC, American Psychiatric Press, 1997Google Scholar
28. Wechsler D: Wechsler Memory Scale—Revised. New York, Psychological Corp, 1987Google Scholar
29. Sugishita M: Wechsler Memory Scale—Revised (Japanese). Tokyo, Nihonbunkakagakusya, 2001Google Scholar
30. Mori E, Hirono N, Yamashita H, Imamura T, Ikejiri Y, Ikeda M, Kitagaki H, Shimomura T, Yoneda Y: Premorbid brain size as a determinant of reserve capacity against intellectual decline in Alzheimer’s disease. Am J Psychiatry 1997; 154:18-24Link, Google Scholar
31. Arndt S, Cohen G, Alliger RJ, Swayze VW II, Andreasen NC: Problems with ratio and proportion measures of imaged cerebral structures. Psychiatry Res Neuroimaging 1991; 40:79-89Crossref, Medline, Google Scholar
32. Wang PP, Jernigan TL: Morphometric studies using neuroimaging. Neurol Clin 1994; 12:789-802Crossref, Medline, Google Scholar



