Impaired Olfactory Identification in Relatives of Patients With Familial Schizophrenia
Abstract
OBJECTIVE: Impaired olfactory identification ability has previously been demonstrated in patients with schizophrenia. This study assessed olfactory function in psychotic and nonpsychotic members of multigenerational families with familial schizophrenia to determine whether deficits were present in both groups. METHOD: The University of Pennsylvania Smell Identification Test was administered birhinally to three groups of subjects aged less than 65 years: 19 psychotic and 27 nonpsychotic members of families with familial schizophrenia and 43 age- and sex-matched healthy volunteers. RESULTS: Nonpsychotic family members had significantly higher mean University of Pennsylvania Smell Identification Test scores than psychotic family members but were impaired relative to the healthy volunteer group. These group differences could not be accounted for by age, sex, or smoking habit. Fifty-eight percent of the psychotic and 34% of the nonpsychotic family members performed in the microsmic (impaired) range, compared to 9% of the healthy volunteers. CONCLUSIONS: Impaired olfactory deficits may aggregate in families with schizophrenia and may be indicative of a genetic predisposition to psychosis.
Olfactory function may serve as a behavioral probe for determining the functional integrity of brain regions associated with schizophrenia. Olfactory identification deficits with intact acuity (olfactory agnosia) have been reported in both medicated and neuroleptic-naive individuals with schizophrenia (1–5). Brain regions reported to have functional or structural abnormalities in schizophrenia overlap with major olfactory systems and include the orbitofrontal and entorhinal cortex, as well as the dorsomedial nucleus of the thalamus. Measures of brain function such as olfactory identification may provide important insights into the neurobiological phenotype of familial schizophrenia (6, 7).
In an investigation of olfactory identification ability in which the University of Pennsylvania Smell Identification Test (8) was administered to monozygotic twins discordant for schizophrenia (9), we found that affected twins were impaired and that unaffected co-twins had significantly lower olfactory identification scores than age- and sex-matched healthy volunteers. The unaffected twins’ performance fell midway between that of the affected twins and that of the healthy volunteers. Moberg et al. (10) reported lower olfactory identification scores in siblings of patients with schizophrenia who met criteria for schizotypal personality disorder, compared with relatives who did not meet those criteria. Taken together, the data suggested that impaired olfactory identification ability might be an indicator of abnormal brain functioning in patients with schizophrenia as well as in their biological relatives. In this regard, olfactory identification may serve as a marker for genetic vulnerability to psychosis (11).
The purpose of the current study was to assess olfactory function in multigenerational families with familial schizophrenia. Schizophrenia in families is unlikely to be explained by a single major gene. One approach is to consider familial schizophrenia as the convergence of a limited number of traits that can be quantified. In this regard, Brzustowicz et al. (12) reported linkage of positive symptoms of schizophrenia, as measured by the Positive and Negative Syndrome Scale (13), to markers on chromosome 6p in a set of familial schizophrenia families. The current study assessed olfactory function in the same families to investigate whether impaired olfactory identification ability appears to function as a genetic trait in familial schizophrenia.
On the basis of our prior findings for monozygotic twins with schizophrenia (9), we hypothesized that psychotic family members would have the most severe olfactory identification deficits (with intact acuity) and that nonpsychotic family members would have less severe deficits than the psychotic family members but more severe deficits than a healthy comparison group.
Method
Subjects
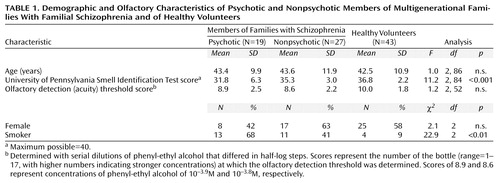
Forty-six subjects (21 men and 25 women) between the ages of 20 and 64 (Table 1) were recruited from 10 large families participating in a genetic linkage study of familial schizophrenia, described elsewhere (12, 14–16). Briefly, families were selected for inheritance consistent with possible autosomal dominant transmission of schizophrenia and related disorders. Families with bipolar disorder or bilineal inheritance were excluded. Data obtained from semistructured interviews (with the patient and nonpatient versions of the Structured Clinical Interview for DSM-III-R [17] and the Structured Clinical Interview for DSM-III-R Personality Disorders [18]) together with collateral information and medical records were used to make diagnoses according to DSM-III-R. On the basis of DSM-III-R lifetime diagnoses, subjects were classified as either psychotic family members (N=19) (schizophrenia, N=11; schizoaffective disorder, N=5; psychosis not otherwise specified, N=3) or nonpsychotic family members (N=27). The nonpsychotic family members were first- or second-degree relatives of the psychotic subjects and came from the affected side of the family. Of the 27 nonpsychotic family members, 10 had never had mental illness, 10 had a previous major depressive episode, three had a history of alcohol abuse, and four had axis II disorders (none of which were schizophrenia spectrum conditions). No subjects had concomitant medical problems that might affect the sense of smell, including current alcohol abuse, allergic rhinitis, upper respiratory tract infection, or a history of significant head injury or facial fracture.
Healthy volunteer subjects (N=43), randomly drawn from our database, were sex and age (within 5 years) matched with each psychotic and nonpsychotic family member. An adequate match for three family members could not be found. The comparison group members met the same inclusion and exclusion criteria as the family members; none had a family history of schizophrenia or psychotic disorder in first or second-degree relatives. The history was obtained by direct questioning of the volunteer. After a complete description of the study to the subjects, written informed consent was obtained. The protocol was approved by the Ethics Committees of both the University of Toronto and Dalhousie University.
Olfactory Measures
The three study groups were compared on measures of olfactory function. The University of Pennsylvania Smell Identification Test (8) is a widely used, well-standardized measure of the ability to match odors to written labels. The test has 40 scratch-and-sniff items. Each item consists of an odor-impregnated patch glued on a page. Along with each patch are four descriptive words, one of which matches the odor. The subject is instructed to guess even if he or she cannot match the odor to its proper descriptor (forced choice). The score is the number of items correctly identified
To rule out compromise of more peripheral olfactory sensing structures, an olfactory detection (acuity) threshold task was administered (8). Subjects were presented with a series of solutions that differed in concentration of phenyl-ethyl alcohol by half-log steps. For each trial in the staircase procedure, the subject was presented with two bottles, one containing a strong concentration of phenyl-ethyl alcohol and one a weak or no concentration, and was asked to choose the bottle containing the stronger odor. The subject’s score was the number of the bottle (range=1–17, with higher numbers indicating stronger concentrations) at which the olfactory detection threshold was determined. A trained individual (K.M.), who was blind to group membership, administered both olfactory tests to the familial schizophrenia family members; another trained individual (K.P.G.) assessed the healthy volunteer subjects.
Statistics
We used mixed model analysis of covariance (ANCOVA) for analysis of the University of Pennsylvania Smell Identification Test scores in affected and unaffected relatives to control for clustered sampling. As the observations came from families, they could not be considered independent. The ANCOVA model used age and sex as covariates, diagnosis as a fixed factor, and family membership and its interaction with diagnosis as random factors. The parameters of the model were estimated with a maximum-likelihood method. Subsequently, we tested restricted models in which the individual factors were removed. These restricted models were compared with the unrestricted model by the criterion of –2(lnLu – lnLr) where Lu and Lr are the likelihoods under unrestricted and restricted models, respectively. This criterion has an asymptotic chi-square distribution with the number of degrees of freedom equal to the difference in number of parameters. A nonsignificant chi-square value indicates a nonsignificant loss of information resulting from removal of the appropriate factor. As the family effect was not significant (see Results), we further performed an analysis of covariance on the smell identification test scores with diagnosis as the independent variable and age and sex as covariates for the three groups. From this, pairwise comparisons were completed to compare the groups’ scores on the smell identification test. The contrasts of interest were between the patients and their relatives (psychotic versus nonpsychotic family members) and between the relatives and the healthy volunteers (nonpsychotic family members versus healthy volunteers).
Secondary analyses were completed to rule out the effects of smoking status on olfactory identification ability. Within the family groups, a two-way analysis of variance (ANOVA) was performed on the smell identification test scores, with smoking status and diagnosis as factors.
Results
Demographic and olfactory data are presented in Table 1. There were no differences in mean age among the three groups. The difference among groups in the percentage of smokers was significant and was accounted for by the low number of smokers in the healthy volunteer group. Among family members who were smokers, there were no differences between the psychotic group and the nonpsychotic group in the amount smoked, as measured in pack-years (average number of packs smoked per day times the number of years of smoking) (t=0.76, df=22, n.s.). Olfactory detection (acuity) threshold scores, a measure of peripheral sensing ability, were not significantly different among the three groups.
The first analysis sought to examine the effect of family membership on olfactory performance. The ANCOVA performed on University of Pennsylvania Smell Identification Test scores showed that the effect of diagnosis (psychotic versus nonpsychotic) was significant (χ2=4.8, df=1, p<0.03) but the effect of family membership and the interaction of family membership and diagnosis were not (χ2=0.002, df=1, n.s.; and χ2<0.01, df=1, n.s., respectively). The effect of age was also significant (beta=–0.12, p<0.04), while the effect of sex was not (beta=2.01, n.s.).
As the effect of family membership was nonsignificant, we performed a second analysis using data from all subjects to test for differences in smell identification test scores between groups (psychotic and nonpsychotic family members and healthy volunteers). There was an overall significant difference (Table 1), with pairwise differences between the psychotic patients and their nonpsychotic relatives (t=2.5, df=44, p<0.02) and between the nonpsychotic relatives and the healthy volunteers (t=2.5, df=68, p<0.02).
Because there were so few smokers in the healthy volunteer group, we examined the effects of smoking status on olfactory performance within each of the two family groups. A two-way ANOVA was performed on the smell identification test scores, with smoking status (current smoker versus nonsmoker) and diagnosis (psychotic versus nonpsychotic) as between-group factors. The main effect for diagnosis was significant (F=4.8, df=1, 45, p<0.05). Neither the main effect of smoking status nor the interaction of smoking status and diagnosis was significant (F=0.04, df=1, 45, n.s.; and F=0.08, df=1, 34, n.s., respectively).
Another analysis was completed to determine if olfactory identification ability differed between relatives with or without a prior diagnosis of mental disorder. No difference was observed (mean score=35.4, SD=2.8, for relatives with a diagnosis versus mean=35.1, SD=3.3, for relatives without a diagnosis; t=0.21, df=25, n.s.)
We used the standardization data for the University of Pennsylvania Smell Identification Test derived by Doty et al. (8) to classify subjects by whether they had an olfactory deficit (were microsmic), represented by a score of less than 34 of 40 for men and less than 35 of 40 for women. A high percentage of family members were microsmic, compared with comparison subjects (Figure 1).
Discussion
The main findings of this study indicate that more psychotic and nonpsychotic members of families with familial schizophrenia had impairments in olfactory identification ability (i.e., were microsmic) than did age- and sex-matched comparison subjects. In addition, olfactory identification in nonpsychotic family members was impaired relative to that in the healthy volunteers. The nonpsychotic family members scored midway between the two other groups. These findings are consistent with our hypothesis. The pattern of mean scores on the University of Pennsylvania Smell Identification Test for the three subject groups was consistent with results from a study of monozygotic twins discordant for schizophrenia (9). That study found that unaffected twins had mean University of Pennsylvania Smell Identification Test scores midway between those of affected twins and those of healthy volunteers (9).
The results for psychotic family members in the current study are consistent with previous reports of olfactory identification impairment in individuals with schizophrenia (3, 19–21). The percentage of microsmic patients in the present study was somewhat higher (58%) than the 30%–50% reported in previous studies (2, 19). This discrepancy could be related to the fact that the psychotic family members in our study were slightly older than subject groups previously studied. In addition, a prior study found that monozygotic twin pairs with a family history of serious mental disorders in first- or second-degree relatives had more marked impairment on the University of Pennsylvania Smell Identification Test than twins without such a family history (9). Since all family members in the current study would have a similar family history, this factor also may have contributed to the observed levels of olfactory impairment.
Factors such as symptom presentation (22), task complexity (21), and smoking history are not likely to have contributed to the observed olfactory identification deficits. Within the family groups, current smoking status did not appear to affect olfactory identification ability, as smokers performed no differently than nonsmokers. In prior studies of schizophrenia, smoking habits have not been shown to alter olfactory function (2, 9, 23).
As olfactory deficits have been reported for patients with schizophrenia who are experiencing a first psychotic episode and for neuroleptic-naive and neuroleptic-withdrawn patients (2, 23), neuroleptic medications are also unlikely to account for the olfactory deficits observed. Regarding age, olfactory identification ability in the healthy population is known to diminish beyond the age of 65 years (24). In an effort to minimize this effect, we included only individuals younger than age 65 years and employed age- and sex-matched comparison subjects. Among the three groups, age was not related to score on the University of Pennsylvania Smell Identification Test. However, within the families, a significant effect for age was present. It is possible that patients and their relatives experience age-related deterioration of olfactory function earlier than healthy volunteers (25).
Overall, the results suggest that impaired olfactory identification aggregates in family members with schizophrenia and related disorders and could serve as a marker for abnormal brain function (9, 10). A number of developmental brain disorders such as Kallmann’s syndrome include abnormalities of olfactory function as part of the phenotype, and anatomical and molecular dissection of these syndromes has helped clarify the pathogenesis of olfactory dysfunction (26). Developmental abnormalities of the medial temporal lobe could be an anatomical substrate for the olfactory dysfunction in familial schizophrenia. Several studies of the entorhinal cortex in patients with schizophrenia have reported disorganization of the cytoarchitecture in this region (as reviewed in reference 27). The entorhinal cortex of one individual from the families participating in the present study showed evidence of developmental abnormalities (28). While the functional significance of these abnormalities remain uncertain, mutant mouse models of disturbed cortical organization, such as “reeler” and “barrelless,” demonstrate impaired neural connectivity and behavioral or sensory abnormalities (29, 30).
Olfactory function and structural brain abnormalities are measures that are independent of diagnosis and hence allow for a novel approach for examining families with schizophrenia and related disorders. Measures such as these may contribute to the development of quantitative trait measures for genetic linkage studies of schizophrenia.
 |
Received July 26, 2000; revision received Jan. 29, 2001; accepted Feb. 12, 2001. From the Department of Psychiatry, Dalhousie University; the Department of Pediatrics, IWK-Grace Health Centre, Halifax, N.S., Canada; the Department of Psychiatry, University of Toronto, Toronto; and the Department of Psychiatry, University of British Columbia, Vancouver, B.C., Canada. Address reprint requests to Dr. Kopala, Department of Psychiatry, Dalhousie University, 9091, AJLB, 5909 Veterans’ Memorial Lane, Halifax, NS, Canada B3H 2E2; [email protected] (e-mail). Supported by the Stanley Foundation Scholars Program (Drs. Kopala, Honer, and Morrison), the Medical Research Council of Canada (Drs. Kopala, Bassett, and Honer), the Ontario Mental Health Foundation (Dr. Bassett), the Ian Douglas Bebensee Foundation (Dr. Bassett), the National Alliance for Research on Schizophrenia and Depression (Dr. Good), and the Department of Health, Province of Nova Scotia. The authors thank J. Hogan, M. Woschee, A. Feldstein, M. Brennan, L. Ford, and L. Maughan for their assistance.
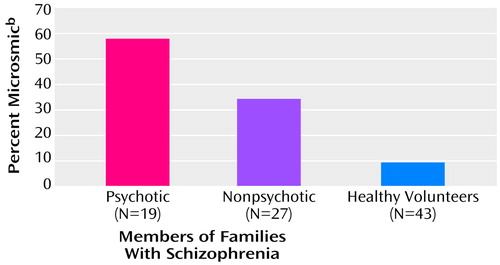
Figure 1. Percentage of Microsmic Subjects Among Psychotic and Nonpsychotic Members of Multigenerational Families With Familial Schizophrenia and Among Healthy Volunteersa
aSignificant difference between groups (χ2=166, df=2, p<0.001).
bUniversity of Pennsylvania Smell Identification Test score less than 34 of 40 for men and less than 35 of 40 for women.
1. Hurwitz T, Kopala L, Clark C, Jones B: Olfactory deficits in schizophrenia. Biol Psychiatry 1988; 23:123-128Crossref, Medline, Google Scholar
2. Kopala L, Clark C, Hurwitz T: Olfactory deficits in neuroleptic naive patients with schizophrenia. Schizophr Res 1992; 8:245-250Crossref, Google Scholar
3. Seidman LJ, Talbot NL, Kalinowski AG, McCarley RW, Faraone SV, Kremen WS, Pepple JR, Tsuang MT: Neuropsychological probes of fronto-limbic system dysfunction in schizophrenia: olfactory identification and Wisconsin Card Sorting performance. Schizophr Res 1991; 6:55-65Crossref, Medline, Google Scholar
4. Martzke J, Kopala L, Good K: Olfactory dysfunction in neuropsychiatric disorders: review and methodological considerations. Biol Psychiatry 1997; 42:231-237Crossref, Google Scholar
5. Moberg P, Agrin R, Gur R, Gur R, Turetsky B, Doty R: Olfactory dysfunction in schizophrenia: a qualitative and quantitative review. Neuropsychopharmacology 1999; 21:325-340Crossref, Medline, Google Scholar
6. Honer W, Bassett A, Kopala L, Kennedy J: A genotype-phenotype research strategy for schizophrenia. Can J Psychiatry 1990; 35:776-783Crossref, Medline, Google Scholar
7. Tsuang M: Genotypes, phenotypes and the brain: a search for connections in schizophrenia: Br J Psychiatry 1993; 163:299-307Google Scholar
8. Doty R, Shaman P, Dann M: Development of the University of Pennsylvania Smell Test: standardized microencapsulated test for olfactory function. Physiol Behav 1984; 32:489-502Crossref, Medline, Google Scholar
9. Kopala LC, Good KP, Torrey EF, Honer WG: Olfactory function in monozygotic twins discordant for schizophrenia. Am J Psychiatry 1998; 155:134-136Link, Google Scholar
10. Moberg P, Doty R, Turetsky B, Wylonis T, Acosta T, Gur E: Olfactory functioning in siblings discordant for schizophrenia (abstract). Biol Psychiatry 1996; 39:571-572Crossref, Google Scholar
11. Park S, Raine A, Lencz T, Bihrle S, LaCasse L: Structural and functional correlates of working memory and olfactory identification in schizotypal subjects (abstract). Schizophr Res 1997; 24:135Google Scholar
12. Brzustowicz L, Honer W, Chow E, McAlduff J, Hodgkinson K, Bassett A: Use of a quantitative trait to map a locus associated with the severity of positive symptoms to chromosome 6p in familial schizophrenia. Am J Hum Genet 1997; 61:1388-1396Google Scholar
13. Kay S, Sevy S: Pyramidical model of schizophrenia. Schizophr Bull 1990; 16:537-544Crossref, Medline, Google Scholar
14. Bassett A, Collins E, Nuttall S, Honer W: Positive and negative symptoms in families with schizophrenia. Schizophr Res 1993; 11:9-19Crossref, Medline, Google Scholar
15. Bassett A, Honer W: Evidence for anticipation in schizophrenia. Am J Hum Genet 1994; 54:864-870Medline, Google Scholar
16. Brzustowicz LM, Honer WG, Chow EW, Little D, Hogan J, Hodgkinson K, Bassett AS: Linkage of familial schizophrenia to chromosome 13q32. Am J Hum Gen 1999; 65:1096-1103Google Scholar
17. Spitzer RL, Williams JBW, Gibbon M, First MB: Structured Clinical Interview for DSM-III-R. Washington, DC, American Psychiatric Press, 1990Google Scholar
18. Spitzer RL, Williams JBW, Gibbon M, First MB: Structured Clinical Interview for DSM-III-R Personality Disorders (SCID-II). Washington, DC, American Psychiatric Press, 1990Google Scholar
19. Kopala L, Clark C, Hurwitz TA: Sex differences in olfactory function in schizophrenia. Am J Psychiatry 1989; 146:1320-1322Google Scholar
20. Kopala L, Good K, Honer W: Olfactory identification ability in pre- and postmenopausal women with schizophrenia. Biol Psychiatry 1995; 38:57-63Crossref, Medline, Google Scholar
21. Kopala L, Good K, Martzke J, Hurwitz T: Olfactory deficits in schizophrenia are not a function of task complexity. Schizophr Res 1995; 17:195-199Crossref, Medline, Google Scholar
22. Good K, Martzke J, Honer W, Kopala L: Left nostril olfactory identification impairment in a subgroup of male patients with schizophrenia. Schizophr Res 1998; 33:45-53Crossref, Medline, Google Scholar
23. Wu J, Buchsbaum MS, Moy K, Denlea N, Kesslak P, Tseng H, Plosnaj D, Hetu M, Potkin S, Bracha S, Cotman C: Olfactory memory in unmedicated schizophrenics. Schizophr Res 1993; 9:41-47Crossref, Medline, Google Scholar
24. Doty R, Shaman P, Applebaum S, Giberson R, Siksorski L, Rosenberg L: Smell identification ability: changes with age. Science 1984; 226:1441-1443Google Scholar
25. Moberg PJ, Doty RL, Turetsky BI, Arnold SE, Mahr RN, Gur RC, Bilker W, Gur RE: Olfactory identification deficits in schizophrenia: correlation with duration of illness. Am J Psychiatry 1997; 154:1016-1018Google Scholar
26. Franco B, Guioli S, Pragliola A: A gene deleted in Kallmann’s syndrome shares homology with neural cell adhesion and axonal path-finding molecules. Nature 1991; 353:529-536Crossref, Medline, Google Scholar
27. Falkai P, Schneider-Axmann T, Honer W: Entorhinal cortex pre-alpha clusters in schizophrenia: quantitative evidence of a developmental abnormality. Biol Psychiatry 2000; 47:937-943Crossref, Medline, Google Scholar
28. Honer W, Bassett A, Falkai P, Beach T, LaPointe J: A case study of temporal lobe development in familial schizophrenia. Psychol Med 1996; 26:191-195Crossref, Medline, Google Scholar
29. Rakic P, Caviness V: Cortical development: view from neurological mutants two decades later. Neuron 1995; 14:1101-1104Google Scholar
30. Welker E, Armstrong-James M, Bronchti G, Ourednik W, Gheorghita-Baechler F, Dubois R, Guernsey DL, Van der Loos H, Neumann PE: Altered sensory processing in the somatosensory cortex of the mouse mutant barrelless. Science 1996; 271:1864-1867Google Scholar



