Spatial Working Memory Deficits in Schizophrenia: Relationship With Tardive Dyskinesia and Negative Symptoms
Abstract
OBJECTIVE: This study examined the interrelationship between negative symptoms, orofacial tardive dyskinesia, and specific neurocognitive processes, particularly those involved in memory and executive function, in patients with schizophrenia. METHOD: A set of computerized neurocognitive tasks, the Cambridge Neuropsychological Test Automated Battery, was used to assess executive and memory function in 54 hospitalized patients with chronic schizophrenia. Analysis of covariance was used to examine differences between groups with or without the topographical syndromes of orofacial tardive dyskinesia and between groups with high or low negative symptom scores. Principal-components and path analyses were used to examine further the influence of negative symptoms and orofacial tardive dyskinesia on performance on tests of memory and executive function. RESULTS: Both orofacial tardive dyskinesia and negative symptoms were significantly and independently associated with deficits on measures of spatial working memory span derived from principal-components analysis, but only orofacial tardive dyskinesia was associated with deficits on measures of spatial working memory strategy. Both were also associated with impairment on the delayed-matching-to-sample task, a test of memory. These associations were not explained by deficits in global intellectual function. Path analysis suggested that the relationships between the clinical symptoms and performance on the delayed-matching-to-sample task were mediated entirely through their relationship with the spatial working memory measures. CONCLUSIONS: In schizophrenia, orofacial tardive dyskinesia and evident negative symptoms are relatively independent markers of compromise of the cerebral systems that mediate spatial working memory. Candidate neural circuits include the frontal-striatal-thalamic systems, particularly those involving the dorsolateral prefrontal cortex.
The prevalence of tardive dyskinesia in patients receiving antipsychotic medication is 15%–20% (1), although in older patients the figure may reach 60% (2). The two subsyndromes that have been identified—orofacial tardive dyskinesia and trunk and limb tardive dyskinesia—may be pathophysiologically distinct (3), as suggested by the differences in clinical correlates and in their associations with particular symptoms and neurocognitive deficits (4). Orofacial tardive dyskinesia has been consistently and specifically associated with both negative symptoms and neuropsychological deficits (2, 5). Early studies examining the latter relationship (6–9) used short-form measures of global cognition (e.g., Mini-Mental State Examination [MMSE]), but they did not assess conceptual abilities or abstraction, and assessment of memory was limited to tests of orientation and immediate memory (see reference 4 for discussion). In the few more detailed studies of memory and learning in tardive dyskinesia, the findings have been inconsistent (e.g., references 10, 11; see reference 4 for review), partly owing to the small numbers of subjects, the limited age ranges in some studies, or the failure to assess dyskinesia subsyndromes separately. Further, the use of global memory measures, without specific hypotheses about which memory systems were implicated, has not helped to clarify the nature of the associations with tardive dyskinesia. However, in a study comparing patients with and without tardive dyskinesia, Myslobodsky (12) found that patients with tardive dyskinesia were impaired on recall tasks rather than on recognition memory tasks, a pattern that has been described in disorders of the basal ganglia (13). Granholm et al. (14) found that severity of tardive dyskinesia was associated with poorer procedural learning and, in magnetic resonance imaging studies, also found shortened T2 relaxation times in the caudate nuclei of these patients, further implicating the basal ganglia.
Other studies have demonstrated that patients with orofacial tardive dyskinesia tend to show deficits on tasks of executive functioning, such as the Trail Making Test (5, 15) and the Wisconsin Card Sorting Test (16–18). We have argued previously that frontal-striatal-thalamic circuits are implicated in the pathophysiology of schizophrenia and that deficits in executive function (and some memory functions) result from disruption of these systems (13, 19, 20). Further, we have suggested that this disruption may also explain the high incidence of tardive dyskinesia in schizophrenia (4). Therefore, we would predict that patients with tardive dyskinesia (especially orofacial tardive dyskinesia) would manifest cognitive deficits related to disturbances of frontal-striatal systems, such as impairments of working memory and procedural learning. In contrast we would expect little or no relationship between tardive dyskinesia and cognitive functions subserved primarily by temporal lobe structures (e.g., delayed-matching-to-sample tasks [21–24]).
The negative symptoms of schizophrenia, which have also been associated with neurocognitive deficits, have emerged as a risk factor for the early development of tardive dyskinesia, suggesting that these schizophrenic symptoms increase the risk of developing this condition (4, 25). Studies examining the relationship between tardive dyskinesia, negative symptoms, and impaired cognitive function have generally found significant associations (17, 26), although few studies have systematically examined the interrelationships of all three factors. On the basis of such findings, it has been proposed that several different pathological processes might act synergistically in patients with schizophrenia to bring forward in time what is essentially, at least in the case of orofacial tardive dyskinesia, an inherent motor pattern. A complex interaction is postulated between the aging process, exposure to dopamine antagonist drugs, and the pathological changes underlying the schizophrenic illness, particularly those implicated in negative symptoms (27). Pantelis et al. (13) have argued further that in patients with schizophrenia, the pattern of neurocognitive impairment seen with tardive dyskinesia and negative symptoms is similar to that of patients with basal ganglia pathology. They hypothesized more specifically that a shared underlying pathology for negative symptoms and orofacial tardive dyskinesia, as well as for neurocognitive deficits, involves frontal-striatal-thalamic circuits. The study reported here was designed to explore this hypothesis further by examining the relationship between negative symptoms, tardive dyskinesia, and specific neurocognitive measures of memory and executive function, which are thought to be mediated by these frontal-subcortical circuits (20). Further, we hypothesized that any relationship between tardive dyskinesia, negative symptoms, and performance on tasks of memory considered to be mediated by medial temporal lobe structures (e.g., the delayed-matching-to-sample task) would be explainable by previously demonstrated deficits in working memory (28, 29), which have been attributed to dysfunction of the dorsolateral prefrontal cortex (30).
Method
Patients
Patients were members of a study group described elsewhere (29, 31). Briefly, 111 inpatients with DSM-III-R schizophrenia were identified among all inpatients with an illness duration of more than 2 years who were being treated in a long-stay psychiatric hospital. Fifty-one were excluded owing to recent drug abuse (assessed with urine drug screening); poor eyesight; history of significant head injury, leukotomy, or neurological disorder; or a medical condition affecting cognition (detailed in reference 29). Fifty-four of the 60 remaining patients completed neuropsychological and clinical testing.
The Riverside Ethics Committee (London) approved the study, and all patients provided written informed consent.
Assessments
Basic demographic data on age, gender, illness duration, and duration of hospitalization were collected. Clinical symptoms were assessed with the Manchester Scale (32). On the basis of the combined score on the two negative symptom items (flatness of affect and poverty of speech), the patients were assigned to low (score <3) or high (score ≥3) negative symptom groups.
Movement Disorder
Tardive dyskinesia was assessed with the Tardive Dyskinesia Rating Scale (33); ratings for trunk and limb tardive dyskinesia were limited to choreiform or choreoathetoid movements. One investigator (T.R.E.B.), who was unaware of patients’ cognitive assessments, completed all movement disorder ratings. Data on the scale’s interrater and test-retest reliability and level of agreement with assessments of expert clinicians are provided elsewhere (33). The presence of orofacial tardive dyskinesia was defined by a total score of 2 or more on the orofacial items of the Tardive Dyskinesia Rating Scale. The presence of trunk and limb tardive dyskinesia was similarly defined by using the total score on the trunk and limb items.
Neuropsychological Assessments
Each patient was assessed with the MMSE (34), the WAIS-R (35), and the National Adult Reading Test (36). The National Adult Reading Test score was converted to a WAIS-R full-scale IQ to provide an estimate of premorbid intelligence that has high test-retest reliability in patients with chronic schizophrenia (37). Patients scoring fewer than 10 correct words on the National Adult Reading Test were administered the Schonell Graded Word Reading Test (38) to improve the estimate of premorbid IQ.
Cambridge Neuropsychological Test Automated Battery
Each patient was given a series of computerized tests from the Cambridge Neuropsychological Test Automated Battery (39). The tests were run on an Acorn BBC Master microcomputer (Acorn, Cambridge, U.K.) with a high-resolution Microvitec color monitor and Touchtec 501 touch-sensitive screen (Microvitec Display, Bradford, U.K.). Executive and memory function were assessed with the following five tasks:
1. The spatial short-term memory task assessed the patient’s ability to remember a sequence of squares presented on the screen. The patient’s visuospatial span was the highest level (measured as the number of squares from two to nine) at which the patient successfully remembered at least one sequence of squares. This measure indexed the ability to hold information on-line in order to plan moves on tasks such as the spatial working memory task.
2. The spatial working memory task required the patient to search through an increasing number (two, three, four, six, and eight) of boxes to locate hidden tokens. A box with a token held only one token, so that once the token was located in a particular box in a sequence, that box would not need to be searched again. A “between-search error” occurred when a patient returned to search a box where a token had already been located. A “strategy” score, ranging from 1 (best) to 37 (worst), calculated for the more difficult six- and eight-box levels (29), reflected the extent to which the patient adopted a systematic search.
3. The pattern recognition task required the patient to observe 12 abstract line patterns presented individually, followed by 12 pairs of stimuli that were sequentially in reverse order relative to the target stimuli. The patient had to point to the previously seen target pattern, rather than to the incorrect distractor. Each patient was tested with two sets of 12 novel stimuli. The percentage of correct responses for the 24 trials was determined.
4. The spatial recognition task measured the ability to recognize the spatial location of target stimuli. A sequence of five white boxes with different spatial locations was presented, followed by two white squares at two locations that were sequentially the reverse of the locations of the target stimuli. The patient had to point to the previously seen target location, rather than to the distractor position. A total of four sequences that used novel positions for the target stimuli were presented. The percentage of correct responses for the 20 trials was determined.
5. The delayed-matching-to-sample task measured the ability to remember a target stimulus (a complex, multicolored pattern) presented for 4 seconds. After presentation of the stimulus, the patient was required to choose the identical pattern from a set of four stimuli (one correct and three distractors) after variable delay periods (0, 4, and 12 seconds) or during a simultaneous matching condition in which the target and four choices appeared together. Four sets of 10 trials were presented for each of the simultaneous and delay conditions, and the percentage of correct responses across each condition was determined. Theoretical analysis suggests that working memory and strategic ability are critical to performance on this task.
Data Processing and Analysis
Data analysis was performed with SPSS (SPSS Inc., Chicago). The data from the spatial short-term memory task, spatial working memory task, and delayed-matching-to-sample task were analyzed with analysis of variance (ANOVA) and repeated measures ANOVA, as appropriate, with age as a covariate (unless otherwise stated). Multivariate F tests are labeled as such. For correlations, Pearson’s product-moment correlation coefficients (r) were calculated. The Bonferroni correction was used in the analysis of data from the spatial working memory task (five difficulty levels) and the delayed-matching-to-sample task (three difficulty levels); significant interactions were further examined with p set to <0.01 and <0.016, respectively.
On the basis of an initial analysis of the neuropsychological task results, we identified key intervening variables relating specifically to working memory. These were used to model the relationship between negative symptoms, orofacial tardive dyskinesia, and performance on the delayed-matching-to-sample task. The items related to working memory (29) were reduced by principal-components analysis to produce independent factors to simplify subsequent analyses, given the small number of subjects. Path analysis was then used to examine how negative symptoms and orofacial tardive dyskinesia might affect performance on the delayed-matching-to-sample task by means of these working memory factors. The structural equation modeling program that was employed (AMOS) (SPSS Inc., Chicago) uses asymptotic standard errors to specify 95% confidence intervals for the path coefficients.
Results
Patients
The demographic and clinical characteristics of the study patients, grouped by presence or absence of orofacial and trunk and limb tardive dyskinesia and by severity of negative symptoms, are summarized in Table 1. There were no significant differences between the groups in the proportion of patients who received anticholinergics or in the mean dose of antipsychotics. The groups with and without orofacial or trunk and limb tardive dyskinesia did not differ on negative symptom scores, and, similarly, the groups with low and high negative symptom scores did not differ significantly on mean orofacial or trunk and limb tardive dyskinesia scores. Thus, negative symptoms and tardive dyskinesia were not closely associated in this study group, a finding that was also demonstrated by the lack of a significant correlation between these measures. There were no differences between the groups in mean MMSE score or premorbid IQ (estimated from the National Adult Reading Test score). In the overall patient group, there were no significant correlations between MMSE scores and total scores for orofacial tardive dyskinesia, trunk and limb tardive dyskinesia, or negative symptoms.
Executive Function
Spatial short-term memory task
Patients with and without orofacial or trunk and limb tardive dyskinesia and those with high versus low negative symptom scores did not differ on measures of spatial short-term memory span (Table 1).
Spatial working memory
Figure 1 shows the mean number of between-search errors for the two orofacial tardive dyskinesia groups and the two negative symptom groups at various stages of the task. For the orofacial tardive dyskinesia groups, there was a significant interaction between group and level of difficulty across the five stages of the task (multivariate F=3.05, df=4, 40, p=0.03), but no main group effect. Post hoc analyses indicated that the two orofacial tardive dyskinesia groups differed significantly in number of between-search errors at the most difficult level of the task (F=7.79, df=2, 43, p=0.008). There was no significant effect of antipsychotic medication dose on these results. There were no significant differences between the two trunk and limb tardive dyskinesia groups in their performance on the task.
There was no significant difference between the negative symptom groups in the number of between-search errors with increasing difficulty of the task (F=3.62, df=1, 43, p=0.06). However, total negative symptom score was significantly correlated with the number of between-search errors at the four- and eight-box levels (r=0.34, df=46, p<0.02, and r=0.44, df=44, p=0.002, respectively), even after covarying for age and antipsychotic dose.
There was no significant difference between the orofacial tardive dyskinesia groups, the trunk and limb tardive dyskinesia groups, or the negative symptom groups in their strategy scores on this task. This suggests that differences in performance cannot be attributed to differences in the use of a systematic search strategy.
The interaction between orofacial tardive dyskinesia group, negative symptom group, and task performance was examined. The analysis revealed a significant interaction of orofacial tardive dyskinesia and task difficulty (multivariate F=2.77, df=4, 38, p=0.04), but the interactions of negative symptom group and task difficulty and of orofacial tardive dyskinesia group, negative symptom group, and task difficulty were not significant.
Memory Function
Pattern and spatial recognition tasks
There were no significant differences in the percentage of correct responses on the pattern and spatial recognition tasks between the orofacial tardive dyskinesia groups, the trunk and limb tardive dyskinesia groups, or the negative symptom groups (Table 1).
Delayed-matching-to-sample task
The percentage of correct responses in the simultaneous matching condition of the delayed-matching-to-sample task did not differ significantly between the orofacial tardive dyskinesia groups, the trunk and limb tardive dyskinesia groups, and the negative symptom groups. Comparing the performance of the same groups in the delayed matching conditions, there were no significant effects of delays of 0, 4, and 12 seconds.
There was a significant difference between the negative symptom groups during the delay matching conditions (F=4.55, df=1, 35, p=0.04) but no interaction between group and delay period. Post hoc analysis indicated that the patients with a high negative symptom score had a significantly lower percentage of correct responses than those with a low negative symptom score at 0 seconds, i.e., no delay (F=8.24, df=1, 37, p=0.007); the difference between the orofacial tardive dyskinesia groups in the percentage of correct responses at 0 seconds did not reach significance (F=3.70, df=1, 36, p=0.06) (Figure 2). There were no significant three-way interactions between performance on the delay matching conditions, negative symptom group, and orofacial tardive dyskinesia group.
The simultaneous matching condition may be conceptualized as a control task for the delayed matching conditions, i.e., the simultaneous and delay conditions have the same neurocognitive requirements except that the ability to internally represent the stimuli is required in the delay conditions. On this basis, and also taking into consideration the lack of any delay-dependent effects on the task, we conducted a repeated measures ANOVA of performance at the simultaneous and subsequent delay conditions to identify the contribution that internal representation makes in the delay conditions. For the negative symptom groups there was now a significant effect of delay (multivariate F=2.96, df=3, 33, p<0.05) and a significant interaction of delay and group (multivariate F=4.13, df=3, 33, p=0.01), but the difference between the groups was not significant (F=3.66, df=1, 35, p=0.06). Similarly, for the orofacial tardive dyskinesia groups, there was a significant effect of delay (multivariate F=3.55, df=3, 34, p=0.02) but no significant difference between the groups (F=3.81, df=1, 36, p=0.06) and no interaction of delay and group. There were no significant three-way interactions.
Correlational analyses (controlling for age) revealed significant relationships between performance on the delay conditions and both negative symptom score and orofacial tardive dyskinesia score (for scores at 0 seconds delay, r=–0.46, df=34, p=0.003, and r=–0.54, df=34, p=0.001, respectively; for summary scores across delay periods: r=–0.47, df=34, p=0.003, and r=–0.38, df=34, p=0.02, respectively).
Principal-components analysis
On the basis of these analyses and on the theorized nature of working memory (29, 40), three measures of working memory were selected for subsequent principal-components analysis. They were the number of between-search errors at level 8 of the spatial working memory task, the spatial short-term memory span score, and the spatial working memory task strategy score. Principal-components analysis with varimax rotation produced two independent factors that explained 80% of the variance in these three measures. As shown in Table 2, the first factor (spatial working memory span factor) had high loadings from spatial short-term memory span and between-search errors at level 8 of the spatial working memory task. The second factor (spatial working memory strategy factor) had a unique high loading from the strategy score. These factors were therefore conceptualized as two independent aspects of working memory, one related to spatial working memory and span and the other related to spatial working memory strategy. For both factors, higher scores represented greater impairment. To simplify the subsequent path analysis, we estimated the spatial working memory span and spatial working memory strategy factor scores for each patient using the linear regression estimation method.
Pearson’s correlations for the relationships between the two working memory factors and performance on the delayed-matching-to-sample task, negative symptom scores, and orofacial tardive dyskinesia scores are also presented in Table 2.
Path analysis
Given the hypothesized requirements of the delayed-matching-to-sample task, we used path analysis to investigate how working memory mediated the observed relationships between both negative symptoms and orofacial tardive dyskinesia and performance on the delayed-matching-to-sample task. Figure 3 shows the path diagram and path coefficients. Because the two working memory factors (spatial working memory span and spatial working memory strategy) were independent, no path between them was incorporated in the model. The final path model was constructed by successively eliminating nonsignificant paths from a full model. The nonsignificant paths were the direct paths between negative symptoms and performance on the delayed-matching-to-sample task (beta=–0.06, p=0.65), between negative symptoms and the spatial working memory strategy factor (beta=0.09, p=0.56), and between orofacial tardive dyskinesia and performance on the delayed-matching-to-sample task (beta=–0.17, p=0.27). Elimination of these paths did not result in any significant reduction in the fit of the model (χ2=1.24, df=3, p=0.26). Further, while age was significantly associated with orofacial tardive dyskinesia (r=0.48, df=52, p<0.001), the contribution of age to the variables in the path analysis was minimal and nonsignificant. Further, partial correlations showed that orofacial tardive dyskinesia, rather than age, was the explanatory variable. Therefore, orofacial tardive dyskinesia scores were not adjusted for age in the path analysis.
Both negative symptoms and orofacial tardive dyskinesia were independently and positively associated with the spatial working memory span factor score (all path coefficients in the diagram are significant at p<0.05). Only orofacial tardive dyskinesia was related to the spatial working memory strategy factor score. The strategy factor score and the span factor score contributed almost equally to the delayed-matching-to-sample task score, but there was no direct contribution from either negative symptoms or orofacial tardive dyskinesia. The orofacial tardive dyskinesia score was linearly related to the span factor, but closer examination revealed a nonlinear relationship between this measure and the strategy factor, in the form of an apparent ceiling effect on spatial working memory strategy at higher levels of orofacial tardive dyskinesia. We examined this nonlinearity using quadratic regression and found that the raw correlation between these variables rose from 0.44 to 0.53. However, it was not possible to incorporate this effect into the model by using a nonlinear transformation on either of the measures because this change would have attenuated the linear correlations between other variables in the model.
Discussion
Specific neurocognitive deficits have been reported in patients with chronic schizophrenia, particularly in the areas of executive function and memory (41). Such deficits appear to be present at an early stage of the illness (42, 43) and appear to be relatively stable over time (44). In the current study we examined the relative contribution of negative symptoms and tardive dyskinesia to such deficits in patients with chronic schizophrenia. The results suggest that, compared to patients with chronic schizophrenia who do not have orofacial tardive dyskinesia or evident negative symptoms, those who do show significantly greater deficits in performance on a spatial working memory task that is considered a test of executive function. This effect was particularly evident when the demands on working memory were greatest. These associations with working memory were not explained by age, current dose of antipsychotic medication, or any global deficit in intellectual functioning in these patients.
Further, orofacial tardive dyskinesia and negative symptoms were associated with significantly poorer performance on the delayed-matching-to-sample task, a test of visual recognition memory. When the effects of age were controlled, these patients showed no delay-dependent effects on this task, unlike the deficits observed in patients with medial temporal lesions or Alzheimer’s dementia (21, 22). Rather, orofacial tardive dyskinesia and negative symptoms were associated with relatively marked impairments in the first delay condition at which the stimulus information must be held “on line” in short-term memory. These symptoms were not related to impairment in performance during the simultaneous condition of the matching-to-sample task. Further, when we examined the hypothesis that these patients were impaired in their ability to form an internal representation of the stimuli in the matching task, significant effects were observed, including a significant interaction effect with negative symptoms and a nearly significant effect for orofacial tardive dyskinesia, while both groups of symptoms were significantly associated with performance on the delayed matching task. These findings suggest that the apparent memory deficit in patients with negative symptoms and in those with orofacial tardive dyskinesia may be understood as an inability to represent the information internally. This inability is represented in the working memory component of the task, consistent with the theoretical requirements of the task (see Method section). The model tested in the path analysis supported this interpretation by showing that the significant associations of both orofacial tardive dyskinesia and negative symptoms with impaired performance on the delayed-matching-to-sample task could be mediated by the mnemonic and strategic components of working memory. These relationships implicate the involvement of frontal executive systems, which have been identified as relevant to working memory function (for discussion, see reference 29). The lack of delay-dependent effects may suggest that abnormalities in medial temporal areas are less relevant to the deficits observed. These findings are supported by a recent functional magnetic resonance imaging study that used a similar delayed matching task and found that medial temporal lobe areas were activated during the delay component of the task (24). Further, deficits on the delayed-matching-to-sample task we used have been identified in patients with both frontal lobe lesions and disorders of the basal ganglia (22, 45, 46). It is important to note that patients with Parkinson’s disease have also shown delay-independent deficits on the task (22). The observed relationships between negative symptoms and orofacial tardive dyskinesia and the delay-independent effects of the tasks, together with the relationships with working memory, would be consistent with involvement of relevant frontal-striatal systems in both negative symptoms and tardive dyskinesia (4, 13).
Unlike orofacial tardive dyskinesia, trunk and limb tardive dyskinesia was not significantly associated with task performance on any of the executive or memory function tasks. This finding provides further support for the idea that orofacial tardive dyskinesia and trunk and limb tardive dyskinesia are separate topographical subsyndromes, with the former showing the more consistent association with neurocognitive impairment (2, 4, 6). Any interpretation of such a finding as evidence that these clinical subsyndromes are pathophysiologically distinct should take account of the greater specificity of assessment items for orofacial tardive dyskinesia compared to trunk and limb tardive dyskinesia (3). Assessment of the latter may be potentially confounded by the presence of other behavioral and neurological problems, such as dystonia, akathisia, and stereotypies and mannerisms. This issue was acknowledged in the present study by basing the ratings for trunk and limb tardive dyskinesia on choreiform or choreoathetoid movements, to the extent that these can be clinically discriminated. Although there may be a closer association between frontal-striatal circuitry involving orofacial tardive dyskinesia and that involving working memory, it is not possible to speculate further about the anatomical details from this study.
Any possible confounding effect of medication in this study was minimized by the lack of a significant difference in current mean daily dose of antipsychotic medications between patients in the orofacial tardive dyskinesia and negative symptom groups. The significant findings of the study were not changed when antipsychotic dose was covaried. Further, the proportion of patients receiving anticholinergic agents did not differ between these groups. Although the prevalence of orofacial tardive dyskinesia increases with age, covarying for current age did not affect the results.
This study suggests that, in patients with chronic schizophrenia, orofacial tardive dyskinesia and negative symptoms act as independent factors influencing performance on spatial working memory tasks. Path analysis showed that these symptoms also had a significant influence on performance on a delayed-matching-to-sample task, operating largely through the components of working memory. The results of the path analysis should be interpreted with caution because of the relatively small number of subjects in this study. However, this analysis suggested that, while negative symptoms operated mainly by means of the short-term memory component of working memory, orofacial tardive dyskinesia operated through both the mnemonic and the strategic components of working memory. One interpretation is that orofacial tardive dyskinesia and evident negative symptoms are relatively independent markers of compromise of the cerebral systems that mediate spatial working memory. However, other studies have identified significant relationships between orofacial tardive dyskinesia and negative symptoms (6, 9, 47), although this relationship was not apparent in our study. It has been suggested that such a relationship is only evident in younger patients, in whom the presence of negative symptoms is a risk factor for the development of orofacial tardive dyskinesia, while in older patients this relationship is lost (25). Taken together with the results of the present investigation, these findings suggest that both overlapping and separable mechanisms are involved in the production of negative symptoms and orofacial tardive dyskinesia. Candidate neural systems that mediate the mnemonic and strategic components of working memory include frontal-striatal-thalamic systems, particularly involving the ventrolateral and adjacent dorsolateral prefrontal cortices, respectively (48). The dorsolateral prefrontal cortex has previously been associated with negative symptoms (20, 49), but to our knowledge no studies have examined ventrolateral areas. Our findings provide some support for pathophysiological independence between orofacial tardive dyskinesia and negative symptoms in patients with chronic schizophrenia. However, there is also evidence to support an element of shared pathophysiology between these clinical domains (4), with dysfunction of the ventrolateral prefrontal-striatal-thalamic circuit accounting for the co-occurrence of negative symptoms, orofacial tardive dyskinesia, and working memory specifically.
 |
 |
Presented at the satellite symposium on cognition in schizophrenia, immediately preceding the International Congress on Schizophrenia Research, Santa Fe, N.M., April 15–16, 1999. Received Jan. 12, 2000; revision received Jan. 29, 2001; accepted Feb. 5, 2001. From the Cognitive Neuropsychiatry Research & Academic Unit, Department of Psychiatry, University of Melbourne, and the Applied Schizophrenia Division, Mental Health Research Institute; the Division of Neuroscience and Psychological Medicine, Imperial College School of Medicine, London, U.K.; the Department of Psychology, Horton Hospital, Surrey, U.K.; and the Department of Experimental Psychology, Cambridge University, Cambridge, U.K. Address reprint requests to Dr. Pantelis, Cognitive Neuropsychiatry Research & Academic Unit, Department of Psychiatry, University of Melbourne, Cognitive Neuropsychiatry Research and Academic Unit, Sunshine Hospital, P.O. Box 294, St. Albans, Victoria 3021, Australia; [email protected] (e-mail). Supported by the Horton Hospital League of Friends and Westminster Hospital League of Friends. Development of the Cambridge Neuropsychological Test Automated Battery was supported by a program grant from the Wellcome Trust to Dr. Robbins. The authors thank Fiona (Barber) Ambery, Susan Bodger, and Sîan Thrasher for help with the study, Dr. Stephen Wood for comments on the manuscript, and Jo Iddon for help with data queries.
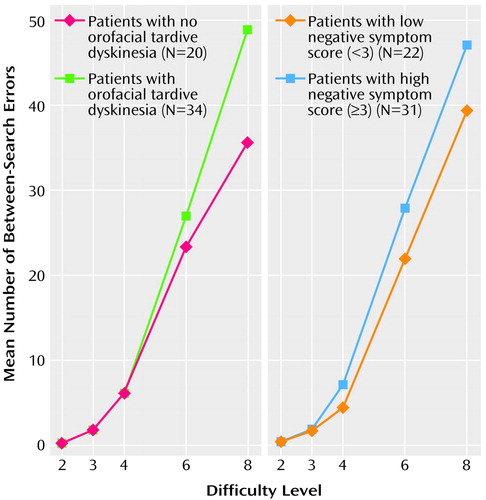
Figure 1. Performance on a Spatial Working Memory Taska of Schizophrenic Patients With and Without Orofacial Tardive Dyskinesiab and Patients With Low or High Negative Symptom Scoresc
aPatients searched through an increasing number of boxes to find a hidden token. A between-search error occurred when a patient searched again through a box where a token had already been found. Difficulty level was the number of boxes available to be searched.
bPresence of orofacial tardive dyskinesia indicated by a total score of 2 or more on the orofacial items of the Tardive Dyskinesia Rating Scale (33).
cTotal score on the Manchester Scale (32) items for flatness of affect and poverty of speech.
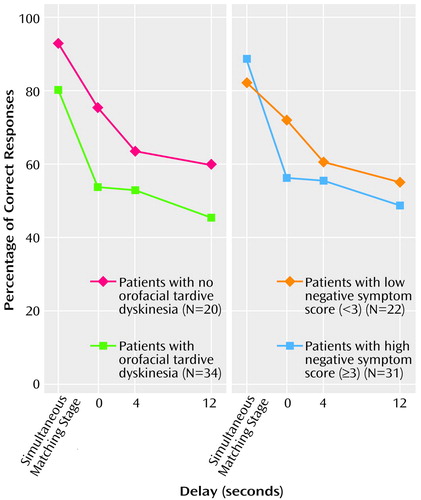
Figure 2. Performance on a Delayed-Matching-to-Sample Taska of Schizophrenic Patients With and Without Orofacial Tardive Dyskinesiab and Patients With Low or High Negative Symptom Scoresc
aPatients were shown a complex, multicolored pattern for 4 seconds and then were required to choose the same pattern from a set of four images shown simultaneously with the original pattern or after a delay of 0, 4, or 12 seconds.
bPresence of orofacial tardive dyskinesia indicated by a total score of 2 or more on the orofacial items of the Tardive Dyskinesia Rating Scale (33).
cTotal score on the Manchester Scale (32) items for flatness of affect and poverty of speech.
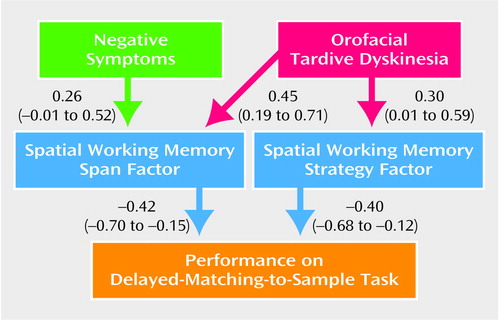
Figure 3. Path Analysisa of the Relationship of Orofacial Tardive Dyskinesia and Negative Symptoms to Spatial Working Memory and Performance on a Delayed-Matching-to-Sample Task in Patients With Schizophreniaa
aPath coefficients (beta) significant at p<0.05 and 95% confidence intervals are shown for each path.
bData on presence of orofacial tardive dyskinesia available for 54 patients; data on negative symptom score available for 53 patients.
1. Tardive Dyskinesia: A Task Force Report of the American Psychiatric Association. Washington, DC, APA, 1992Google Scholar
2. Byne W, White L, Parella M, Adams R, Harvey PD, Davis KL: Tardive dyskinesia in a chronically institutionalized population of elderly schizophrenic patients—prevalence and association with cognitive impairment. Int J Geriatr Psychiatry 1998; 13:473-479Crossref, Medline, Google Scholar
3. Barnes TRE: Movement disorders associated with antipsychotic drugs: the tardive syndromes. Int Rev Psychiatry 1990; 2:355-366Crossref, Google Scholar
4. Collinson SC, Pantelis C, Barnes TRE: Abnormal involuntary movements in schizophrenia and their association with cognitive impairment, in Schizophrenia: A Neuropsychological Perspective. Edited by Pantelis C, Nelson HE, Barnes TRE. London, John Wiley & Sons, 1996, pp 237-258Google Scholar
5. Waddington JL, O’Callaghan E, Larkin C, Kinsella A: Cognitive dysfunction in schizophrenia: organic vulnerability factor or state marker for tardive dyskinesia? Brain Cogn 1993; 23:56-70Crossref, Medline, Google Scholar
6. Waddington JL, Youssef HA, Dolphin C, Kinsella A: Cognitive dysfunction, negative symptoms, and tardive dyskinesia in schizophrenia: their association in relation to topography of involuntary movements and criterion of their abnormality. Arch Gen Psychiatry 1987; 44:907-912Crossref, Medline, Google Scholar
7. Waddington JL, Youssef HA: An unusual cluster of tardive dyskinesia in schizophrenia: association with cognitive dysfunction and negative symptoms. Am J Psychiatry 1986; 143:1162-1165Google Scholar
8. Waddington JL, Youssef HA: Late onset involuntary movements in chronic schizophrenia: relationship of “tardive” dyskinesia to intellectual impairment and negative symptoms. Br J Psychiatry 1986; 149:616-620Crossref, Medline, Google Scholar
9. Davis EJB, Borde M, Sharma LN: Tardive dyskinesia and type II schizophrenia. Br J Psychiatry 1992; 160:253-256Crossref, Medline, Google Scholar
10. Wolf ME, Ryan JJ, Mosnaim AD: Cognitive functions in tardive dyskinesia. Psychol Med 1983; 13:671-674Crossref, Medline, Google Scholar
11. Paulsen JS, Heaton RK, Jeste DV: Neuropsychological impairment in tardive dyskinesia. Neuropsychology 1994; 8:227-241Crossref, Google Scholar
12. Myslobodsky MS: Cognitive impairment in patients with tardive dyskinesia. J Nerv Ment Dis 1985; 173:156-160Crossref, Medline, Google Scholar
13. Pantelis C, Barnes TRE, Nelson HE: Is the concept of frontal-subcortical dementia relevant to schizophrenia? Br J Psychiatry 1992; 160:442-460Crossref, Medline, Google Scholar
14. Granholm E, Bartzokis G, Asarnow RF, Marder SR: Preliminary associations between motor procedural learning, basal ganglia T2 relaxation times, and tardive dyskinesia in schizophrenia. Psychiatry Res 1993; 50:33-44Crossref, Medline, Google Scholar
15. Wegner JT, Catalano F, Gibralter J, Kane JM: Schizophrenics with tardive dyskinesia—neuropsychological deficit and family psychopathology. Arch Gen Psychiatry 1985; 42:860-865Crossref, Medline, Google Scholar
16. Waddington JL, O’Callaghan E, Buckley P, Madigan C, Redmond O, Stack JP, Kinsella A, Larkin C, Ennis JT: Tardive dyskinesia in schizophrenia: relationship to minor physical anomalies, frontal lobe dysfunction and cerebral structure on magnetic resonance imaging. Br J Psychiatry 1995; 167:41-44Crossref, Medline, Google Scholar
17. Brown KW, White T: The association among negative symptoms, movement disorders, and frontal lobe psychological deficits in schizophrenic patients. Biol Psychiatry 1991; 30:1182-1190Google Scholar
18. Brown KW, White T, Palmer D: Movement disorders and psychological tests of frontal lobe function in schizophrenic patients. Psychol Med 1992; 22:69-77Crossref, Medline, Google Scholar
19. Robbins TW: The case for frontostriatal dysfunction in schizophrenia. Schizophr Bull 1990; 16:391-402Crossref, Medline, Google Scholar
20. Pantelis C, Brewer W: Neuropsychological and olfactory dysfunction in schizophrenia—relationship of frontal syndromes to syndromes of schizophrenia. Schizophr Res 1995; 17:35-45Crossref, Medline, Google Scholar
21. Owen AM, Sahakian BJ, Semple J, Polkey CE, Robbins TW: Visuo-spatial short-term recognition memory and learning after temporal lobe excisions, frontal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia 1995; 33:1-24Crossref, Medline, Google Scholar
22. Sahakian BJ, Morris RG, Evenden JL, Heald A, Levy R, Philpot M, Robbins TW: A comparative study of visuospatial memory and learning in Alzheimer-type dementia and Parkinson’s disease. Brain 1988; 111:695-718Crossref, Medline, Google Scholar
23. Fowler KS, Saling MM, Conway EL, Semple JM, Louis WJ: Computerized delayed matching to sample and paired associate performance in the early detection of dementia. Appl Neuropsychol 1995; 2:72-78Crossref, Medline, Google Scholar
24. Elliott R, Dolan RJ: Differential neural responses during performance of matching and nonmatching to sample tasks at two delay intervals. J Neurosci 1999; 19:5066-5073Google Scholar
25. Liddle PF, Barnes TE, Speller J, Kibel D: Negative symptoms as a risk factor for tardive dyskinesia in schizophrenia. Br J Psychiatry 1993; 163:776-780Crossref, Medline, Google Scholar
26. van Os J, Fahy T, Jones P, Harvey I, Toone B, Murray R: Tardive dyskinesia: who is at risk? Acta Psychiatr Scand 1997; 96:206-216Crossref, Medline, Google Scholar
27. Waddington JL: Schizophrenia, affective psychoses, and other disorders treated with neuroleptic drugs: the enigma of tardive dyskinesia, its neurobiological determinants, and the conflict of paradigms. Int Rev Neurobiol 1989; 31:297-353Crossref, Medline, Google Scholar
28. Park S, Holzman PS: Schizophrenics show spatial working memory deficits. Arch Gen Psychiatry 1992; 49:975-982Crossref, Medline, Google Scholar
29. Pantelis C, Barnes TRE, Nelson HE, Tanner S, Weatherley L, Owen AM, Robbins TW: Frontal-striatal cognitive deficits in patients with chronic schizophrenia. Brain 1997; 120:1823-1843Google Scholar
30. Goldman Rakic PS: Prefrontal cortical dysfunction in schizophrenia: the relevance of working memory, in Psychopathology and the Brain. Edited by Carroll BJ, Barrett JE. New York, Raven Press, 1991, pp 1-23Google Scholar
31. Pantelis C, Barber FZ, Barnes TRE, Nelson HE, Owen AM, Robbins TW: Comparison of set-shifting ability in patients with chronic schizophrenia and frontal lobe damage. Schizophr Res 1999; 37:251-270Crossref, Medline, Google Scholar
32. Krawiecka M, Goldberg D, Vaughan M: A standardized psychiatric assessment scale for rating chronic psychotic patients. Acta Psychiatr Scand 1977; 55:299-308Crossref, Medline, Google Scholar
33. Barnes TRE, Trauer T: Reliability and validity of a tardive dyskinesia videotape rating technique. Br J Psychiatry 1982; 140:508-515Crossref, Medline, Google Scholar
34. Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189-198Crossref, Medline, Google Scholar
35. Wechsler D: Wechsler Adult Intelligence Scale—Revised. New York, Psychological Corp, 1981Google Scholar
36. Nelson HE: National Adult Reading Test (NART): Test Manual. Windsor, UK, National Foundation for Educational Research-Nelson, 1982Google Scholar
37. Smith D, Roberts S, Brewer W, Pantelis C: Test-reset reliability of the National Adult Reading Test (NART) as an estimate of premorbid IQ in patients with schizophrenia. Cognitive Neuropsychiatry 1998; 3:71-80Crossref, Google Scholar
38. Schonell F: Backwardness in the Basic Subjects. London, Oliver and Boyd, 1942Google Scholar
39. Robbins TW, James M, Owen AM, Sahakian BJ, McInnes L, Rabbitt P: Cambridge Neuropsychological Test Automated Battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia 1994; 5:266-281Medline, Google Scholar
40. Petrides M: Impairments on nonspatial self-ordered and externally ordered working memory tasks after lesions of the mid-dorsal part of the lateral frontal cortex in the monkey. J Neurosci 1995; 15:359-375Crossref, Medline, Google Scholar
41. Heinrichs RW, Zakzanis KK: Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology 1998; 12:426-445Crossref, Medline, Google Scholar
42. Hutton SB, Puri BK, Duncan LJ, Robbins TW, Barnes TRE, Joyce EM: Executive function in first-episode schizophrenia. Psychol Med 1998; 28:463-473Crossref, Medline, Google Scholar
43. Brewer WJ, Pantelis C, Anderson V, Velakoulis D, Singh B, Copolov DL, McGorry PD: Stability of olfactory identification deficits in neuroleptic-naive patients with first-episode psychosis. Am J Psychiatry 2001; 158:107-115Link, Google Scholar
44. Rund BR: A review of longitudinal studies of cognitive functions in schizophrenic patients. Schizophr Bull 1999; 24:425-436Crossref, Google Scholar
45. Lawrence AD, Sahakian BJ, Hodges JR, Rosser AE, Lange KW, Robbins TW: Executive and mnemonic functions in early Huntington’s disease. Brain 1996; 119:1633-1645Google Scholar
46. Owen AM, Beksinska M, James M, Leigh PN, Summers BA, Marsden CD, Quinn NP, Sahakian BJ, Robbins TW: Visuospatial memory deficits at different stages of Parkinson’s disease. Neuropsychologia 1993; 31:627-644Crossref, Medline, Google Scholar
47. Barnes TRE, Liddle PF: Tardive dyskinesia: implications for schizophrenia? in Schizophrenia: New Pharmacological and Clinical Developments: Royal Society of Medicine International Congress and Symposium Series 94. Edited by Schiff AA, Roth M, Freeman HL. London, Royal Society of Medicine, 1985, pp 81-87Google Scholar
48. Owen AM, Evans AC, Petrides M: Evidence for a two-stage model of spatial working memory processing within the lateral frontal cortex: a positron emission tomography study. Cereb Cortex 1996; 6:31-38Crossref, Medline, Google Scholar
49. Liddle PF, Morris DL: Schizophrenic syndromes and frontal lobe performance. Br J Psychiatry 1991; 158:340-345Crossref, Medline, Google Scholar



