Impact of Clozapine on Completed Suicide
Abstract
OBJECTIVE: Clozapine has been found to be superior to typical neuroleptics in ameliorating the symptoms of refractory schizophrenia. This study evaluated clozapine’s effect on the rate of death due to suicide. METHOD: All patients over a 4-year period who initiated treatment with clozapine while hospitalized within the Department of Veterans Affairs (VA) system (N=1,415) were matched with a schizophrenic control group (N=2,830) by propensity scoring—a widely accepted statistical method that has been used relatively little in psychiatric research. Centralized VA databases and a national death registry were used to identify all deaths within the two groups, along with listed causes, for the 3 years after discharge. RESULTS: Veterans exposed to clozapine while inpatients were significantly less likely to die during the follow-up period than those in the control group, but this was entirely attributable to the much lower rate of death due to respiratory disorders in the clozapine group. There were no significant differences in rates of suicide or accidental death. CONCLUSIONS: These results fail to support the hypothesis that clozapine treatment is associated with significantly fewer deaths due to suicide.
Schizophrenia is the most disabling, prolonged, and costly of mental illnesses, striking 1% of all Americans, most often during adolescence or the young adult years. There is now substantial evidence that the atypical antipsychotic drug clozapine is superior to standard neuroleptic drugs in reducing the symptoms of refractory schizophrenia while inducing fewer extrapyramidal side effects (1–7).
However, the effectiveness of clozapine must be balanced against its association with serious adverse side effects. Clozapine has been found to produce an approximately 1% incidence of potentially fatal agranulocytosis (8), which necessitates initial weekly WBC counts to assure early detection of its development. Clozapine is also associated with a comparatively high incidence of sedation, excessive salivation, hypotension, tachycardia, and, at higher doses, seizures (9). Clozapine is also expensive. However, it has been suggested that clozapine treatment may be associated with a reduction in both attempted and completed suicides (10–12). Such an effect could significantly alter the risk/benefit equation of using this medication.
Since Bleuler’s time, suicide has been recognized as a frequent feature in schizophrenia (13). It has been estimated that up to 10% of all patients with schizophrenia will commit suicide, a rate comparable to that seen in mania and depression (14) and 36 times that observed in the general population (15). It does not appear that treatment with typical neuroleptics has changed the suicide rate in schizophrenia appreciably (16).
Three previous studies have examined clozapine’s effect on suicidal behavior. Meltzer and Okayli (10) compared suicidal thoughts and attempts in a group of 88 patients before and after receiving clozapine. While they observed an 86% decrease in suicide attempts after 2 years of treatment, the absence of a control group precludes determining whether this decrease represents regression to the mean or a medication effect. Walker et al. (11) utilized the Clozaril National Registry to classify all patients exposed to clozapine through 1993 as “current,” “recent,” or “past” users of clozapine. These patients were matched with the National Death Index to determine deaths that had occurred subsequent to clozapine initiation. After eliminating patients over 55 years old, current users of clozapine had a standardized mortality rate (deaths per 100,000 person-years) from suicide of 39 while recent and past users had standardized mortality rates from suicide of 246 and 222, respectively. However, like Meltzer and Okayli, Walker et al. failed to identify a control group of similar patients not exposed to clozapine. In addition, employing a comparison group consisting of patients who had discontinued the medication could introduce bias, since several studies (17, 18) have demonstrated that patients who discontinue medications have a poorer clinical outcome. In the third study, Reid et al. (12) compared annual rates of death due to suicide of patients maintained on regimens of clozapine with rates of suicide among all patients treated within the Texas Department of Mental Health and Mental Retardation and among a subgroup of patients with schizophrenia or schizoaffective disorder. They observed an annual suicide rate (per 100,000 patients) for the clozapine-treated group of 12.7 while finding annual suicide rates of 60.2 and 63.1 among all patients and for the schizophrenic/schizoaffective group, respectively. While Reid et al. did report suicide rates for a schizophrenic control group, the equivalence of the comparison group to the clozapine group on even basic demographic characteristics (such as age, gender, or race) was not systematically established, thereby making interpretation of the results uncertain.
Methodological weaknesses thus limit the conclusions that can be drawn from these reports. The most rigorous method for evaluating the effect of clozapine on suicide would be a random assignment study that compared clozapine to a conventional neuroleptic. However, since suicide is a rare occurrence even among patients with schizophrenia, this study would need a very large number of patients who would then need to be followed for several years. Such a study would be very difficult to complete, since other experimental studies have reported that from 22% (7) to 45% (19) of patients initially assigned to treatment with conventional neuroleptics received clozapine within the first 12–18 months after randomization. Treatment with other atypical neuroleptics in either group would further confound the interpretation of the results.
This study reports on an attempt to examine the effect of clozapine treatment on the rate of completed suicides by using a more rigorous nonexperimental design and national databases from the Department of Veterans Affairs (VA). This study has several strengths. First, it used a comprehensive national database to identify a cohort of schizophrenic patients exposed to clozapine during an inpatient stay as well as a large pool of potential control subjects. It employed a sophisticated statistical technique, propensity scoring, to match clozapine-treated and control patients. It used the National Death Index to identify all deaths and the causes of death for both schizophrenic patient groups in the 3 years following hospital discharge. The last strength of the study is that it systematically addressed the issue of dropouts by using two analyses—one that included dropouts from the clozapine-treated group in an “intent-to-treat” type analysis and one that used a three-group analysis to compare patients on the basis of clozapine exposure.
Method
Sources
Data for this study were obtained from the discharge abstracts of all 73,700 VA psychiatric hospitalizations of at least 1 day for the fiscal years 1992 through 1995 in which the primary discharge diagnosis was schizophrenia. These hospitalizations represented the treatment of 45,917 unduplicated individuals.
All treatment information was retrieved from centralized VA databases. The Patient Treatment File is an automated discharge record of all episodes of VA inpatient care, while the Outpatient Care File documents all outpatient service delivery. For those patients who received clozapine, continued clozapine receipt was monitored through records from the National Clozapine Coordinating Center, which monitors all clozapine dispensed within the VA. With these data, clozapine initiation and termination dates were determined.
We used the National Clozapine Coordinating Center registry to identify 1,415 patients within this group whose Patient Treatment File indicated that clozapine treatment was initiated for the first time during a hospitalization within the years 1992 and 1995. These “index” episodes were identified when the date of clozapine initiation, according to the National Clozapine Coordinating Center, fell between the admission and discharge dates for an episode recorded in the Patient Treatment File. The 44,502 patients who were hospitalized but not identified as having received clozapine at any time during the study period were eligible for selection as control subjects. Eligible control subjects with multiple inpatient episodes had one episode chosen at random for inclusion in the pool of potential matches.
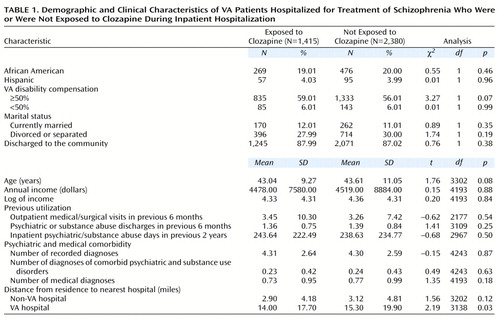
Demographic information such as age, race, marital status, income, and VA disability compensation was available from the Patient Treatment File and Outpatient Care File. Information was also available on the number of medical diagnoses, presence of comorbid psychiatric and substance abuse diagnoses (i.e., dual diagnosis patients), and previous inpatient and outpatient service utilization of psychiatric, substance abuse, medical, or surgical programs. Information concerning the index admission included dates of admission and discharge, discharge status (whether to the community or another residential facility), and zip code of residence. The distances to the nearest VA and non-VA hospitals were calculated by using the patient’s zip code and data from a survey conducted by the American Hospital Association.
The Schizophrenic Control Group
Construction of a control group had to address the issue that patients were presumably selected for clozapine treatment because their symptoms had been unresponsive to conventional neuroleptic treatment. Our ability to ascribe differences in outcome to treatment effects rather than selection biases depends on the degree to which the control group was equivalent to the clozapine group on relevant clinical variables. We used propensity scoring, a widely accepted statistical technique, to maximize the comparability of the clozapine and control groups. With this method, a group of potentially confounding covariates in an observational study is replaced by a single score that measures the propensity of a subject to be similar to the subjects in the treatment group (20–22). This score is then used to match treatment and control groups in an attempt to minimize the effect of selection bias.
Because of the large difference between the clozapine and control groups in the number of recorded inpatient hospital days in the 2 years preceding the index hospitalization, the sample was stratified, and propensity score matching was conducted separately for patients with more than 75 inpatient days during that time and those with 75 days or less.
To apply these methods, logistic regression was performed in which the dependent variable was a dichotomous indicator of clozapine exposure and the predictor variables were potential confounders of the association between clozapine treatment and outcome: age; race; marital status; number of psychiatric, medical, and surgical outpatient visits in the previous 6 months; number of discharges from psychiatric inpatient units in the previous 6 months; medical or surgical hospitalization in the previous 6 months; days of psychiatric inpatient hospitalization in the preceding 2 years; comorbid substance abuse diagnoses; number of medical diagnoses; number of total diagnoses; number of times a patient was transferred between services while an inpatient; receipt of VA disability compensation (no compensation, less than 50% compensation, compensation of 50% or more); whether a patient was discharged to the community or to another institution; and the distance from the centrum of the zip code of residence to the nearest VA and non-VA hospitals. Although administrative databases were used for these data, the data concerning these variables have been shown to be reliable (23).
Fiscal year matching was used to address the change in the supply of VA inpatient services, since there were extensive bed closures at the time of this study (24). Propensity score matching was thus carried out separately within each of eight groups: four fiscal years by two levels of previous inpatient utilization. The size of the pool of potential control patients allowed for the selection of two matching control subjects for each clozapine subject.
The propensity scores of all subjects were partitioned into five subsets by quintiles, and clozapine subjects were randomly matched with control subjects within each quintile. Rosenbaum and Rubin (20) have demonstrated that a five-strata model yields optimal results.
To determine whether the matching had effectively balanced the potential confounders between the clozapine subjects and the selected control sample, univariate t tests for continuous variables and chi-square tests for discrete variables were conducted (Table 1). The only significant difference on any of the variables on which the two groups were matched was the distance to the nearest VA hospital, indicating that the potential confounders had been successfully balanced between the 1,415 clozapine subjects and 2,830 control subjects.
Determination of Mortality Status
Social Security numbers, date of birth, and gender were used to merge the administrative records from the VA sample with the National Death Index to determine vital status of both the clozapine and control groups as of December 31, 1998, the most recent date that vital status information was available. The National Death Index is a comprehensive list of all reported deaths in the nation. Death certificates are required for every death, and states are required to forward death data to the National Death Index.
Searches for matching records were conducted for the year of discharge from the hospital, and every subsequent year through the end of 1998. National Death Index records were returned for all individuals who could have been a possible match with VA records, based upon very loose matching criteria, including Soundex code matching of last names. Determinations of “true” matches were made manually by one of the authors (R.D.). True matches were defined as those in which the gender matched, at least eight out of nine digits of the Social Security number matched, and the year of birth matched to within 1 year. We identified a total of 345 matches in this data set.
The National Death Index also records death certificate information on the reported causes of death, as indicated by the certifying physician. However, death certificate data can be confusing and misleading, since multiple causes of death are often recorded (both primary and underlying), and they are not necessarily reported in order of importance. The National Center for Health Statistics, which maintains the National Death Index and uses the data for reporting national mortality statistics, has developed a coding algorithm that takes all reported causes of death into consideration for a single patient and uses standardized principles to determine the most likely primary cause of death. It is these recoded primary causes of death that we used in our cause-specific mortality analyses.
Mortality Analysis
Analysis of mortality associated with clozapine treatment was conducted in several steps. First, we compared overall all-cause mortality rates among those never exposed to clozapine to the rates for those treated with clozapine. All-cause mortality rates were calculated as the total number of deaths in each group divided by the person-years of follow-up time in each group. Follow-up time for each individual was calculated as the time between discharge from the hospital until either date of death or December 31, 1998. The statistical significance of differences in mortality rates was determined by using a Cox proportional hazards model. The assumption that the underlying hazards for each group were proportional to each other (i.e., there was a consistent effect of group over time) was tested through the use of a time interaction term in the model. Since the time interaction was nonsignificant, the hazards were assumed to be proportional and analysis proceeded without the time interaction. Although the study groups were propensity-score matched on a series of potential confounders, there could still be residual confounding of factors such as age and race. Therefore, the Cox proportional hazards model was also adjusted for the potential confounders of age and race.
We also performed analyses of cause-specific mortality by using a similar statistical model. Cause-specific mortality was grouped by ICD-9 codes into system subcategories (e.g., respiratory, circulatory). Certain disorders (e.g., agranulocytosis, pulmonary embolism) were isolated because of their particular relevance with regard to clozapine use (25). Death rates were calculated as the total number of deaths due to a specific cause divided by the total follow-up time. Differences between the study groups were again tested with Cox proportional hazards models. Patients who died of alternate causes were censored at their time of death, and those who did not die were censored at December 31, 1998.
Using the National Clozapine Coordinating Center database, we were able to identify how long each of the clozapine-exposed patients continued to receive the drug from the VA. Thus, in addition to comparing those receiving clozapine for any length of time to those never exposed to clozapine, we were able to compare those with short-term (less than 12 months) and long-term (12 months or longer) clozapine exposure with each other and with the never-exposed group.
A significance level of p<0.05 was used for all analyses, in spite of the fact that multiple tests were performed. This liberal criterion was employed to give the benefit of the doubt to a treatment effect.
Results
Patients who were exposed to clozapine for any length of time had a lower overall age- and race-adjusted mortality rate than those with no clozapine exposure, and the Cox proportional hazards model indicated that this was a statistically significant difference (Table 2).
When specific causes of death were examined, the clozapine-exposed group had a significantly lower death rate due to respiratory diseases than the control group, specifically chronic obstructive pulmonary disease. This lower respiratory-related death rate was the most likely explanation for the overall mortality rate difference, as evidenced by the fact that when respiratory deaths were eliminated from all-cause analyses there was no longer a significant difference between the two groups (data not shown). There were no significant differences in rates of suicide, injury and poisoning, or undetermined injury (Table 2).
There were 397 (28%) patients who received short-term clozapine treatment (less than 1 year) and 1,018 (72%) who received long-term clozapine treatment (1 year or longer). Table 2 indicates that the all-cause mortality rate was significantly lower for the patients with long-term clozapine exposure relative to the never-exposed group, whereas it was higher for patients with short-term exposure compared to the never-exposed group.
Table 2 also presents comparisons in cause-specific mortality rates for patients with short-term, long-term, or no exposure to clozapine. The patients with short-term clozapine exposure, when compared with the never-exposed group, had significantly higher rates of death attributed to mental disorders. We believe that the deaths attributed to mental disorders were also likely to have been suicides, since mental illness in itself does not directly result in death, and some clinicians may be reluctant to identify their patients as suicides in the public record. The patients with long-term exposure, when compared with the never-exposed group, had significantly lower rates of death due to respiratory disorders. When deaths due to respiratory disorders were removed from all-cause analyses the long-term exposure group still demonstrated a significantly lower death rate than the never-exposed group (11.6 versus 17.01) (χ2=6.92, df=1, p=0.01).
Figure 1 presents survival curves adjusted per the Cox proportional hazards model for patients with short-term, long-term, or no exposure to clozapine. This is a graphical presentation of the all-cause mortality proportional hazards model. Adjusting for age and race, there was a significant difference in survival as determined by the log rank test (χ2=10.84, df=2, p=0.004). Compared to the never-exposed group, the short-term group was 1.13 times more likely to die over the follow-up period, whereas the long-term group was 0.63 times as likely to die.
With this sample size, there was 80% power to detect a relative rate ratio as low as 1.22—this would correspond to a 3.5-point difference in the death rates.
Discussion
This study combined the use of a sophisticated statistical technique and national healthcare and mortality databases to examine differences in both overall mortality and, more specifically, suicide rates between a schizophrenic patient group that initiated clozapine treatment while inpatients and a matched schizophrenic control group that never received clozapine. While inpatient clozapine initiation was associated with a significant decrease in the overall rate of death (primarily attributable to fewer deaths due to respiratory illness), it was not associated with a significant decrease in the rate of completed suicide.
It is noteworthy that the only significant difference in rate of death among the subcategories was for respiratory diseases. We think this is most likely because patients with respiratory diseases such as chronic obstructive pulmonary disease were in manifestly poor health so clinicians may have been hesitant to initiate treatment with a potentially toxic agent. The potential for such a selection bias is reinforced by the VA requirements for a medical history, recent physical, ECG, and blood work before clozapine is prescribed. This hypothesis is supported by the observation that patients who were prescribed clozapine were significantly less likely to have a diagnosis of chronic obstructive pulmonary disease at the time of their index admission (χ2=8.87, df=1, p=0.003). This finding suggests that our matching model, with its emphasis on psychiatric and service utilization variables, did not sufficiently account for selection bias due to severity of medical illness.
Suicide did account for a substantial number of deaths in this study. Twenty-three (9.2%) of the 250 deaths in the never-exposed group and 10 (10.5%) of the 95 deaths in the clozapine group were ruled suicides, confirming the high risk of this outcome in schizophrenia. However, expressing these results in terms of suicides per 100,000 patient-years yields rates of 175 and 150 per 100,000 patient-years for the never-exposed and clozapine groups, respectively—a difference that is not statistically significant.
The absence of a significant difference in suicide rates between all patients treated with clozapine and the control group is consistent with at least two interpretations. First, clozapine could have no greater protective effect on suicide rate than other medications. Second, because we did not employ random assignment, it could be that the clozapine-exposed group had a greater risk of suicide to begin with (and perhaps had even been placed on the medication for this reason) and that the clozapine lowered the suicide rate to match that of the control subjects.
The issue of selection bias in the clozapine group with respect to suicidality deserves closer scrutiny. While the two groups were matched on a host of variables (Table 1), the principal question for this study is whether the two groups were adequately matched on variables representing the risk of suicide. Many risk factors have been identified for suicide in schizophrenia (26). Our matching method accounted for many demographic, vocational, substance abuse, and course-of-illness measures but did not enable us to match directly for previous suicide attempts, depressive and nondepressive symptoms, or awareness of illness. While previous suicide attempts have been considered the most robust predictor of subsequent suicide attempts (27), their ability to predict imminent suicidality has been questioned (28). And while depression and feelings of hopelessness have been associated with a greater risk of suicide (29), this has not been a universal finding (30). However, in comparing the effects of clozapine and standard treatment on suicide, perhaps the most relevant matching question is the role of refractoriness. It has been observed that there is no significant difference in suicide thoughts, plans, or attempts between treatment-responsive and treatment-resistant patients hospitalized with schizophrenia (10).
Other limitations of our study deserve mention. Any study that examines a relatively rare event (in this case death) over a relatively limited period of follow-up would benefit from extending the period of follow-up. Similarly, if there were periods of time in which it was more likely that death would occur, including this period would be desirable. All of the patients in this study, whether receiving clozapine or not, were followed after their discharge from inpatient hospitalization—an event associated with a higher risk for suicide (15, 31). With an average age of 43 for the study population, it is doubtful that our patients were within the first decade of their illness. While this period has been associated with a higher risk for suicide (32), there is also evidence that the risk for suicide, while perhaps decreasing over time, remains high for patients with schizophrenia even after decades of follow-up (14).
There is also the question as to how generalizable these results are. These patients were all discharged inpatients, and their experience cannot necessarily be generalized to inpatients who were not discharged or to outpatients. At the time of this study, access to clozapine was tightly controlled in the VA, as it was in many state systems (19, 33). There is also the issue of comparability between VA patients and other patients with schizophrenia. A recent study has demonstrated that VA patients with schizophrenia and men with schizophrenia treated in non-VA programs do not differ on measures of clinical severity, although they are older and have higher incomes (34).
When patients exposed to clozapine were categorized according to the duration of treatment, patients who stopped receiving clozapine within a year (short-term exposure group) showed a nonsignificantly greater risk of suicide. If a significant risk of suicide had been observed, as it has elsewhere (11), we do not think this would necessarily reflect a medication effect. Poorer outcomes have been consistently observed among patients who are not compliant with medication regimens, including placebo treatment arms in randomized clinical trials (in one particular lithium treatment trial [35], 18%–32% of patients either withdrew consent or could not be located). While we lacked data on the reasons for termination of clozapine treatment in our sample, published reports suggest that patient refusal accounts for a relatively similar proportion (approximately 34%) (36).
To our knowledge, this study is the first attempt to use a carefully matched control group to examine the effect of clozapine on the rate of suicide in patients with schizophrenia. Clozapine treatment was not associated with significant protection against suicide. Although clozapine treatment was associated with an overall lower risk of dying, this appears to be an artifact of imperfect matching on medical illness.
 |
 |
Received June 5, 2000; revisions received Oct. 31 and Dec. 14, 2000; accepted Dec. 28, 2000. From the Psychiatry Service, VA Connecticut Healthcare System; the VA Northeast Program Evaluation Center, New Haven, Conn.; and the Departments of Psychiatry and Public Health, Yale University School of Medicine, New Haven, Conn. Address reprint requests to Dr. Sernyak, Psychiatry Service – 116A, VA Connecticut Healthcare System, West Haven Campus, 950 Campbell Ave., West Haven, CT 06516; [email protected] (e-mail). Funded in part by the Veterans Integrated Service Network (VISN) 1 Mental Illness Research, Education and Clinical Center of the Department of Veterans Affairs. The authors thank Janet Tekell, M.D., David Garver, M.D., and Gary Ripper for their assistance in data acquisition.
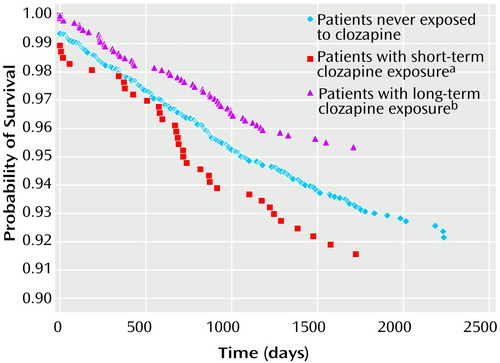
Figure 1. Postdischarge Survival Curves of VA Patients Hospitalized for Treatment of Schizophrenia Who Had Short-Term, Long-Term, or No Exposure to Clozapine
aTreatment with clozapine for less than 12 months.
bTreatment with clozapine for 12 months or longer.
1. Kane J, Honigfeld G, Singer J, Meltzer H: Clozapine for the treatment-resistant schizophrenic: a double-blind comparison with chlorpromazine. Arch Gen Psychiatry 1988; 45:789–796Crossref, Medline, Google Scholar
2. Meltzer H, Burnett S, Bastani B, Ramirez L: Effects of six months of clozapine treatment on the quality of life of chronic schizophrenic patients. Hosp Community Psychiatry 1990; 41:892–897Abstract, Google Scholar
3. Pickar D, Owen RR, Litman RE, Konicki E, Gutierrez R, Rapaport MH: Clinical and biological response to clozapine in patients with schizophrenia: crossover comparison with fluphenazine. Arch Gen Psychiatry 1992; 49:345–353Crossref, Medline, Google Scholar
4. Breier A, Buchanan RW, Irish D, Carpenter W Jr: Clozapine treatment of outpatients with schizophrenia: outcome and long-term response patterns. Hosp Community Psychiatry 1993; 44:1145–1149Google Scholar
5. Breier A, Buchanan RW, Kirkpatrick B, Davis OR, Irish D, Summerfelt A, Carpenter WT Jr: Effects of clozapine on positive and negative symptoms in outpatients with schizophrenia. Am J Psychiatry 1994; 151:20–26Link, Google Scholar
6. Lieberman JA, Safferman AZ, Pollack S, Szymanski S, Johns C, Howard A, Kronig M, Bookstein P, Kane JM: Clinical effects of clozapine in chronic schizophrenia: response to treatment and predictors of outcome. Am J Psychiatry 1994; 151:1744–1752Google Scholar
7. Rosenheck R, Cramer J, Xu W, Thomas J, Henderson W, Frisman L, Fye C, Charney D (Department of Veterans Affairs Cooperative Study Group on Clozapine in Refractory Schizophrenia): A comparison of clozapine and haloperidol in hospitalized patients with refractory schizophrenia. N Engl J Med 1997; 337:809–815Crossref, Medline, Google Scholar
8. Alvir JM, Lieberman JA, Safferman AZ, Schwimmer JL, Schaaf JA: Clozapine-induced agranulocytosis: incidence and risk factors in the United States. N Engl J Med 1993; 329:162–167Crossref, Medline, Google Scholar
9. Lieberman JA, Safferman AZ: Clinical profile of clozapine: adverse reactions and agranulocytosis. Psychiatr Q 1992; 63:51–70Crossref, Medline, Google Scholar
10. Meltzer HY, Okayli G: Reduction of suicidality during clozapine treatment of neuroleptic-resistant schizophrenia: impact on risk-benefit assessment. Am J Psychiatry 1995; 152:183–190Link, Google Scholar
11. Walker AM, Lanza LL, Arellano F, Rothman KJ: Mortality in current and former users of clozapine. Epidemiology 1997; 8:671–677Crossref, Medline, Google Scholar
12. Reid WH, Mason M, Hogan T: Suicide prevention effects associated with clozapine therapy in schizophrenia and schizoaffective disorder. Psychiatr Serv 1998; 49:1029–1033Google Scholar
13. Bleuler E: Dementia Praecox or the Group of Schizophrenias (1911). Translated by Zinkin J. New York, International Universities Press, 1950Google Scholar
14. Tsuang MT, Woolson RF: Excess mortality in schizophrenia and affective disorders: do suicides and accidental deaths solely account for this excess? Arch Gen Psychiatry 1978; 35:1181–1185Google Scholar
15. Black DW: Mortality in schizophrenia—the Iowa Record-Linkage Study: a comparison with general population mortality. Psychosomatics 1988; 29:55–60Crossref, Medline, Google Scholar
16. Winokur G, Tsuang M: The Iowa 500: suicide in mania, depression, and schizophrenia. Am J Psychiatry 1975; 132:650–651Link, Google Scholar
17. Meltzer HY, Cola P, Way L, Thompson PA, Bastani B, Davies MA, Snitz B: Cost effectiveness of clozapine in neuroleptic-resistant schizophrenia. Am J Psychiatry 1993; 150:1630–1638Google Scholar
18. Roy A: Suicide in chronic schizophrenia. Br J Psychiatry 1982; 141:171–177Crossref, Medline, Google Scholar
19. Essock SM, Hargreaves WA, Covell NH, Goethe J: Clozapine’s effectiveness for patients in state hospitals: results from a randomized trial. Psychopharmacol Bull 1996; 32:683–697Medline, Google Scholar
20. Rosenbaum P, Rubin D: Reducing bias in observational studies using subclassification on the propensity score. J Am Statistical Association 1984; 79:516–524Crossref, Google Scholar
21. Rosenbaum P, Rubin D: The central role of the propensity score in observational studies for causal effects. Biometrika 1983; 70:41–55Crossref, Google Scholar
22. Rosenbaum P, Rubin D: Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Statistician 1985; 39:33–38Google Scholar
23. Kashner T: Agreement between administrative files and written medical records: a case of the Department of Veterans Affairs. Med Care 1998; 36:1324–1336Google Scholar
24. Rosenheck R, Horvath T: The impact of VA reorganization on patterns of mental health care. Psychiatr Serv 1998; 49:53Link, Google Scholar
25. Hagg S, Spigset O, Soderstrom T: Association of venous thromboembolism and clozapine. Lancet 2000; 355:1155–1156Google Scholar
26. Meltzer HY: Suicide in schizophrenia: risk factors and clozapine treatment. J Clin Psychiatry 1998; 59(suppl 3):15–20Google Scholar
27. Meltzer H: Suicide and schizophrenia: clozapine and the InterSePT study. J Clin Psychiatry 1999; 60(suppl 12):47–50Google Scholar
28. Breier A, Astrachan B: Characterization of schizophrenic patients who commit suicide. Am J Psychiatry 1984; 141:206–209Link, Google Scholar
29. Drake RE, Cotton PG: Depression, hopelessness and suicide in chronic schizophrenia. Br J Psychiatry 1986; 148:554–559Crossref, Medline, Google Scholar
30. Allebeck P, Varla A, Kristjansson E, Wistedt B: Risk factors for suicide among patients with schizophrenia. Acta Psychiatr Scand 1987; 76:414–419Crossref, Medline, Google Scholar
31. Axelsson R, Lagerkvist-Briggs M: Factors predicting suicide in psychotic patients. Eur Arch Psychiatry Clin Neurosci 1992; 241:259–266Crossref, Medline, Google Scholar
32. Caldwell CB, Gottesman II: Schizophrenics kill themselves too: a review of risk factors for suicide. Schizophr Bull 1990; 16:571–589Crossref, Medline, Google Scholar
33. Reid WH, Pham VA, Rago W: Clozapine use by state programs: public mental health systems respond to a new medication. Hosp Community Psychiatry 1993; 44:739–743Abstract, Google Scholar
34. Rosenheck R, Desai R, Steinwachs D, Lehman A: Benchmarking treatment of schizophrenia: a comparison of service delivery by the national government and by state and local providers. J Nerv Ment Dis 2000; 188:209–216Crossref, Medline, Google Scholar
35. Dorus W, Ostrow D, Anton R, Cushman P, Collins J, Schaefer M, Charles H, Desai P, Hayashida M, Malkerneker U, Willenbring M, Fiscella R, Sather M: Lithium treatment of depressed and nondepressed alcoholics. JAMA 1989; 262:1646–1679Google Scholar
36. Zito J, Volavka J, Craig T, Czobor P, Banks S, Vitrai J: Pharmacoepidemiology of clozapine in 202 inpatients with schizophrenia. Pharmacoepidemiology 1993; 27:1262–1269Google Scholar



