No Support for Regional Selectivity in Clozapine-Treated Patients: A PET Study With [11C]Raclopride and [11C]FLB 457
Abstract
OBJECTIVE: The authors’ goal was to test the hypothesis of extrastriatal D2 receptor selectivity as the mechanism of action of clozapine. METHOD: Positron emission tomography (PET) was used to examine extrastriatal as well as striatal dopamine D2 receptor occupancy in four patients treated with clozapine and three patients treated with haloperidol. The reference radioligand [11C]raclopride was used for determination of D2 receptor occupancy in the striatum. The radioligand [11C]FLB 457 was chosen for determination of D2 receptor occupancy in the thalamus, the temporal cortex, and the frontal cortex. RESULTS: In patients treated with haloperidol the D2 receptor occupancy was high in all examined brain regions. In clozapine-treated patients the D2 receptor occupancy was relatively low in both the striatum and the extrastriatal regions. CONCLUSIONS: The results from the present study give no support for the hypothesis of regional selectivity as the mechanism of action for clozapine.
The dopamine D2 receptor subtype is generally considered to be the biochemical target of antipsychotic drugs (1–3). This hypothesis has been supported by consistent findings of high dopamine D2 receptor occupancy in positron emission tomography (PET) studies of patients treated with classic antipsychotics (4–8).
The prototype atypical drug clozapine is effective in some patients who do not respond to classic antipsychotics (9). In addition, the incidence of extrapyramidal side effects with clozapine is very low (10), and treatment with clozapine may have an effect on negative symptoms (11–13). Interestingly, the striatal D2 receptor occupancy during clozapine treatment is significantly lower (20%–67%) than that during treatment with classic antipsychotics (70%–90%) (14–16). This low D2 occupancy supports the view that clozapine acts by a different mechanism from that of classic antipsychotic drugs.
Despite the low striatal D2 receptor occupancy, it has been suggested that the dopamine system is essential for the mechanism of action of clozapine. In addition to occupancy of the D2 receptors, clozapine treatment induces high occupancy of the serotonin 2A (5-HT2A) receptors. It has been suggested that the high 5-HT2A/D2 ratio underlies the atypical properties of clozapine (11). An early hypothesis was that clozapine has preferential effects in the limbic and cortical dopaminergic systems (17–21). However, the low resolution of older PET systems and the lack of suitable high-affinity radioligands have not allowed detection of the low D2 densities in extrastriatal regions.
We have developed [11C]FLB 457, a substituted benzamide with the very high affinity of 20 pM for D2 and D3 dopamine receptors in vitro (22, 23). In a preliminary study using an old PET system (24, 25), we found that the extrastriatal binding of [11C]FLB 457 is reduced by treatment with haloperidol and clozapine. The D2 occupancy was at the same level in the thalamus and the temporal cortex as that determined with [11C]raclopride in the striatum. Opposite results were demonstrated by Pilowski and co-workers (26) using single photon emission tomography (SPET) and [123I]epidepride in seven clozapine-treated patients. Pilowski et al. reported that the D2 receptor occupancy was low in the striatum but high in the temporal cortex. The results were taken as support for the hypothesis of limbic selectivity of clozapine.
The aim of the present study was to examine the hypothesis of higher D2 receptor occupancy induced by clozapine in extrastriatal regions than in the striatum. Seven patients, three treated with haloperidol and four with clozapine, were examined with high resolution PET. [11C]FLB 457 was the radioligand used for determination of D2 receptor occupancy in the extrastriatal regions, and [11C]raclopride was used for determination of D2 receptor occupancy in the striatum.
Method
The study was approved by the Ethics and Radiation Safety Committees of the Karolinska Hospital. The subjects were examined at the Department of Clinical Neuroscience of Karolinska Hospital and participated after giving written informed consent.
Healthy Comparison Subjects
Eight male subjects, age 23–38 years, participated in two PET examinations with [11C]raclopride and [11C]FLB 457, respectively. They were healthy according to history, physical examination, psychiatric interview, blood and urine analysis, and magnetic resonance imaging (MRI) of the brain. They did not use any medication (23).
Subjects With Schizophrenia
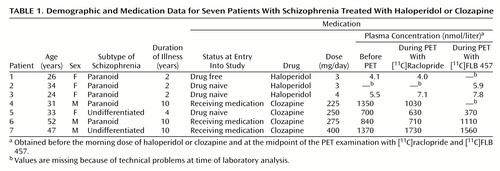
Seven patients with schizophrenia diagnosed according to DSM-IV were recruited (Table 1). The duration of illness ranged between 2 and 10 years. Exclusion criteria were organic mental disorder, drug abuse, brain injury, and somatic illness. None of the patients was receiving concomitant treatment with psychotropic drugs other than the two drugs studied. None had received depot injections during the preceding 6 months. Duration of illness, the case history, and the history of neuroleptic treatment were confirmed by patient records and by interviews of the relatives.
Three patients were treated with haloperidol. Two of these patients were drug naive at the time of entry into the study. The third patient had been drug free for 2 years before the study and had previously received treatment with classic antipsychotics for 1 year. The D2 receptor occupancy was examined with PET when the patients had been on monotherapy with haloperidol for 5–7 weeks. The dose of haloperidol was clinically titrated according to a low-dose strategy; the occurrence of extrapyramidal side effects during clinical titration was a reason for dose reduction.
Four patients were treated with clozapine. One was drug naive and three were on the medication when they entered the study. At the time of the PET examination they had been on monotherapy with clozapine for 1–5 years.
Experimental Procedure
Each of the seven patients participated in two PET examinations during antipsychotic drug treatment. The two examinations, with [11C]FLB 457 and [11C]raclopride, respectively, were performed on the same day. The first examination started 3 hours after the morning dose of haloperidol or clozapine and the second 6 hours later. As part of a separate study on D2 receptors in neuroleptic-free patients, the drug-naive and drug-free patients (patients 1, 2, 3, and 5 in Table 1) had already been examined before they received haloperidol or clozapine.
A plastic helmet was made for each subject and used with a head fixation system to obtain optimal positioning and reliable transfer of regions of interest between PET and MRI (27). To obtain anatomical correlates, the patients were examined with the MR Advantage System on a 1.5-T GE Signa scanner. A fast spin-echo sequence (echo train length=6) was used with a moderately T2-weighted protocol. The series of sections were the same as in the PET examinations.
[11C]Raclopride was prepared by methylation of the desmethyl precursor analogue with [11C]methyl iodide (28). The specific radioactivity of [11C]raclopride was 400–1300 Ci/mmol at the time of injection, and the radioactivity injected was 285–326 Mbq. [11C]FLB 457 was prepared by methylation of the desmethyl precursor (FLB 604) with [11C]methyl iodide or [11C]methyl triflate (22, 29). The specific radioactivity was 700–3000 Ci/mmol, and the radioactivity injected was 229–333 Mbq. The radioligand was injected into the right cubital vein as a bolus for 2 seconds. The cannula was then immediately flushed with 10 ml of saline.
The PET system in use was the Siemens ECAT EXACT 47, which was run in the three-dimensional data mode. The resolution in plane was 3.8 mm, and the axial resolution was 4.0 mm full width half maximum (30). The frame sequence consisted of three 1-minute, four 3-minute, and six ([11C]raclopride) or eight ([11C]FLB 457) 6-minute frames. Brain radioactivity was thus measured for 51 minutes ([11C]raclopride) and 63 minutes ([11C]FLB 457), respectively, after injection of radioligand. The reconstructed data were displayed as 47 horizontal sections with a center-to-center distance of 3.125 mm.
Plasma Drug Concentration
Venous blood samples for determination of plasma drug concentration were drawn before the morning dose and in connection with the PET examinations. The blood samples were drawn into heparin-treated glass tubes and centrifuged. Plasma was frozen at –20°C until analyzed. Haloperidol plasma concentration was analyzed with high-performance liquid chromatography (31). Clozapine plasma concentration was determined by gas chromatography/mass spectrometry with single ion detection (32).
Determination of D2 Receptor Occupancy
Regions of interest were drawn on the MRIs and transferred to the reconstructed PET images. Regions of interest were defined for the frontal cortex and temporal cortex, the thalamus, the putamen, and the cerebellar cortex. The first section in which the superior collicle could be visualized was identified on the MRI, and the thalamus was drawn in four sections above that level. The striatal region of interest (putamen) was drawn in the first three of these four sections. In addition, the frontal and temporal cortex were drawn in three sections at the same level as the thalamus. For the cerebellar region of interest, the first appearance of the petrosal bone was identified on the MRIs and the cerebellar cortex was drawn in four sections below that level. Data from the series of sections were pooled to obtain the average radioactivity concentration of the whole volume of interest and were plotted versus time (24, 25).
[11C]FLB 457 binding to D2 receptors in the extrastriatal regions was analyzed by using the simplified reference tissue model proposed by Lammertsma and Hume (33). The model accounts for regional differences in the time course for free and nonspecifically bound radioligand (33). D2 receptor occupancy was defined as the percentage reduction of the binding potential during drug treatment compared with the binding potential in the absence of treatment.
The general approach was to use the mean regional binding potential values from eight healthy comparison subjects as an estimate of the baseline value. In addition, receptor occupancy was also calculated by using the individual baseline binding potential in the four drug-naive or drug-free patients (patients 1, 2, 3, and 5 in Table 1).
The simplified reference tissue model was used to analyze [11C]raclopride binding to D2 receptors. In addition, to enable comparison with previously published data on D2 receptor occupancy in the striatum, the equilibrium ratio method was applied as described elsewhere (6). Briefly, specific [11C]raclopride binding in the striatum (Cb ) was defined as the difference between the radioactivity in the putamen and that in the cerebellum (Cf). The time curves for the putamen and the cerebellum were integrated for the time interval 9–45 minutes after radioligand injection and a ratio (R) was obtained according to the following  :
:
D2 receptor occupancy in the striatum was defined as the percentage reduction of the ratio during drug treatment compared with the mean values in eight healthy comparison subjects. In addition, individual baseline values were used for four of the patients (patients 1, 2, 3, and 5 in Table 1).
Statistics
The simplified reference tissue model and the ratio method were compared by using an analysis of variance method for estimation of measurement error or reliability (34). This method yields an intraclass coefficient that is based on the within-subject standard deviation and the between-methods standard deviation. The coefficient varies between 0 (no agreement) and 1.0 (perfect agreement). Differences in occupancy between drugs were tested by independent Student’s t tests.
Results
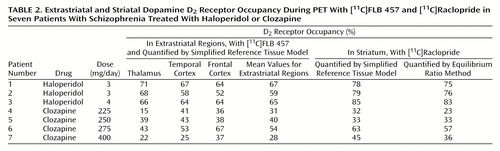
Using the simplified reference tissue model, we found that the D2 receptor occupancy in the striatum of the haloperidol-treated patients was between 78% and 85% (Table 2). In the striatum of the four clozapine-treated patients the D2 receptor occupancy was lower, ranging from 32% to 63% (t=4.29, df=5, p=0.008). The D2 receptor occupancy values determined with the simplified reference tissue model and the ratio method were very similar (Table 2). The coefficient of correspondence between methods was 0.98 (a very high agreement).
In the haloperidol-treated patients, the D2 receptor occupancy in the thalamus was 66%–71% (Table 2). The thalamic D2 receptor occupancy was lower in clozapine-treated patients, ranging from 15% to 43% (t=4.81, df=5, p=0.005). The D2 receptor occupancy in the temporal cortex was 58%–67% in the haloperidol group and 25%–53% in the clozapine group (t=3.12, df=5, p=0.003). In the frontal cortex the range of the D2 receptor occupancy values was 52%–64% for the haloperidol-treated patients and between 36% and 67% for the clozapine treated patients (t=1.53, df=5, p=0.17).
The plasma concentrations of haloperidol and clozapine for all patients were in accordance with reference values from previous studies (32, 35, 36) (Table 1).
Discussion
Previous PET studies have consistently demonstrated high D2 receptor occupancy in the striatum during treatment with classic antipsychotics (4, 7, 8). In patients treated with the atypical antipsychotic drug clozapine, however, a significantly lower striatal D2 receptor occupancy has been reported (15, 16). In the present study, which used a PET system with high resolution and three-dimensional data acquisition, the striatal D2 receptor occupancy was higher in patients treated with haloperidol (78%–85%) than in patients treated with clozapine (32%–63% ). The results for [11C]raclopride binding are thus consistent with earlier studies (15, 16).
The D2 receptor occupancy in the extrastriatal regions for the haloperidol-treated patients was approximately at the same level as in the striatum, which is consistent with previous results (25).
Limbic selectivity has been proposed as a possible mechanism of action of clozapine, the prototype atypical antipsychotic drug. This hypothesis, first formulated in the 1970s, was originally based on animal data and postulated that there is a limbic selectivity in the functional effects of clozapine (17, 18). A receptor ligand study of both rat and human brain tissue (19) suggested that clozapine binds preferentially to D2 receptors in extrastriatal regions. This view was further supported by the finding that clozapine has a different affinity for distinct isoforms of the D2 receptor (37). In the present study, PET was used to test the hypothesis of preferential binding of clozapine to extrastriatal D2 receptors. We found that the mean D2 receptor occupancy in clozapine-treated patients ranged from 28% to 54% in the three extrastriatal regions (Table 2). The corresponding values for the striatum in this group ranged from 32% to 63% (t=0.97, df=3, p=0.41). Preferential extrastriatal D2 receptor occupancy induced by clozapine treatment was thus not supported by our data.
Determination of extrastriatal D2 receptor occupancy was made with the radioligand [11C]FLB 457. The high affinity of [11C]FLB 457 enables quantification of D2 receptor binding in extrastriatal regions with low density of D2 receptors (22). In previous studies, striatal D2 receptor occupancy has routinely been quantified by using the ratio equilibrium method, which requires a peak equilibrium model in radioligand binding during the time of the PET examination (24). The time required for equilibrium is dependent on the density of the receptors (23, 25). In the striatum, a region with very high density of D2 receptors, equilibrium with [11C]FLB 457 will not be reached until several hours after the 63-minute data acquisition time used in this study (38). As previously shown, the short acquisition time for [11C]FLB 457 binding in the striatum is thus not sufficient for reliable calculations of the binding potential according to the peak equilibrium or the simplified reference tissue model (23). Therefore, [11C]FLB 457 binding in the striatum was not included in the present analysis.
For calculation of the D2 receptor occupancy in the extrastriatal regions, we used the simplified reference tissue model developed by Lammertsma and Hume (33), who described it as stable for calculation of small receptor quantities. To allow comparison with previous [11C]raclopride data in the striatum, we used not only the simplified reference tissue model but also the well established equilibrium ratio method (6). There was a significant correspondence in the results of the two methods, suggesting that the methods are comparable.
For calculation of receptor occupancy during drug treatment, reference values obtained in a drug-free state are required. The optimal reference value is that obtained from a recent examination of the same patient before initiation of drug treatment. However, since clozapine is used mainly for patients whose illness is resistant to classic neuroleptics, it may not be possible to perform PET examinations in a drug-free state for ethical reasons. Accordingly, in the clozapine group, where three of the four patients were receiving medication when they entered the study, a mean value obtained in healthy comparison subjects was used as a reference value. The error introduced by this procedure is within a few percent and has been discussed in detail elsewhere (15).
In a study by Pilowski et al. (26), who used SPET and the high-affinity radioligand [123I]epidepride, the D2 receptor occupancy was estimated in seven patients treated with clozapine and five patients treated with typical antipsychotic drugs. Pilowski et al. reported that in all clozapine-treated patients the D2 receptor occupancy was low in the striatum but high in the temporal cortex. In the patients treated with typical antipsychotics, the D2 receptor occupancy was high both in the temporal cortex and the striatum. The results were taken as a support for the hypothesis of limbic selectivity as the mechanism of action of clozapine.
In contradiction to this SPET study, the present findings do not support preferential high occupancy in the extrastriatal regions. There are several methodological differences between these studies that may contribute to the discrepant results. One important issue is the different imaging systems, PET versus SPET. Another issue concerns the choice of radioligand and the method used for quantification of receptor occupancy. A simple ratio approach using a high-affinity radioligand such as [123I]epidepride without validation of equilibrium conditions may yield an underestimation of the D2 receptor occupancy in the high-density striatum in comparison with the D2 receptor occupancy in the low-density extrastriatal regions.
In conclusion, this study confirms that classic as well as atypical antipsychotics induce D2 receptor occupancy in extrastriatal regions. However, the hypothesis of preferential extrastriatal D2 receptor occupancy as the mechanism of action of clozapine was not supported by our data.
 |
 |
Received July 18, 2000; revision received Dec. 11, 2000; accepted Dec. 19, 2000. From the Department of Clinical Neuroscience, Psychiatry Section, Karolinska Hospital. Address reprint requests to Dr. Talvik, Department of Clinical Neuroscience, Psychiatry Section, Karolinska Hospital, S-171 76 Stockholm, Sweden; [email protected] (e-mail). This work was supported by grants from the Swedish National Institute for Mental Health (Ro-41205-12), the BIO-MED II program (BMH4-CT96-0220), the Swedish Medical Research council (B93-21X-09114-05C), and the Swedish Natural Science Research Council (K-KU 9973-308) and by the Karolinska Institute. The authors thank Dr. Gunnar Edman for statistical advice.
1. Creese I, Burt DR, Snyder SH: Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science 1976; 192:481–483Crossref, Medline, Google Scholar
2. Seeman P, Lee T, Chau-Wong M, Wong K: Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature 1976; 261:717–719Crossref, Medline, Google Scholar
3. Carlsson A: Antipsychotic drugs, neurotransmitters, and schizophrenia. Am J Psychiatry 1978; 135:165–173Link, Google Scholar
4. Farde L, Hall H, Ehrin E, Sedvall G: Quantitative analysis of D2 dopamine receptor binding in the living human brain by PET. Science 1986; 231:258–261Crossref, Medline, Google Scholar
5. Cambon H, Baron JC, Boulenger JP, Loc’h C, Zarifian E, Maziere B: In vivo assay for neuroleptic receptor binding in the striatum. Br J Psychiatry 1987; 151:824–830Crossref, Medline, Google Scholar
6. Farde L, Wiesel F-A, Halldin C, Sedvall G: Central D2-dopamine receptor occupancy in schizophrenic patients treated with antipsychotic drugs. Arch Gen Psychiatry 1988; 45:71–76Crossref, Medline, Google Scholar
7. Smith M, Wolf A, Brodie J, Arnett C, Barouche F, Shiue C-Y, Fowler J, Russell J, MacGregor R, Wolkin A, Angrist B, Rotrosen J, Peselow E: Serial [18F]N-methylspiroperidol PET studies to measure changes in antipsychotic drug D-2 receptor occupancy in schizophrenic patients. Biol Psychiatry 1988; 23:653–663Crossref, Medline, Google Scholar
8. Baron JC, Martinot JL, Cambon H, Boulenger JP, Poirier MF, Caillard V, Blin J, Huret JD, Loc’h C, Maziere B: Striatal dopamine receptor occupancy during and following withdrawal from neuroleptic treatment: correlative evaluation by positron emission tomography and plasma prolactin levels. Psychopharmacology (Berl) 1989; 99:463–472Crossref, Medline, Google Scholar
9. Kane J, Honigfeld G, Singer J, Meltzer H: Clozapine for the treatment-resistant schizophrenic: a double-blind comparison with chlorpromazine. Arch Gen Psychiatry 1988; 45:789–796Crossref, Medline, Google Scholar
10. Casey DE: Clozapine: neuroleptic-induced EPS and tardive dyskinesia. Psychopharmacology (Berl) 1989; 99(suppl):S47–S53Google Scholar
11. Meltzer HY: Clinical studies on the mechanism of action of clozapine: the dopamine-serotonin hypothesis of schizophrenia. Psychopharmacology (Berl) 1989; 99(suppl):S18–S27Google Scholar
12. Meltzer HY: Dimensions of outcome with clozapine. Br J Psychiatry Suppl 1992; 17:46–53Medline, Google Scholar
13. Lindenmayer JP, Grochowski S, Mabugat L: Clozapine effects on positive and negative symptoms: a six-month trial in treatment-refractory schizophrenics. J Clin Psychopharmacol 1994; 14:201–204Crossref, Medline, Google Scholar
14. Farde L, Wiesel F-A, Nordström A-L, Sedvall G: D1- and D2-dopamine receptor occupancy during treatment with conventional and atypical neuroleptics. Psychopharmacology (Berl) 1989; 99(suppl):S28–S31Google Scholar
15. Farde L, Nordström A-L, Wiesel F-A, Pauli S, Halldin C, Sedvall G: Positron emission tomographic analysis of central D1- and D2-dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine—relation to extrapyramidal side effects. Arch Gen Psychiatry 1992; 49:538–544Crossref, Medline, Google Scholar
16. Nordström A-L, Farde L, Nyberg S, Karlsson P, Halldin C, Sedvall G: D1, D2, and 5-HT2 receptor occupancy in relation to clozapine serum concentration: a PET study of schizophrenic patients. Am J Psychiatry 1995; 152:1444–1449Google Scholar
17. Andén N, Stock G: Effect of clozapine on the turnover of dopamine in the corpus striatum and the limbic systems. J Pharm Pharmacol 1973; 25:346–348Crossref, Medline, Google Scholar
18. Baldessarini RJ: The “neuroleptic” antipsychotic drugs, 1: mechanisms of action. Postgrad Med 1979; 65:108–111Crossref, Medline, Google Scholar
19. Borison RL, Diamond BI: Regional selectivity of neuroleptic drugs: an argument for site specificity. Brain Res Bull 1983; 11:215–218Crossref, Medline, Google Scholar
20. Janowsky A, Neve KA, Kinzie JM, Taylor B, de Paulis T, Belknap JK: Extrastriatal dopamine D2 receptors: distribution, pharmacological characterization and region specific regulation by clozapine. J Pharmacol Exp Ther 1992; 261:1282–1290Google Scholar
21. Fibiger HC: Neuroanatomical targets of neuroleptic drugs as revealed by Fos immunochemistry. J Clin Psychiatry 1994; 55(suppl B):33–36Google Scholar
22. Halldin C, Farde L, Högberg T, Mohell N, Hall H, Suhara T, Karlsson P, Nakashima Y, Swahn K-G: Carbon-11-FLB 457: a radioligand for extrastriatal D2 dopamine receptors. J Nucl Med 1995; 36:1275–1281Google Scholar
23. Olsson H, Halldin C, Swahn C-G, Farde L: Quantification of [11C]FLB 457 binding to extrastriatal dopamine receptors in the human brain. J Cereb Blood Flow Metab 1999; 19:1164–1173Google Scholar
24. Farde L, Eriksson L, Blomquist G, Halldin C: Kinetic analysis of central [11C]raclopride binding to D2-dopamine receptors studied by PET—a comparison to the equilibrium analysis. J Cereb Blood Flow Metab 1989; 9:696–708Crossref, Medline, Google Scholar
25. Farde L, Suhara T, Nyberg S, Karlsson P, Nakashima Y, Hietala J, Halldin C: A PET study of [11C]FLB 457 binding to extrastriatal D2-dopamine receptors in healthy subjects and antipsychotic drug-treated patients. Psychopharmacology (Berl) 1997; 133:396–404Crossref, Medline, Google Scholar
26. Pilowski LS, Mulligan RS, Acton PD, Eli PJ, Costa DC, Kerwin RW: Limbic selectivity of clozapine (letter). Lancet 1997; 350:490–491Crossref, Medline, Google Scholar
27. Greitz T, Bergström M, Boëtius J, Kingsley D, Ribbe T: Head fixation system for integration of radiodiagnostic and therapeutic procedures. Neuroradiology 1980; 19:1–6Medline, Google Scholar
28. Halldin C, Farde L, Högberg T, Hall H, Ström P, Ohlberger A, Solin O: A comparative PET-duty of five carbon-11 or fluorine-18 labelled salicylamides: preparation and in vitro dopamine D-2 receptor binding. Int J Rad Appl Instrum B 1991; 18:871–881Crossref, Medline, Google Scholar
29. Lundkvist C, Sandell J, Någren K, Pike VW, Halldin C: Improved synthesis of the PET radioligands, [11C]FLB 457, [11C]MDL 100907 and [11C]B-CIT-FE, by the use of [11C]methyl triflate. J Labelled Compounds and Radiopharmaceuticals 1998; 41:545–556Crossref, Google Scholar
30. Wienhard K, Dahlbom M, Eriksson L, Michel C, Bruckbauer T, Pietrzyk U, Heiss W: The ECAT EXACT HR: performance of a new high resolution positron scanner. J Comput Assist Tomogr 1994; 18:110–118Crossref, Medline, Google Scholar
31. LLerena A, Alm C, Dahl M-L, Ekqvist B, Bertilsson L: Haloperidol disposition is dependent on debrisoquine hydroxylation phenotype. Ther Drug Monit 1992; 14:92–97Crossref, Medline, Google Scholar
32. Bondesson U, Lindström L: Determination of clozapine and its N-demethylated metabolite in plasma by use of gas chromatography-mass spectrometry with single ion detection. Psychopharmacology (Berl) 1988; 95:472–475Crossref, Medline, Google Scholar
33. Lammertsma AA, Hume SP: Simplified reference tissue model for PET receptor studies. Neuroimage 1996; 4(3 part 1):153–158Google Scholar
34. Bland JM, Altman DG: Measurement error and correlation coefficients. Br Med J 1996; 313:41–42Crossref, Medline, Google Scholar
35. Smith RC, Vroulis G, Shvartsburd A, Allen R, Lewis N, Schoolar JC, Chojnacki M, Johnson R: RBC and plasma levels of haloperidol and clinical response in schizophrenia. Am J Psychiatry 1982; 139:1054–1056Google Scholar
36. Mavroidis ML, Kanter DR, Hirschowitz J, Garver DL: Clinical response and plasma haloperidol levels in schizophrenia. Psychopharmacology (Berl) 1983; 81:354–356Crossref, Medline, Google Scholar
37. Malmberg Å, Jackson DM, Eriksson A, Mohell N: Unique binding characteristics of antipsychotic agents, interacting with human dopamine D2A, D2B, and D3 receptors. Mol Pharmacol 1993; 43:749–754Medline, Google Scholar
38. Loc’h C, Halldin C, Bottlaender M, Swahn CG, Moresco RM, Maziere M, Farde L, Maziere B: Preparation of [76Br]FLB 457 and [76Br]FLB 463 for examination of striatal and extrastriatal dopamine D-2 receptors with PET. Nucl Med Biol 1996; 23:813–819Crossref, Medline, Google Scholar



