Urinary Free Cortisol in Chronic Fatigue Syndrome
Abstract
OBJECTIVE: The authors measured 24-hour urinary free cortisol in a group of well-characterized patients with chronic fatigue syndrome. METHOD: They obtained 24-hour urine collections from 121 consecutive clinic patients with chronic fatigue syndrome and 64 comparison subjects without the syndrome. RESULTS: Urinary free cortisol was significantly lower in the subjects with chronic fatigue syndrome regardless of the presence or absence of current or past comorbid psychiatric illness. Lower levels of urinary free cortisol were not related to medication use, sleep disturbance, or disability levels. CONCLUSIONS: There is mild hypocortisolism in chronic fatigue syndrome. Whether a primary feature or secondary to other factors, hypocortisolism may be one factor contributing to the symptoms of chronic fatigue syndrome.
Most clinicians agree that chronic fatigue syndrome is a heterogeneous, multifactorial syndrome with a biopsychosocial origin. One component may encompass hypothalamic-pituitary-adrenal (HPA) axis dysfunction. Two studies have found reduced 24-hour urinary free cortisol, three found reduced plasma cortisol, and one found reduced salivary cortisol in chronic fatigue syndrome, but other studies have failed to confirm this mild hypocortisolism (see reference 1 for detailed review). Several factors may underlie this inconsistency, including poorly characterized subject groups (particularly psychiatric comorbidity, illness severity, and illness duration), use of single plasma measures (poor indicators of adrenal function), and small numbers of subjects (none of the studies included more than 35 patients).
Method
All subjects attending our chronic fatigue syndrome clinic at King’s College Hospital in London between January 1997 and March 2000 were asked to provide a 24-hour specimen of urine if they fulfilled the 1994 Centers for Disease Control criteria for chronic fatigue syndrome (2). These criteria exclude patients with a past or current diagnosis of psychotic, melancholic, or bipolar depression; psychosis; dementia; or eating disorder. No subjects had alcohol or substance abuse for 2 years before the onset of symptoms or any time thereafter. Patients underwent thorough medical screening to exclude an alternative cause for their fatigue, and a medication history was obtained.
Patients were interviewed with a semistructured interview for chronic fatigue syndrome and psychological disorder (3) and categorized as having primary chronic fatigue syndrome, i.e., without significant comorbidity, or chronic fatigue syndrome with comorbid diagnoses. Comorbid DSM-IV diagnoses were present in 32 (26%) of the 121 patients, specifically, depression (N=24) and anxiety disorder (panic disorder, agoraphobia, or generalized anxiety disorder [N=7] and fibromyalgia [N=5]). Several patients had more than one comorbid condition. A further 28 (23%) of the 121 patients gave a past history of psychiatric disorder, defined as seeking or receiving treatment for an emotional disorder. Simple phobias were not assessed.
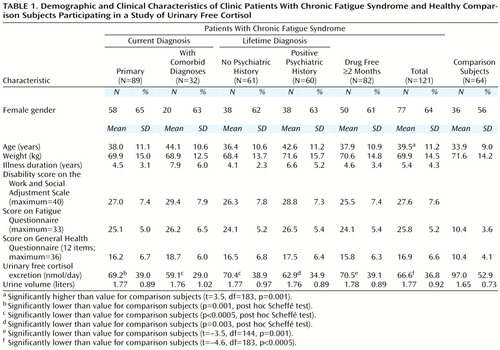
One hundred thirteen of the 121 patients completed questionnaires (Table 1). The questionnaires included the General Health Questionnaire (4), Work and Social Adjustment Scale (5), and Fatigue Questionnaire (6).
Sixty-four ambulant healthy comparison subjects were recruited from volunteers and staff members in our institutions. None gave a history of substantial medical problems, chronic fatigue syndrome, or a major psychiatric disorder during an assessment by a psychiatric nurse using a semistructured interview. The hospital ethics committee approved all procedures. After a complete description of the study, we obtained written informed consent from all participants.
Urinary free cortisol was assayed by using the Technicon Immuno-1 assay (Bayer PLC, Newbury, U.K.) on extracted and reconstituted samples. Statistical analyses comprised two-tailed t tests, one-way analysis of variance (ANOVA) with post hoc Scheffé tests, and Pearson’s correlation coefficients. SPSS version 8.0 (SPSS, Inc., Chicago) was used to conduct the statistical analyses.
Results
Table 1 shows demographic and clinical characteristics of the subjects, including questionnaire scores and urinary free cortisol values. The patients (N=121) and comparison subjects (N=64) were well matched for gender and weight, although patients with chronic fatigue syndrome were slightly older (Table 1). The patients with chronic fatigue syndrome had significantly lower levels of urinary free cortisol than the comparison subjects; this significant difference held when only the medication-free patients were analyzed (Table 1). Mean urinary volume was no different between groups (t=0.83, df=171, p=0.41), reducing the possibility of incomplete collections.
To examine the effect of psychiatric comorbidity, we entered comparison subjects, patients with primary chronic fatigue syndrome, and patients with chronic fatigue syndrome and comorbid psychiatric diagnoses into an ANOVA. The group effect was significant (F=11.6, df=2, 182, p<0.0005); post hoc testing revealed that both the patients with primary chronic fatigue syndrome and those who had chronic fatigue syndrome and comorbid diagnoses had lower urinary free cortisol values than comparison subjects (Table 1). Similarly, examination of the effect of previous psychiatric history showed a significant group difference (F=11.0, df=2, 180, p<0.0005); lower urinary free cortisol levels were found in both the patients with and the patients without previous psychiatric disorder (Table 1).
We used questionnaire data to examine the effect of sleep disturbance and disability. Forty subjects reported substantial insomnia on the General Health Questionnaire item 2. Their urinary free cortisol (mean=70.1 nmol/day, SD=36.1) was no different from that of the 73 subjects without insomnia (mean=64.2 nmol/day, SD=39.0) (t=–0.78, df=111, p=0.44). After we calculated quartiles of disability, we found that mean urinary free cortisol was 72.1 nmol/day (SD=46.1) in the least disabled quartile (score=22 or less out of a possible total of 40) and 65.0 nmol/day (SD=35.7) in the rest, a nonsignificant difference (t=–0.87, df=111, p=0.38). Similarly, urinary free cortisol was 64.9 nmol/day (SD=30.3) in the most disabled quartile (score=34 or higher out of a possible total of 40) and 67.5 nmol/day (SD=40.2) in the rest (t=0.32, df=111, p=0.75), a nonsignificant difference. Finally, there were no significant correlations between urinary free cortisol and fatigue, General Health Questionnaire score, Work and Social Adjustment Scale score, age, weight, or illness duration (Table 1).
We repeated the above analyses including only patients with primary chronic fatigue syndrome who had been medication free for 2 months or more (N=62). Comparisons of the most disabled quartile against the rest (mean=73.4 nmol/day, SD=28.3, versus mean=74.7 nmol/day, SD=47.2) (t=0.11, df=60, p=0.91) and the least disabled against the rest (mean=74.8 nmol/day, SD=56.2, versus mean=74.2 nmol/day, SD=37.2) (t=–0.05, df=60, p=0.96) revealed no significant differences. Urinary free cortisol in the subjects with insomnia (mean=77.2 nmol/day, SD=40.5) was not significantly different from that of the subjects without insomnia (mean=71.6 nmol/day, SD=43.9) (t=–0.47, df=60, p=0.64). There were no significant correlations between urinary free cortisol and the other variables.
Discussion
Patients with chronic fatigue syndrome, whether suffering from comorbid psychiatric illness or not, had lower levels of urinary free cortisol than healthy comparison subjects. This study assessed basal adrenocortical function in the largest group of chronic fatigue syndrome patients to date and adds to the previous findings of lower urinary free cortisol in this disorder. Only one study, using 22 chronic fatigue syndrome subjects, has failed to find lower levels of urinary free cortisol (1).
A possible limitation of our study is the use of a tertiary care population, with inherent referral biases. These results may not apply to patients in primary or secondary care, although one study of patients with chronic fatigue syndrome in the community did find mild hypocortisolism (1). In addition, although a widely used measure, urinary free cortisol represents only a proportion of total cortisol output; shifts in cortisol metabolic pathways could theoretically cause reduced urinary free cortisol, although no such shifts have been found to date in chronic fatigue syndrome.
The origin of the reduced adrenocortical function remains unclear. This study was cross-sectional, so we cannot say whether it is a cause or effect of chronic fatigue syndrome. We did not find self-reported insomnia or disability to be associated with differential effects on urinary free cortisol. Nevertheless, in an acute setting, sleep or circadian rhythm disruption (7) can cause HPA axis changes similar to those seen in chronic fatigue syndrome. Prospective cohort studies are needed to explore whether such factors are important at the onset of chronic fatigue syndrome. Another factor that may have long-lasting effects on the HPA axis is previous trauma or abuse (8). Given the evidence linking such trauma to both physical and psychiatric symptoms, future studies might investigate if this underlies the HPA axis disturbance in chronic fatigue syndrome.
From whatever cause, low circulating cortisol is associated with fatigue (e.g., in Addison’s disease); furthermore, raising cortisol levels can reduce fatigue in chronic fatigue syndrome (9). Thus, this study provides further evidence that adrenocortical dysfunction in chronic fatigue syndrome, whatever the etiology and whether primary or secondary, may be one piece of the multifactorial jigsaw underlying the production of symptoms in chronic fatigue syndrome.
 |
Received April 7, 2000; revisions received Aug. 15 and Sept. 20, 2000; accepted Oct. 5, 2000. From the Department of Psychological Medicine, Guy’s King’s and St. Thomas’ School of Medicine and the Institute of Psychiatry; and the Department of Clinical Biochemistry, King’s College Hospital, London. Address reprint requests to Dr. Cleare, Department of Psychological Medicine, Guy’s King’s and St. Thomas’ School of Medicine and the Institute of Psychiatry, 103 Denmark Hill, London SE5 8AZ, UK; [email protected] (e-mail). Supported in part by the Linbury Trust. The authors thank members of the Chronic Fatigue Syndrome Research and Treatment Unit as well as Roy Sherwood and Tim Peters, King’s College Hospital, London, for help in running this study and comments on the manuscript.
1. Parker A, Wessely S, Cleare AJ: The neuroendocrinology of chronic fatigue syndrome. Psychol Med (in press)Google Scholar
2. Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A (International Chronic Fatigue Syndrome Study Group): The chronic fatigue syndrome: a comprehensive approach to its definition and study. Ann Intern Med 1994; 121:953–959Crossref, Medline, Google Scholar
3. Sharpe M, Chalder T, Palmer I, Wessely S: Chronic fatigue syndrome: a practical guide to assessment and management. Gen Hosp Psychiatry 1997; 19:185–199Crossref, Medline, Google Scholar
4. Goldberg D: The Detection of Psychiatric Illness by Questionnaire. London, Oxford University Press, 1972Google Scholar
5. Marks I: Behavioural Psychotherapy: Maudsley Pocket Book of Clinical Management. Bristol, UK, Wright, 1986Google Scholar
6. Chalder T, Berelowitz G, Pawlikowska T, Watts L, Wessely S, Wright D, Wallace EP: Development of a fatigue scale. J Psychosom Res 1993; 37:147–153Crossref, Medline, Google Scholar
7. Leese G, Chattington P, Fraser W, Vora J, Edwards R, Williams G: Short-term night-shift working mimics the pituitary-adrenocortical dysfunction of chronic fatigue syndrome. J Clin Endocrinol Metab 1996; 81:1867–1870Google Scholar
8. De Bellis M, Chrousos G, Dorn L, Burke L, Helmers K, Kling M, Trickett P, Putnam F: Hypothalamic-pituitary-adrenal axis dysregulation in sexually abused girls. J Clin Endocrinol Metab 1994; 78:249–255Medline, Google Scholar
9. Cleare AJ, Heap E, Malhi GS, Wessely S, O’Keane V, Miell J: Low-dose hydrocortisone in chronic fatigue syndrome: a randomised crossover trial. Lancet 1999; 353:455–458Crossref, Medline, Google Scholar



