Elevated D8/17 Expression on B Lymphocytes, a Marker of Rheumatic Fever, Measured With Flow Cytometry in Tic Disorder Patients
Abstract
OBJECTIVE: Elevated D8/17 expression on B lymphocytes is a known susceptibility marker of rheumatic fever. Previous studies have reported higher than usual D8/17 expression on B lymphocytes of patients with tic disorders. The purpose of this study was to assess D8/17 expression on B lymphocytes of tic disorder patients by using an objective method in which no operator variability was involved. METHOD: D8/17 expression on B lymphocytes was assessed with flow cytometry by using an immunoglobulin M (IgM) monoclonal D8/17-specific antibody in an unselected group of Dutch patients with tic disorders (N=33) and healthy volunteers (N=20). Binding of this monoclonal antibody was compared with binding of an irrelevant IgM monoclonal antibody, and the shift in mean fluorescence intensity of the D8/17-specific antibody compared to that of the irrelevant IgM monoclonal antibody was used as a measure of D8/17 overexpression. For the patients, Yale Global Tic Severity Scale scores were used to assess disease severity. RESULTS: D8/17 overexpression in the patient group (mean=16.8 arbitrary units, SD=30.5) was significantly higher than in the comparison group (mean=3.2, SD=3.0). A significant minority of the patients (N=13, 39.4%), however, had levels of D8/17 overexpression within the range of that of the healthy comparison subjects. Flow cytometric analysis did not indicate a separate subpopulation of D8/17-positive B cells. CONCLUSIONS: These data confirm the utility of D8/17 B cell overexpression as a peripheral blood marker in patients with tic disorders and are compatible with a streptococcus-related pathogenesis for at least a subgroup of patients with tic disorders.
The pathogenesis of childhood-onset tic disorders is not well understood. In addition to genetic factors, autoimmunity may be involved in these disorders (1). Sydenham’s chorea has been postulated as a medical model for tic disorders (2). A possible indicator of autoimmunity is the greater than usual binding of a D8/17-specific monoclonal antibody to B lymphocytes (3, 4). D8/17-specific monoclonal antibody is a mouse immunoglobulin M (IgM) monoclonal antibody originally prepared from fusions of spleen cells from mice that had been repeatedly immunized with isolated human B cells obtained from patients with rheumatic fever or rheumatic heart disease (5). D8/17-specific monoclonal antibody has been reported to bind to a small percentage of B lymphocytes in normal comparison subjects (averaging 5%–7%), but in patients with rheumatic fever, the percentage of D8/17-positive B lymphocytes was found to be much higher (mean=33.5%) (5). It has been proposed that an individual could be classified as “D8/17-positive” when his D8/17 B cell expression exceeded the mean plus one standard deviation of that of the healthy comparison subjects (that is, 12%). The D8/17-positive status has been suggested as a susceptibility marker for rheumatic fever, a well-known complication of infections with group A beta-hemolytic streptococci; 60%–100% of the subjects with rheumatic fever studied so far have been reported to be D8/17-positive (6–11).
Two research centers reported elevated D8/17 expression on B cells in patients with tic disorders. A National Institute of Mental Health group (4) investigated 27 children who fulfilled the criteria for pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS), nine children with Sydenham’s chorea, and 24 healthy comparison subjects. The children with PANDAS represent a separate subgroup of patients, from the spectrum of illness encompassing Tourette’s syndrome and obsessive-compulsive disorder (OCD), whose tics and obsessive-compulsive symptoms are shown to arise in response to beta-hemolytic streptococcal infections. They found that 85% of the children with PANDAS, 89% of the children with Sydenham’s chorea, and only 17% of the comparison subjects were D8/17-positive (4). Murphy et al. (3) compared 31 patients with childhood onset OCD, Tourette’s syndrome, or chronic tic disorders with 21 healthy comparison subjects and found that all patients and only one comparison subject were D8/17-positive. They suggested that D8/17-positivity may serve as a susceptibility marker for the development of tics or obsessive-compulsive symptoms.
In both studies, D8/17 expression on B cells was assessed with indirect immunofluorescence, after which D8/17-positive cells were scored by means of fluorescent microscopy. In the study of Murphy et al. (3), the investigators were not always completely blind to the subject’s disease status. Given the subjective nature of the use of indirect immunofluorescence, we intended to improve the methodology of assessing D8/17 B cell expression by applying a method in which no operator variability was involved and controlling for nonspecific binding.
We report here on D8/17 expression on B lymphocytes in an unselected group of Dutch patients with tic disorders and in a group of healthy volunteers by means of flow cytometry. In addition, we correlated D8/17 expression with disease severity as measured by the Yale Global Tic Severity Scale (12).
Method
Subjects
Two groups of subjects were chosen for the study: healthy volunteers and patients with a definite tic disorder per the Tourette’s Syndrome Research Criteria (13). According to these criteria, observable tics must be present during the clinical interview to allow for study entry. The aim and procedure of the study were fully explained to the subjects before written consent was requested. If the subjects were under 18, the parents were informed as well, and the written informed consent of the parents and the subject’s assent were obtained.
None of the comparison subjects or their families was reported to show symptoms of a tic disorder or any other neuropsychiatric disorder. Another exclusion criterion for the comparison group was any known history or family history of an autoimmune disorder, including a history of rheumatic fever. Twenty healthy volunteers entered the study (13 male, seven female), ranging in age from 8 to 32 years (mean=13.9, SD=5.0).
The patients were recruited from the outpatient clinic of the Child and Adolescent Psychiatry Center or from members of the Tourette’s Syndrome Patients Association of the Netherlands. None of the patients reported a history of autoimmune disorder, including a history of rheumatic fever. Thirty-three patients entered the study (19 men, 14 women), ranging in age from 6 to 59 years (mean=20.6, SD=14.5). The presence of psychiatric disorders other than autism was not an exclusion criterion. Psychiatric screening was performed by two of the authors, an experienced physician in the field of tic disorders (P.J.H.) and a board-certified child psychiatrist (P.W.T.). Blood was sampled from the subjects within a week of the psychiatric screening.
To assess tic severity, we used the Yale Global Tic Severity Scale (12), which consists of separate scales for motor and vocal tics complemented with a separate rating of impairment. The mean total Yale Global Tic Severity Scale score for the patients was 37.81 (SD=24.33, range=5–93), with a mean motor score of 13.44 (SD=5.09, range=5–24), a mean vocal score of 6.88 (SD=6.41, range=0–22), and a mean impairment score of 17.5 (SD=16.1, range=0–50). Ten of the patients were taking psychotropic medication, either clonidine (N=3) or various antipsychotic agents (N=7); 23 were free of medication.
Immunology
Both groups were assessed for D8/17 B cell expression by an examiner (J.B.) who was blind to subject status. Blood was collected in acid citrate dextran tubes (acid citrate dextran solution B tubes, Terumo Europe, Leuven, Belgium), and the blood-staining procedure was carried out on fresh cells. Flow cytometric analysis was performed within 24 hours.
Staining was performed by adding 30 μl of an irrelevant IgM monoclonal antibody, MOC32, directed against neuroendocrine antigens of epithelial origin of small cell lung cancer cells (tube A) or 30 μl of the D8/17-specific monoclonal antibody (tube B), to 100 μl of whole blood. Both the D8/17-specific monoclonal antibody and MOC32 were available in a concentration of 150 μg/ml and were used in an undiluted form. After incubation for 1 hour at 4°C, the suspension was washed with 2 ml of phosphate-buffered saline with 0.5% bovine serum albumin (Sigma Aldrich, Zwijndrecht, the Netherlands) and centrifuged at 2,500 rpm for 2 minutes. To both pellets, 5 μl of phycoerythrin-conjugated CD19 (IQP, Groningen, the Netherlands) as well as 5 μl of fluorescein isothiocyanate-conjugated goat antimouse IgM (SBA, Birmingham, Ala.) was added for half an hour at room temperature. Phycoerythrin-conjugated CD19 was used as a marker of the total B cell subpopulation, whereas the fluorescein isothiocyanate conjugated goat antimouse IgM was able to detect binding of the D8/17-specific monoclonal antibody and of the irrelevant monoclonal antibody, respectively. After incubation, the cells were lysed with 3 ml of fluorescence-activated cell sorter lysing solution (BD, Leiden, the Netherlands) for 10 minutes, centrifuged, and washed. The pellets were resuspended in 100 μl of phosphate-buffered saline with 0.5% bovine serum albumin and stored at 4°C until measured on a FACSstar (Becton Dickinson, Woerden, the Netherlands).
Measuring was performed by placing a gate around the CD19-positive B cells and counting 2,000 cells. As shown in Figure 1, intersecting perpendicular lines were placed to define the R2 quadrant with the comparison IgM monoclonal antibody at 1% (top row) in order to examine D8/17-positive B cells (bottom row). Overexpression of D8/17 B cells was calculated by subtracting the mean fluorescence intensity of the comparison monoclonal antibody from the mean fluorescence intensity of the D8/17-specific monoclonal antibody. Negative values of this shift in mean fluorescence intensity were set at 0. Individuals were classified as D8/17-negative (<95th percentile of the D8/17 B cell overexpression of the comparison subjects) or D8/17-positive (≥95th percentile of the D8/17 B cell overexpression of the comparison subjects).
Data Analysis
We used the Mann-Whitney U test to test differences between the D8/17 B cell overexpression of patients and of comparison subjects. To rule out medication effects, we also tested the differences between D8/17 B cell overexpression of the patients who were not taking medication and of healthy comparison subjects by means of the Mann-Whitney U test. Spearman’s rank correlation test was used to assess the relationship between D8/17 overexpression and the total as well as motor and phonic scores of the Yale Global Tic Severity Scale (12).
To assess the relationship between D8/17 B cell overexpression and patient age, we used Spearman’s rank correlation test. To investigate possible differences between D8/17 B cell overexpression and sex in patients, we used the Mann-Whitney U test. All tests of significance used the 0.05 level of significance and were two-tailed.
Results
We assessed D8/17 overexpression on B cells by means of flow cytometry in subjects with tic disorders and a healthy comparison group. Figure 1 (lower right corner) shows a typical flow cytometric picture of a D8/17-positive patient with a relatively large overexpression of D8/17 compared to the binding of the irrelevant monoclonal antibody. Also, the results obtained with a comparison subject are shown, demonstrating a slight overexpression of D8/17. Figure 1 shows that the population of B cells stained with D8/17-specific monoclonal antibody is fairly homogeneous: no separate subpopulation of D8/17-positive cells can be distinguished, despite the marked shift in mean fluorescence intensity of the D8/17 B cell expression compared to the expression of the comparison monoclonal antibody.
Median D8/17 B cell overexpression was significantly (Mann-Whitney U=121.0, df=51, p<0.0001) higher in the patient group (mean=16.8 arbitrary units, SD=30.5, median=11.6, range=0–179.6) than in the comparison group (mean=3.2, SD=3.0, median=2.8, range=0–10.1). For the total group, the median shift in mean fluorescence intensity was 6.3 (arbitrary units) (mean=11.8, SD=24.9, range=0–179.6). Figure 2 shows D8/17 overexpression on B cells in the total group, in tic disorder patients, and in comparison subjects. A comparison of the patient group that was not taking medication (mean=10.6, SD=7.0, median=10.6) with the healthy comparison subjects also revealed significant differences in D8/17 B cell overexpression (Mann-Whitney U=97.5, df=41, p=0.001).
When the cutoff point for an individual’s D8/17-positivity was set at 10.0 (at the 95th percentile of the D8/17 B cell overexpression of the comparison subjects), one (5%) of the comparison subjects was D8/17-positive, whereas 20 (60.6%) of the patients were D8/17-positive.
Furthermore, we tested whether the level of D8/17 overexpression correlated with the severity of the tic disorder. There was no statistically significant positive correlation between the level of D8/17 expression and the severity of motor tics as measured by the motor (r=0.24, df=31, p=0.18), phonic (r=–0.20, df=31, p=0.25), or total (r=0.16, df=31, p=0.37) score on the Yale Global Tic Severity Scale. There was no correlation between D8/17 expression and age. The mean D8/17 expression of the male patients did not differ from that of the female patients (Mann-Whitney U=117.0, df=31, p=0.58).
Discussion
As a group, the patients with a tic disorder showed significantly higher D8/17 overexpression on B lymphocytes than did the healthy volunteers, thus confirming the findings of earlier reports. However, no support could be found for the distinction of a separate subpopulation of D8/17-positive B cells. Given this finding, it is unclear how D8/17-positive B cells could be distinguished and counted manually by means of indirect immunofluorescence, as described in the reports by Murphy et al. (3) and Swedo et al. (4).
Apparently, contrary to earlier concepts (e.g., reference 5), D8/17-positive patients do not show an elevated number of D8/17-positive B cells but, rather, show elevated D8/17 expression on the B cell population as a whole, which can accurately be measured by calculation of the shift in mean fluorescence intensity of the D8/17-specific antibody compared to that of the irrelevant IgM monoclonal antibody.
A significant minority of patients (N=13, 39.4%), however, had levels of D8/17 overexpression that fell within the range of that of the healthy comparison subjects. In this respect, our results differ from those of Murphy et al. (3), who reported, using a different method, that all of their 31 patients had elevated levels of D8/17 expression. However, a recent study in an Afrikaner population in South Africa (14) also reported an overlap in D8/17 expression between healthy comparison subjects and OCD patients. Ten out of 11 patients with OCD and 14 out of 22 comparison subjects were reported to be D8/17-positive (defined as having 12% or more D8/17-positive B lymphocytes) in that study as determined by immunofluorescence microscopy. If the cutoff point for D8/17-positivity in that study had been defined as the 95th percentile for the comparison subjects, the results would have been virtually identical to our data.
The present study is, to our knowledge, the first using flow cytometry with a comparison IgM monoclonal antibody for assessing D8/17 expression on B lymphocytes. Flow cytometry is an objective method in which no operator variability is involved that allows for analysis of many more cells than is possible by counting D8/17-positive cells by using a fluorescent microscope. Previously, Chapman and co-workers (15) also described a flow cytometric assay for assessing D8/17 B cell expression in patients with Tourette’s syndrome or OCD, but their method clearly differed from ours, since they did not control binding of the D8/17-specific monoclonal antibody for nonspecific binding by using an irrelevant monoclonal antibody. Therefore, they measured a percentage of D8/17-positive B cells instead of using a shift in mean fluorescence intensity of the D8/17-specific antibody compared to that of the irrelevant IgM monoclonal antibody as a measure of D8/17 overexpression. Chapman and co-workers compared the results of their flow cytometric assay with the results of cell counting using immunofluorescence microscopy and found a significant but not perfect correlation (r=0.82). In their study, D8/17 B cell expression of patients and comparison subjects also clearly overlapped, irrespective of the method of assessment. Those data are in accordance with our data, but they clearly differ from the earlier reports of Swedo et al. (4) and Murphy et al. (3).
The characterization of the antigen recognized by the D8/17-specific monoclonal antibody as well as the pathogenetic meaning of D8/17 B lymphocyte overexpression awaits further study. The D8/17-specific monoclonal antibody has not only been found to bind to B cell surface structures but also to diverse tissue sites in humans such as the myocardium, smooth muscle, skeletal muscle, and epithelium cells. The monoclonal antibody appears to bind to the cytoskeletal helical coil/coiled structures myosin and tropomyosin. It is of interest that the D8/17-specific monoclonal antibody was also found to bind to streptococcal M proteins (16). Because of such cross reactivities, structural similarities may exist between the cytoskeletal proteins myosin and tropomyosin, surface antigens present on a subset of B cells, and streptococcal M proteins. It is unclear how these findings relate to the model of molecular mimicry that is thought to lead to the symptom complex of rheumatic fever. However, elevated D8/17 expression may point to an individual’s susceptibility to experience autoimmune complications in the aftermath of streptococcal infections. Apparently, some individuals with elevated D8/17 expression develop rheumatic fever, whereas others are prone to the emergence of tic disorders. On the other hand, there are considerable numbers of patients with tic disorders who show D8/17 expression in the normal range. It is not known whether D8/17-positive patients with Tourette’s syndrome represent a distinct subgroup within the spectrum of tic disorders in which autoimmunity may be involved or whether alternative disease mechanisms may be involved in D8/17-negative patients with tic disorders. Carefully comparing D8/17-positive with D8/17-negative patients, both clinically and serologically as well as with regard to treatment response, should identify possible differences between these patients.
The specificity of D8/17-positivity for child neuropsychiatric disorders remains to be elucidated, since elevated D8/17 B cell binding in autism has also been reported (17). In this group, a positive correlation was found between the percentage of D8/17-positive B cells and repetitive behavior, the hallmark of tic disorders. We found no correlation between D8/17 B cell expression and tic disorder severity as measured by the Yale Global Tic Severity Scale. In contrast to those of autism, the symptoms of tic disorders are liable to fluctuate, which can possibly obscure this correlation in patients with tic disorders, since it is not known whether percentages of D8/17 expression reflect such fluctuations.
Furthermore, the ethnic background of the patient group might be a relevant factor. For example, 90%–100% of the rheumatic fever patients in the United States (of unspecified ethnic origin) were found to have elevated D8/17 B cell expression, whereas it was found in only 66% of the rheumatic fever subjects in India (18). Specific regional susceptibility markers for the latter population have been found (19). It is therefore of interest to investigate whether different susceptibility markers also exist in tic disorder patients across different regions. To our knowledge, the present study is the first report on D8/17 B cell overexpression in Western Europe. Clearly, more data are needed concerning D8/17 B cell expression in healthy comparison subjects across different population groups with the use of objective measures.
Much work remains to be done in order to elucidate the role of autoimmunity in tic disorders, especially with regard to the role of previously reported autoantibodies (20–22). To further investigate the usefulness of the proposed concept of PANDAS, studies comparing Tourette’s syndrome subjects who fulfill the criteria for PANDAS with non-PANDAS subjects must be performed.
To conclude, the present finding of elevated D8/17 B cell overexpression by an objective method in a psychiatric population is highly intriguing. Elevated D8/17 B cell expression might not only serve as an objective blood marker for at least a subgroup of the tic disorder spectrum, but moreover, it points toward a streptococcus-related pathogenesis with potentially promising implications for further fruitful research, possibly leading to more effective future interventions. Apart from providing insight into possible pathophysiology, a blood marker may stimulate research in other fields. For example, if elevated D8/17 B cell expression is a reliable and stable marker in a subgroup of patients, it could facilitate the composition of homogeneous subgroups for genetic studies.
Presented at the 11th International Congress of the European Society of Child and Adolescent Psychiatry, Hamburg, Germany, Sept. 15–19, 1999. Received April 19, 2000; revision received Sept. 29, 2000; accepted Nov. 28, 2000. From the Child and Adolescent Psychiatry Center, Departments of Clinical Immunology and Biological Psychiatry, University Hospital. Address reprint requests to Dr. Hoekstra, Child and Adolescent Psychiatry Center, Hanzeplein 1, 9713 GZ Groningen, the Netherlands; [email protected] (e-mail). The authors thank Dr. Zabriskie (Rockefeller, N.Y.) for the D8/17-specific monoclonal antibody and Mrs. Chapman (Rockefeller, N.Y.) for advice on the staining procedure.
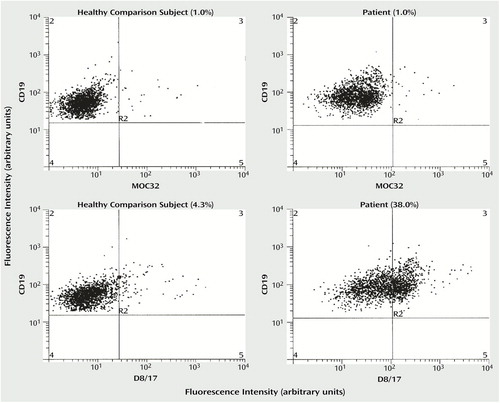
Figure 1. Flow Cytometric Analysis of D8/17 Expression on B Lymphocytes in a Healthy Comparison Subject and in a Tic Disorder Patient With Higher Than Normal D8/17 Expressiona
aEach dot represents one cell. Both axes refer to fluorescence intensity reflecting the magnitude of the respective antibody binding (D8/17-specific monoclonal antibody, MOC32, or CD19) of each cell. The top two panels show expression on the B cells of an irrelevant monoclonal antibody (MOC32). The two bottom panels show expression of D8/17-specific monoclonal antibody, showing that the cell population is fairly homogeneous. Therefore, measurement of cells gated to the R2 quadrant is not entirely precise, indicating that measurement of shift in mean fluorescence intensity is a more accurate measure of D8/17 B cell overexpression. The area of specific binding is defined as the area in which less than 1% of the B cells bind to the irrelevant monoclonal antibody MOC32.
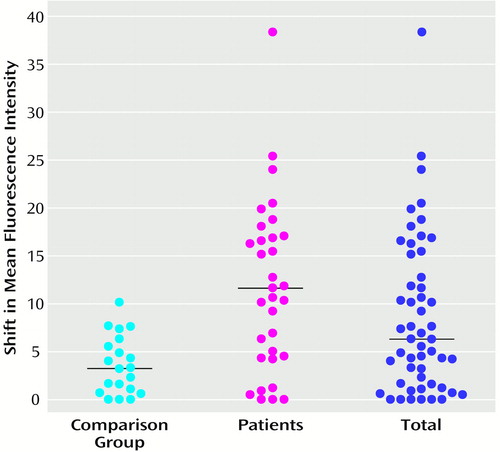
Figure 2. Levels of Overexpression of D8/17 Antigen on B Lymphocytes in 20 Healthy Comparison Subjects, 33 Tic Disorder Patients, and the Total Groupa
aShift in mean fluorescence intensity of the D8/17-specific antibody compared to that of the irrelevant immoglobulin M (IgM) monoclonal antibody. Horizontal lines represent median values.
1. Hallett JJ, Kiessling LS: Neuroimmunology of tics and other childhood hyperkinesias. Neurol Clin 1997; 15:333–344Crossref, Medline, Google Scholar
2. Swedo SE: Sydenham’s chorea: a model for childhood autoimmune neuropsychiatric disorders. JAMA 1994; 272:1788–1791Google Scholar
3. Murphy TK, Goodman WK, Fudge MW, Williams RC Jr, Ayoub EM, Dalal M, Lewis MH, Zabriskie JB: B lymphocyte antigen D8/17: a peripheral marker for childhood-onset obsessive-compulsive disorder and Tourette’s syndrome? Am J Psychiatry 1997; 154:402–407Google Scholar
4. Swedo SE, Leonard HL, Mittleman BB, Allen AJ, Rapoport JL, Dow SP, Kanter ME, Chapman F, Zabriskie J: Identification of children with PANDAS by a marker associated with rheumatic fever. Am J Psychiatry 1997; 154:110–112Link, Google Scholar
5. Khanna AK, Buskirk DR, Williams RC Jr, Gibofsky A, Crow MK, Menon A, Fotino M, Reid HM, Poon-King T, Rubinstein P: Presence of a non-HLA B cell antigen in rheumatic fever patients and their families as defined by a monoclonal antibody. J Clin Invest 1989; 83:1710–1716Google Scholar
6. Kilasoniia LO, Chumburidze VB, Tatishvili NI, Polianskaia IS, Tsitlanadze VG: [A new immunogenetic marker of rheumatism.] Klin Med (Mosk) 1989; 67:64–66 (Russian)Medline, Google Scholar
7. Shostak NA, Anokhin VN, Kazakova TV, Morozova EI, Zolkina IV, Voilokova RI: [Detection of B-cell marker of rheumatism using monoclonal antibodies D8/17 in families of patients with rheumatism, report 1]. Revmatologiia (Mosk), July-Sept 1990, pp 27–31 (Russian)Google Scholar
8. Taneja V, Mehra NK, Reddy KS, Narula J, Tandon R, Vaidya MC, Bhatia ML: HLA-DR/DQ antigens and reactivity to B cell alloantigen D8/17 in Indian patients with rheumatic heart disease. Circulation 1989; 80:335–340Crossref, Medline, Google Scholar
9. Shostak NA, Anokhin VN, Zolkina IV, Morozova EI, Kazakova TV: [Detection of B-cell marker of rheumatism using monoclonal antibodies D8/17 in the families of patients with rheumatism, report 2]. Revmatologiia (Mosk), Jan-March 1991, pp 24–26 (Russian)Google Scholar
10. Shostak NA: [The diagnostic significance of the surface B-cell marker carrier state in arthritis and other manifestations of rheumatic fever.] Ter Arkh 1991; 63:49–52 (Russian)Medline, Google Scholar
11. Rodriguez RS, Ontiveros P, Torres S, Khanna AK, Buskirk DR, Zabriskie JB: [Presence of a non-HLA antigen in B-lymphocytes from patients with rheumatic fever and their relatives defined using monoclonal antibodies.] Bol Med Hosp Infant Mex 1990; 47:313–317 (Spanish)Medline, Google Scholar
12. Leckman JF, Riddle MA, Hardin MT, Ort SI, Swartz KL, Stevenson J, Cohen J: The Yale Global Tic Severity Scale: initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry 1989; 28:566–573Crossref, Medline, Google Scholar
13. Kurlan R: Diagnostic criteria for genetic studies of Tourette syndrome. Arch Neurol 1997; 54:517–518Crossref, Medline, Google Scholar
14. Niehaus DJ, Knowles JA, van Kradenberg J, du Toit W, Kaminer D, Seedat S, Daniels W, Cotton M, Brink P, Beyers AD, Bouic P, Chapman F, Zabriskie JB, Stein DJ: D8/17 in obsessive-compulsive disorder and trichotillomania (letter). S Afr Med J 1999; 89:755–756Medline, Google Scholar
15. Chapman F, Visvanathan K, Carreno-Manjarrez R, Zabriskie JB: A flow cytometric assay for D8/17 B cell marker in patients with Tourette’s syndrome and obsessive compulsive disorder. J Immunol Methods 1998; 219:181–186Crossref, Medline, Google Scholar
16. Kemeny E, Husby G, Williams RC Jr, Zabriskie JB: Tissue distribution of antigen(s) defined by monoclonal antibody D8/17 reacting with B lymphocytes of patients with rheumatic heart disease. Clin Immunol Immunopathol 1994; 72:35–43Crossref, Medline, Google Scholar
17. Hollander E, DelGiudice-Asch G, Simon L, Schmeidler J, Cartwright C, DeCaria CM, Kwon J, Cunningham-Rundles C, Chapman F, Zabriskie JB: B lymphocyte antigen D8/17 and repetitive behaviors in autism. Am J Psychiatry 1999; 156:317–320Abstract, Google Scholar
18. Ganguly NK, Anand IS, Koicha M, Jindal S, Wahi PL: Frequency of D8/17 B lymphocyte alloantigen in North Indian patients with rheumatic heart disease. Immunol Cell Biol 1992; 70(part 1):9–14Google Scholar
19. Kaur S, Kumar D, Grover A, Khanduja KL, Kaplan EL, Gray ED, Ganguly NK: Ethnic differences in expression of susceptibility marker(s) in rheumatic fever/rheumatic heart disease patients. Int J Cardiol 1998; 64:9–14Crossref, Medline, Google Scholar
20. Kiessling LS, Marcotte AC, Culpepper L: Antineuronal antibodies: tics and obsessive-compulsive symptoms. J Dev Behav Pediatr 1994; 15:421–425Crossref, Medline, Google Scholar
21. Singer HS, Giuliano JD, Hansen BH, Hallett JJ, Laurino JP, Benson M, Kiessling LS: Antibodies against human putamen in children with Tourette syndrome. Neurology 1998; 50:1618–1624Google Scholar
22. Laurino JP, Hallett J, Kiessling LS, Benson M, Pelletier T, Kuhn C: An immunoassay for anti-neuronal antibodies associated with involuntary repetitive movement disorders. Ann Clin Lab Sci 1997; 27:230–235Medline, Google Scholar



