Impact of Tic Disorders on ADHD Outcome Across the Life Cycle: Findings From a Large Group of Adults With and Without ADHD
Abstract
OBJECTIVE: The impact of tic disorders on the outcome of attention deficit hyperactivity disorder (ADHD) remains a subject of high scientific and clinical interest. To evaluate the impact of comorbid ADHD and tic disorders from a lifespan perspective, the authors systematically examined data from adults with and without ADHD. METHOD: They comprehensively evaluated 312 consecutively referred adults with ADHD and 252 comparison subjects without ADHD. Tic disorders were characterized along with a wide range of neuropsychiatric correlates, including other comorbid disorders as well as indexes of function in the domains of school, cognition, and interpersonal functioning. RESULTS: A significantly greater proportion of adults with ADHD (12%) than those without ADHD (4%) had tic disorders. Tic disorders followed a mostly remitting course and had little impact on functional capacities. In addition, tic disorders were not associated with stimulant use. CONCLUSIONS: These findings in adults with ADHD confirm and extend previous findings in young subjects with ADHD, documenting that although individuals with ADHD are at greater risk for tic disorders, the presence of tic disorders has a limited impact on ADHD outcome.
Despite recurring concerns regarding the impact of tic disorders on the course, outcome, and treatment of ADHD, little empirical information on the subject exists. Recent results from our prospective evaluation of the course of tic disorders in young subjects with ADHD (1) showed that tic disorders follow a remitting course and have limited impact on the outcome of ADHD. Our work also documented that the treatment of ADHD with stimulant drugs had a limited impact on the course of tics and was not associated with de novo development of tics in children with ADHD. These findings are reassuring, but the average age of the subjects in this study was only 15 years; therefore, more information is needed regarding the long-term impact of tic disorders in individuals with ADHD and regarding the potential deleterious impact of treatment for ADHD on the development of tics.
One approach for further evaluation of this gap in knowledge is to use data from adults with ADHD. This is possible because emerging data document that adult ADHD shares with its pediatric counterpart a very similar pattern of functional deficits, psychiatric comorbidity, familiality and genetic underpinnings, neuropsychological deficits, treatment responsivity, and neuroimaging findings. These data indicate diagnostic continuity between the pediatric and the adult phenotype of ADHD (2). For example, adult ADHD has been associated with executive function deficits, school failure, and lower occupational attainment (3); the DRD4 gene (dopamine 4 receptor 7-repeat allele) (4); a high rate (55%) of familiality (5); the same pattern of comorbidity (disruptive behavior, mood, anxiety, and learning disorders) (6, 7); functional impairments in prefrontal and anterior cingulate brain regions (8, 9); and a similar response to stimulant drugs and noradrenergic antidepressants (10–15).
Whether the comorbidity of ADHD and tic disorders has a deleterious impact on ADHD outcome across the life cycle and whether treatment for ADHD has a deleterious impact on tic development have important clinical implications. A finding that tic disorders adversely affect ADHD outcome would underscore the clinical importance of detecting and aggressively treating tic disorders in individuals with ADHD. Similarly, the risk versus benefit analysis of the safety of stimulant treatment in individuals with ADHD with comorbid tics hinges on the unresolved questions of whether stimulant treatment precipitates tic disorders and whether stimulant treatment worsens preexisting tics across the life cycle.
The purpose of this study was to use retrospective data from a large group of well-characterized adults with ADHD to systematically reevaluate the impact of the comorbidity between ADHD and tic disorders on the course and outcome of ADHD in adulthood. On the basis of our previous study (1) and the literature, we hypothesized that 1) tic disorders are overrepresented in adults with ADHD, 2) tic disorders follow a remitting course independent of the course of ADHD, 3) tic disorders have a limited impact on ADHD outcome, and 4) stimulant treatment does not adversely impact the outcome of tic disorders.
Method
Subjects
Subjects were 312 consecutively referred adults between the ages of 19 and 59 who met full DSM-III-R diagnostic criteria for ADHD (lifetime) after clinical assessment that was confirmed with structured diagnostic interview. Each patient had a diagnosis of childhood-onset ADHD that persisted as a full syndrome (eight or more of 14 ADHD symptoms) (87%, N=272) or partial syndrome (five or more of 14 ADHD symptoms) (13%, N=40) in adulthood. For all subjects, ADHD symptoms were clearly associated with both lifetime and current impairment. The comparison subjects were 252 healthy adults participating in ongoing studies at our center who did not have ADHD.
We excluded subjects only if they had major sensorimotor handicaps (paralysis, deafness, blindness), active psychosis, or a full-scale IQ of less than 80. We received approval from the institutional review board to review, analyze, and report on data on referred adults with ADHD treated at our center. After complete description of the study to the comparison subjects, written informed consent was obtained from the comparison subjects.
Diagnostic and Assessment Procedures
The procedures used to evaluate the referred adults with ADHD were identical to those used in our clinical research program and those used extensively in studies of boys and girls as well as adults with ADHD (16). Briefly, we administered the Structured Clinical Interview for DSM-III-R (17) supplemented with modules from the Schedule for Affective Disorders and Schizophrenia for School-Age Children—Epidemiologic Version (K-SADS-E) (18) covering childhood diagnoses. Rates of disorders are lifetime prevalences. The interviewers of referred adults were blind to the subjects’ clinical diagnoses but not to their referral status. For every diagnosis, information was gathered regarding the associated level of impairment (1=mild, 2=moderate, 3=severe), age at onset of symptoms, age at offset of symptoms, number of episodes, and treatment history.
We computed kappa coefficients of agreement by having three experienced, board-certified child and adult psychiatrists diagnose subjects on the basis of audiotaped interviews made by the assessment staff. The median kappa for 173 interviews was 0.86. For the diagnoses of tics and Tourette’s syndrome, perfect reliability was established both between trained raters and between raters and senior clinicians (kappa=1.0); the kappa for ADHD was 0.98. A committee of board-certified child and adult psychiatrists chaired by the program director (J.B.) resolved all diagnostic uncertainties.
To be given a diagnosis of adult ADHD, the subject must have 1) met DSM-III-R criteria for a diagnosis of ADHD by the age of 7, 2) had at least five DSM-III-R symptoms of ADHD at the time of assessment, and 3) described a chronic course of ADHD symptoms from childhood to adulthood. To elicit ADHD symptoms, we used the ADHD module from the K-SADS-E, wording questions in the past tense. If the subject endorsed a symptom to a clinically meaningful degree, we asked whether similar problems were currently present. By using this method we assured that the syndrome observed in adulthood had some continuity with the syndrome reported in childhood.
To be given a diagnosis of Tourette’s syndrome, the subject must have fulfilled all five DSM-III-R criteria: A) both multiple motor tics and one or more vocal tics, B) the tics occur many times a day nearly every day or intermittently throughout a period of more than 1 year, C) the anatomic location, number, frequency, complexity, and severity of the tics change over time, D) onset before age 21, and E) occurrence not due to psychoactive substance intoxication or central nervous system illness (DSM-III-R, p. 80). Non-Tourette’s-syndrome tic disorders were defined as either motor or vocal tics but not both; these tic disorders had to fulfill DSM-II-R criteria B, D, and E. Tic disorders were defined as chronic if the duration was at least 1 year; otherwise, tic disorders were classified as transient.
We used the following interview questions from the tic module of the K-SADS-E: 1) Did you ever have any frequent, jerky, repetitive motor movements such as lip smacking or frequent eye blinking? Other repetitive movements of the face or body (not rhythmic or fidgeting)? What about touching or squatting? 2) Did you ever have vocal tics (grunts or yelps) or utter obscenities? 3) Did the tics (or grunts) occur many times a day (usually in bouts), nearly every day or intermittently throughout a period of more than 1 year? 4) Did the severity and location of the tics change over time? 5) Were you ill at the time the tics occurred? and 6) Were you abusing drugs or alcohol at the time the tics occurred?
As suggested by others (19, 20), we diagnosed major depression only if the depressive episode was associated with marked impairment. Since the anxiety disorders comprise many syndromes with a wide range of severity, we used two or more anxiety disorders to capture the presence of a clinically meaningful anxiety syndrome. We refer to this as “multiple anxiety disorders,” as we have elsewhere (21).
To examine cognitive function, we used a test battery analogous to that used in previous reports of children and adolescents with ADHD (22, 23). We estimated full-scale IQ from the vocabulary and block design subtests of the WAIS-R (24–26). We used the digit symbol, digit span, and arithmetic subtests of the WAIS-R to estimate the freedom from distractibility IQ. Academic achievement was assessed with the arithmetic and reading subtests of the Wide Range Achievement Test, Revised (27). We used the procedure recommended by Reynolds (28) and others to define learning disabilities. To evaluate school functioning, we assessed three straightforward indexes of school failure: placement in special classes, in-school tutoring, and repeated grades. Psychosocial functioning was assessed with the Global Assessment of Functioning Scale (1=worst, 90=best) (DSM-III-R, p. 12). Socioeconomic status was measured with the four-factor Hollingshead scale (1=highest, 4=lowest) (29).
Data Analysis
Categorical data were analyzed by using chi-square tests. Parametric continuous data were analyzed by using one-way analysis of variance, and nonparametric data were analyzed by using the Kruskal-Wallis and the Wilcoxon rank sum test. Continuous and binary dependent variables were analyzed by using regular and logistic regressions when multiple independent variables were accounted for simultaneously. Since the study group consisted of subjects of different ages, we used survival analysis to examine offset of tics. Survival analysis effectively weights data by observational period to account for differing observational periods. The Kaplan-Meier method was used for survival analysis to estimate lifetime prevalence rates and to generate survival curves, which were compared with the Cox proportional hazards model. The software used for analyses was Stata 5 (30). To protect against type II errors, we set a low threshold for statistical significance of 0.01. All statistical tests were two-tailed.
Results
Significantly more of the 312 adults with ADHD (N=36, 12%) had a tic disorder (Tourette’s syndrome or non-Tourette’s-syndrome) than of the 252 adults without ADHD (N=9, 4%) (χ2=12.1, df=1, p<0.001). Since only seven of the 45 subjects with tic disorders met criteria for Tourette’s syndrome, we collapsed the groups with tics and Tourette’s syndrome into one group to gain statistical power. Thus, comparisons were made among three groups: 1) adults with ADHD who had a comorbid tic disorder (N=36), 2) adults with ADHD who did not have a comorbid tic disorder (N=276), and 3) adults without ADHD (N=252) (nine of the comparison subjects had a tic disorder).
As shown in Table 1, there were significant differences in age, social class, and gender distributions among the three groups. These differences were accounted for by the younger age and lower socioeconomic status of both ADHD groups (those with and without tics) compared with the group who did not have ADHD. Although the gender compositions of both ADHD groups and the comparison group were evenly balanced, most of the subjects with ADHD plus a tic disorder group were men.
Clinical Characteristics of ADHD
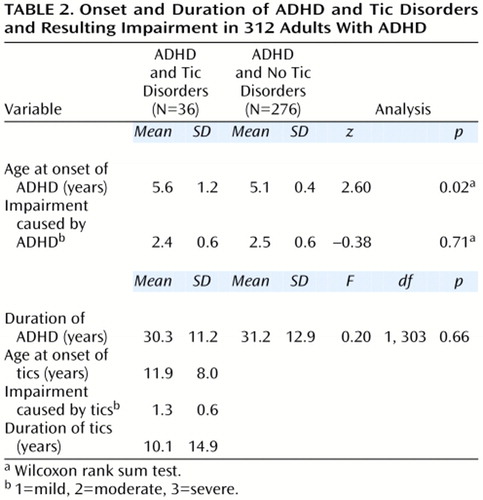
With the exception of a small difference in the age at onset of ADHD between subjects with and without comorbid tics (Table 2), features of ADHD were quite similar in comparisons between adults with ADHD who did or did not have tic disorders. On average, ADHD impairment was rated between moderate and severe (mean=2.4 on a scale on which 1=mild, 2=moderate, and 3=severe) in both groups and did not differ between the groups.
The onset of tic disorders was typically in childhood (Table 2); 26 (72%) were 12 years of age or younger). In addition, 33 (92%) of the adults with ADHD and comorbid tics satisfied criteria for chronic tics (defined as having a tic for a year or more). The mean duration of tics represented approximately 27% of the lifetime of these adults with ADHD. The average impairment attributed to tic symptoms was in the mild to moderate range.
Course of Tic Disorder and ADHD
In adults with both ADHD and tic disorders, the mean age at onset of tics was 6.3 years later than that of ADHD (t=–0.3, df=25, p<0.0001) (Figure 1). ADHD preceded the onset of tic disorder in all but one adult with ADHD and tics. A Cox proportional hazards model showed that the age at onset of ADHD was independent of that of tic disorder status (z=1.50, n.s.).
Figure 1 shows that tic disorders followed a largely remitting course; the age-adjusted rate of complete remission of the tic disorder was 62.2%; the unadjusted rate was 53% (N=19 of 36). Furthermore, a Cox proportional hazards model showed that the offset of tic symptoms was independent of any change in ADHD status from full to partial syndrome (z=–0.60, n.s.).
Comorbidity and Functional Outcome
We used logistic or linear regression to examine the independent contributions of ADHD and tic disorder status to a wide range of outcomes, including frequency of other comorbid disorders, indexes of school function (repeated grades, placement in special classes, in-school tutoring), cognitive function (IQ and achievement tests), frequency of learning disability (reading or mathematics), and measures of social function (Global Assessment of Functioning Scale score). As shown in Table 3 and Table 4, differences between adults with ADHD and the comparison group were accounted for by ADHD and not by tic disorders in all but two of the multiple outcome measures assessed; the exceptions were that tic disorders, but not ADHD, were associated with a higher rate of obsessive-compulsive disorder (OCD) and that both tic disorders and ADHD were associated with a higher rate of bipolar disorder. The rates of OCD in the adults with ADHD were almost identical in men (N=6, 3.3%) and women (N=4, 3.5%) (χ2=0.005, df=1, n.s.).
The analyses were repeated with corrections for potential sociodemographic confounders (age, socioeconomic status, and gender). Full-scale and vocabulary intelligence scores were no longer significantly associated with ADHD (p>0.10); otherwise, the results were unchanged.
Impact of Stimulant Treatment on Tic Disorders
Seventy-five (24.2%) of 310 of the adults with ADHD for whom such data were available were treated with medications, primarily stimulants, for an average of 4 years. The age-adjusted rates of tic disorders in probands with ADHD did not differ by exposure to stimulants (19.1% versus 22.3% for exposure and no exposure to stimulants, respectively) (z=0.43, p=0.67). Nine (12.0%) of the 75 adults with ADHD who were treated with stimulants had tic disorders, compared with 27 (11.5%) of the 235 who were not treated with stimulants.
Nine (25.0%) of the 36 adults with ADHD plus tic disorder were treated with stimulants; only two of these subjects were treated in early childhood at or before the onset of tics. One had stimulant treatment started and stopped before the onset of tics, and the other was treated with stimulants concurrently with the tics. The seven other subjects were treated with stimulants after their tic symptoms remitted and did not suffer a recurrence or worsening of their tic symptoms.
Discussion
In a large group of well-characterized adults with and without ADHD, we found a significant overrepresentation of tic disorders in those who had ADHD compared with those who did not have ADHD. Tic disorders had a mostly remitting course, and their presence did not adversely affect the course of ADHD. In addition, treatment with stimulants was not associated with greater risk for tics. These retrospective findings in adults match very well previously reported results from a prospective pediatric sample of young subjects with and without ADHD who did or did not have comorbid tic disorders. These results provide further support for the conclusion that tic disorders follow a remitting course in the context of ADHD and that their presence has limited impact on ADHD outcome.
Consistent with findings reported in the pediatric literature by us and others, tic disorders in this adult study group had an onset in middle childhood and were clearly chronic. Tic symptoms included frequent and multiple jerky, repetitive, nonrhythmic motor movements, with and without abnormal vocalizations, which varied in location. In addition, we found that OCD was specifically associated with tic disorders in our study group; this finding is also consistent with the pediatric literature.
Despite the balanced gender distribution of our adult ADHD subjects (59% were men and 41% were women), 81% of the adults with ADHD and tic disorders were men. This finding is consistent with the pediatric literature, which has documented an overrepresentation of tics in boys relative to girls (31). However, the rates of OCD in the adults with ADHD were almost identical in men and women.
The findings of a dimorphic distribution of tic disorders and OCD and of a specific relationship between OCD and tic disorders are consistent with the current hypothesis that tics and OCD reflect variable expressions of the same underlying risk factor and provide strong support for the typicality of tic disorders in adults with ADHD. Taken together, the striking homology in clinical characteristics and correlations of tic disorders in these adults with those reported in childhood indicates that the tic disorders in our group of adults were typical.
Our results documenting that comorbidity of ADHD and tic disorders had a limited impact on the morbidity and dysfunction of ADHD in these adults are highly consistent with results from our longitudinal study of children with ADHD who did or did not have tic disorders (32). That study clearly documented that, in sharp contrast to other comorbid disorders, the presence of a tic disorder in children with ADHD had no discernible impact on ADHD outcome assessed in multiple noncompeting domains of functioning. The current findings further support the notion that comorbidity of ADHD and tic disorders does not herald a more compromised functional outcome in individuals with ADHD across the life cycle (33).
Also consistent with our prospective juvenile study are the findings failing to identify meaningful associations between tic disorders and stimulant treatment. In most cases, stimulant treatment occurred after the age of risk for tics and was not associated with reoccurrence of tics. Thus, the current study extends to adults previously reported findings in children, by us and others (34–36), of absence of meaningful associations between stimulant exposure and risk for tic disorders in individuals with ADHD. Despite these reassuring results, it is important to remember that in contrast to research data dealing with averages, clinicians deal with individual patients. Some patients may develop tics secondary to stimulant treatment that can be severe and incapacitating, necessitating careful monitoring of the course of stimulant-treated individuals with ADHD who show signs of a tic disorder.
Although the overrepresentation of tic disorders in adults with ADHD is consistent with findings reported in the pediatric literature (37–39), the rate of tic disorders in adults with ADHD was somewhat lower than that reported in the pediatric literature. The reasons for this discrepancy are unknown, but it may be due to recall bias. Adults may not remember their tics accurately if they were mild and remitted many years earlier. Consistent with this hypothesis is our finding that the base rate of tic disorders in the adult comparison group was also lower than that reported in juvenile samples. It is also possible that the higher rates of tics reported in children and adolescents could be due to a cohort effect. Such a cohort effect has been documented in depression and bipolar disorder, where younger subjects manifest higher rates of the same disorder (40). More work is needed to evaluate these issues further.
The findings presented in this report should be evaluated in the light of their methodological limitations. Because the data in this study were derived from retrospective reports, they could be subject to recall bias. It is possible that subjects underreported both the frequency and the duration of tics and that this led to inclusion of individuals with past tics in the no-tic group. However, these individuals would be more likely to have had milder or transient tics. In addition, the assessment of tics relied on structured diagnostic interviews. It is possible that the use of tic-specific rating scales may have provided greater detail on tic symptoms. However, findings in the current study are strikingly in agreement with those of our earlier, prospective study of tics in juveniles with ADHD (1).
Since our findings are based on a clinical population, they may not generalize to other populations. Clinically referred cases of ADHD may be more likely than nonreferred ones to be more severely ill because they seek treatment from physicians or other health professionals (Berkson’s bias). However, the rates of comorbidity found in this group of clinically referred adults with ADHD are consistent with those observed in nonreferred parents of ADHD children who themselves have ADHD (6, 7). Nevertheless, future studies can benefit from assessment of tic outcome in community samples of adults with and without ADHD.
Despite these limitations, our findings from a large group of referred adults with ADHD extend findings from previous prospective studies of children and adolescents and demonstrate that tic disorders are overrepresented in individuals with ADHD and that tic disorders follow a largely remitting course and have a limited impact on the course and outcome of individuals with ADHD across the life cycle.
 |
 |
 |
 |
Received June 15, 2000; revision received Oct. 17, 2000; accepted Nov. 20, 2000. From the Pediatric Psychopharmacology Unit, Psychiatry Service, Massachusetts General Hospital. Address reprint requests to Dr. Spencer, Pediatric Psychopharmacology Unit (WACC 725), Massachusetts General Hospital, 15 Parkman St., Boston, MA 02114-3117. Supported in part by a grant from the Tourette’s Society Association and by NIMH grant MH-57511 (Dr. Spencer).
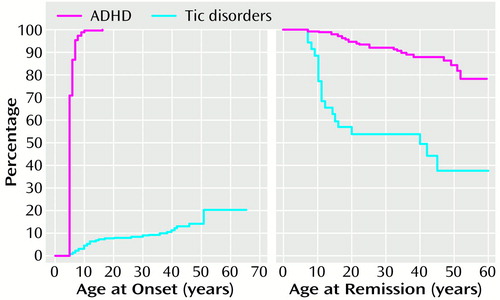
Figure 1. Course of ADHD and Tic Disorders in 312 Adults With ADHD
1. Spencer T, Biederman J, Coffey B, Geller D, Wilens T, Faraone S: The 4-year course of tic disorders in boys with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 1999; 56:842–847Crossref, Medline, Google Scholar
2. Spencer T, Biederman J, Wilens TE, Faraone SV: Adults with attention-deficit/hyperactivity disorder: a controversial diagnosis. J Clin Psychiatry 1998; 59(suppl 7):59–68Google Scholar
3. Seidman LJ, Biederman J, Weber W, Hatch M, Faraone SV: Neuropsychological function in adults with attention-deficit hyperactivity disorder. Biol Psychiatry 1998; 44:260–268Crossref, Medline, Google Scholar
4. Faraone SV, Biederman J, Weiffenbach B, Keith T, Chu MP, Weaver A, Spencer TJ, Wilens TE, Frazier J, Cleves M, Sakai J: Dopamine D4 gene 7-repeat allele and attention deficit hyperactivity disorder. Am J Psychiatry 1999; 156:768–770Abstract, Google Scholar
5. Biederman J, Faraone SV, Mick E, Spencer T, Wilens T, Kiely K, Guite J, Ablon S, Reed E, Warburton R: High risk for attention deficit hyperactivity disorder among children of parents with childhood onset of the disorder: a pilot study. Am J Psychiatry 1995; 152:431–435Link, Google Scholar
6. Biederman J, Faraone SV, Spencer T, Wilens T, Norman D, Lapey KA, Mick E, Lehman BK, Doyle A: Patterns of psychiatric comorbidity, cognition, and psychosocial functioning in adults with attention deficit hyperactivity disorder. Am J Psychiatry 1993; 150:1792–1798Google Scholar
7. Biederman J, Faraone SV, Spencer T, Wilens T, Mick E, Lapey KA: Gender differences in a sample of adults with attention deficit hyperactivity disorder. Psychiatry Res 1994; 53:13–29Crossref, Medline, Google Scholar
8. Bush G, Frazier JA, Rauch SL, Seidman LJ, Whalen PJ, Jenike MA, Rosen BR, Biederman J: Anterior cingulate cortex dysfunction in attention deficit/hyperactivity disorder revealed by fMRI and the counting Stroop. Biol Psychiatry 1999; 45:1542–1552Google Scholar
9. Zametkin AJ, Nordahl TE, Gross M, King AC, Semple WE, Rumsey J, Hamburger S, Cohen RM: Cerebral glucose metabolism in adults with hyperactivity of childhood onset. N Engl J Med 1990; 323:1361–1366Google Scholar
10. Spencer T, Wilens T, Biederman J, Faraone SV, Ablon JS, Lapey K: A double-blind, crossover comparison of methylphenidate and placebo in adults with childhood-onset attention-deficit hyperactivity disorder. Arch Gen Psychiatry 1995; 52:434–443Crossref, Medline, Google Scholar
11. Wilens TE, Biederman J, Spencer TJ, Frazier J, Prince J, Bostic J, Rater M, Soriano J, Hatch M, Sienna M, Millstein RB, Abrantes A: Controlled trial of high doses of pemoline for adults with attention-deficit/hyperactivity disorder. J Clin Psychopharmacol 1999; 19:257–264Crossref, Medline, Google Scholar
12. Wilens TE, Biederman J, Prince J, Spencer TJ, Faraone SV, Warburton R, Schleifer D, Harding M, Linehan C, Geller D: Six-week, double-blind, placebo-controlled study of desipramine for adult attention deficit hyperactivity disorder. Am J Psychiatry 1996; 153:1147–1153Google Scholar
13. Spencer T, Biederman J, Wilens T, Prince J, Hatch M, Jones J, Harding M, Faraone SV, Seidman L: Effectiveness and tolerability of tomoxetine in adults with attention deficit hyperactivity disorder. Am J Psychiatry 1998; 155:693–695Link, Google Scholar
14. Wilens TE, Spencer TJ, Biederman J, Girard K, Doyle R, Prince J, Polisner D, Solhkhah R, Comeau S, Monuteaux MC, Parekh A: A controlled trial of bupropion for attention deficit hyperactivity disorder in adults. Am J Psychiatry 2000; 158:282–288Link, Google Scholar
15. Spencer T, Biederman J, Wilens T, Bostic J, Prince J, Girard K, Doyle R, Parekh A, Kagan J, Bearman S: Efficacy of a mixed amphetamine salts compound in adults with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry (in press)Google Scholar
16. Biederman J, Faraone SV, Keenan K, Benjamin J, Krifcher B, Moore C, Sprich-Buckminster S, Ugaglia K, Jellinek MS, Steingard R, Spencer T, Norman D, Kolodny R, Kraus I, Perrin J, Keller MB, Tsuang MT: Further evidence for family-genetic risk factors in attention deficit hyperactivity disorder: patterns of comorbidity in probands and relatives in psychiatrically and pediatrically referred samples. Arch Gen Psychiatry 1992; 49:728–738Crossref, Medline, Google Scholar
17. Spitzer RL, Williams JBW, Gibbon M, First MB: Structured Clinical Interview for DSM-III-R—Non-Patient Edition (SCID-NP, Version 1.0). Washington, DC, American Psychiatric Press, 1990Google Scholar
18. Orvaschel H: Schedule for Affective Disorders and Schizophrenia for School-Age Children—Epidemiologic Version (K-SADS-E), 5th ed. Fort Lauderdale, Fla, Nova Southeastern University, Center for Psychological Studies, 1994Google Scholar
19. Gershon ES, Hamovit J, Guroff JJ, Dibble E, Leckman JF, Sceery W, Targum SD, Nurnberger JI Jr, Goldin LR, Bunney WE Jr: A family study of schizoaffective, bipolar I, bipolar II, unipolar, and normal control probands. Arch Gen Psychiatry 1982; 39:1157–1167Google Scholar
20. Weissman MM, Gershon ES, Kidd KK, Prusoff BA, Leckman JF, Dibble E, Hamovit J, Thompson WD, Pauls DL, Guroff JJ: Psychiatric disorders in the relatives of probands with affective disorders: the Yale University-National Institute of Mental Health Collaborative Study. Arch Gen Psychiatry 1984; 41:13–21Crossref, Medline, Google Scholar
21. Biederman J, Rosenbaum JF, Hirshfeld DR, Faraone SV, Bolduc EA, Gersten M, Meminger SR, Kagan J, Snidman N, Reznick JS: Psychiatric correlates of behavioral inhibition in young children of parents with and without psychiatric disorders. Arch Gen Psychiatry 1990; 47:21–26Crossref, Medline, Google Scholar
22. Seidman LJ, Biederman J, Faraone SV, Milberger S, Norman D, Seiverd K, Benedict K, Guite J, Mick E, Kiely K: Effects of family history and comorbidity on the neuropsychological performance of children with ADHD: preliminary findings. J Am Acad Child Adolesc Psychiatry 1995; 34:1015–1024Google Scholar
23. Seidman LJ, Biederman J, Faraone SV, Weber W, Ouellette C: Toward defining a neuropsychology of attention deficit-hyperactivity disorder: performance of children and adolescents from a large clinically referred sample. J Consult Clin Psychol 1997; 65:150–160Crossref, Medline, Google Scholar
24. Wechsler D: Manual for the Wechsler Adult Intelligence Scale—Revised. San Antonio, Tex, Psychological Corp, 1981Google Scholar
25. Sattler J: Psychological Assessment, 4th ed. New York, McGraw-Hill, 1988Google Scholar
26. Brooker BH, Cyr JJ: Tables for clinicians to use to convert WAIS-R short forms. J Clin Psychol 1986; 42:983–986Crossref, Google Scholar
27. Jastak S, Wilkinson GS: The Wide Range Achievement Test, Revised. Wilmington, Del, Jastak Associates, 1985Google Scholar
28. Reynolds CR: Critical measurement issues in learning disabilities. J Special Education 1984; 18:451–476Crossref, Google Scholar
29. Hollingshead AB: Four-Factor Index of Social Status. New Haven, Conn, Yale University, Department of Sociology, 1975Google Scholar
30. Stata Reference Manual, Release 5. College Station, Tex, Stata Corp, 1997Google Scholar
31. Leckman J, Peterson B, Anderson G, Arnsten A, Pauls D, Cohen D: Pathogenesis of Tourette’s syndrome. J Child Psychol Psychiatry 1997; 38:119–142Crossref, Medline, Google Scholar
32. Biederman J, Faraone S, Milberger S, Guite J, Mick E, Chen L, Mennin D, Marrs A, Ouellette C, Moore P, Spencer T, Norman D, Wilens T, Kraus I, Perrin J: A prospective 4-year follow-up study of attention-deficit hyperactivity and related disorders. Arch Gen Psychiatry 1996; 53:437–446Crossref, Medline, Google Scholar
33. Spencer T, Biederman J, Harding M, O’Donnell D, Wilens T, Faraone S, Coffey B, Geller D: Disentangling the overlap between Tourette’s disorder and ADHD. J Child Psychol Psychiatry 1998; 39:1037–1044Google Scholar
34. Castellanos FX, Giedd JN, Elia J, Marsh WL, Ritchie GF, Hamburger SD, Rapoport JL: Controlled stimulant treatment of ADHD and comorbid Tourette’s syndrome: effects of stimulant and dose. J Am Acad Child Adolesc Psychiatry 1997; 36:589–596Crossref, Medline, Google Scholar
35. Gadow KD, Sverd J, Sprafkin J, Nolan EE, Grossman S: Long-term methylphenidate therapy in children with comorbid attention-deficit hyperactivity disorder and chronic multiple tic disorder. Arch Gen Psychiatry 1999; 56:330–336Crossref, Medline, Google Scholar
36. Gadow K, Sverd J, Sprafkin J, Nolan E, Ezor S: Efficacy of methylphenidate for attention-deficit hyperactivity disorder in children with tic disorder. Arch Gen Psychiatry 1995; 52:444–455; correction, 52:836Crossref, Medline, Google Scholar
37. Denckla MB, Bemporad JR, MacKay MC: Tics following methylphenidate administration: a report of 20 cases. JAMA 1976; 235:1349–1351Google Scholar
38. Comings DE, Comings BG: A controlled family history study of Tourette’s syndrome, I: attention-deficit hyperactivity disorder and learning disorders. J Clin Psychiatry 1990; 51:275–280Medline, Google Scholar
39. Munir K, Biederman J, Knee D: Psychiatric comorbidity in patients with attention deficit disorder: a controlled study. J Am Acad Child Adolesc Psychiatry 1987; 26:844–848Crossref, Medline, Google Scholar
40. Gershon ES, Hamovit JH, Guroff JJ, Nurnberger JI: Birth-cohort changes in manic and depressive disorders in relatives of bipolar and schizoaffective patients. Arch Gen Psychiatry 1987; 44:314–319Crossref, Medline, Google Scholar



