Velocardiofacial Syndrome: Are Structural Changes in the Temporal and Mesial Temporal Regions Related to Schizophrenia?
Abstract
OBJECTIVE: Velocardiofacial syndrome results from a microdeletion on chromosome 22 (22q11.2). Clinical studies indicate that more than 30% of children with the syndrome will develop schizophrenia. The authors sought to determine whether neuroanatomical features in velocardiofacial syndrome are similar to those reported in the literature on schizophrenia by measuring the volumes of the temporal lobe, superior temporal gyrus, and mesial temporal structures in children and adolescents with velocardiofacial syndrome. METHOD: Twenty-three children and adolescents with velocardiofacial syndrome and 23 comparison subjects, individually matched for age and gender, received brain magnetic resonance imaging (MRI) scans. Analysis of covariance models were used to compare regional brain volumes. Correlations between residualized brain volumes and age were standardized and compared with the Fisher r-to-z transformation. RESULTS: Children with velocardiofacial syndrome had significantly smaller average temporal lobe, superior temporal gyrus, and hippocampal volumes than normal comparison children, although these differences were commensurate with a lower overall brain size in the affected children. In a cross-sectional analysis, children with velocardiofacial syndrome exhibited aberrant volumetric reductions with age that were localized to the temporal lobe and left hippocampal regions. CONCLUSIONS: Abnormal temporal lobe and hippocampal development in velocardiofacial syndrome is potentially concordant with MRI findings in the schizophrenia literature. Temporal lobe and mesial temporal structures may represent a shared substrate for the effects of the 22q11.2 deletion and for the complex etiological pathways that lead to schizophrenia. Longitudinal research may help determine which children with velocardiofacial syndrome are at risk for serious psychiatric illness in adulthood.
The notable increase in the number of published studies on velocardiofacial syndrome over the past 3 years is likely attributable to data suggesting that the syndrome may represent a homogeneous genetic subtype of schizophrenia (1). The concurrent breakthrough in genetic research of the complete sequencing of chromosome 22 (2) will undoubtedly fuel additional investigations of the syndrome and its genetic underpinnings. Caused by a microdeletion on chromosome 22 (22q11.2) (3), velocardiofacial syndrome is a congenital, autosomal dominant condition affecting approximately 1 of 4,000 live births (4). The predominant clinical signs include cleft palate or velopharyngeal insufficiency, cardiac abnormalities, a specific set of facial features, and learning disabilities (5, 6). Given that the syndrome has a known genetic cause and is characterized by a consistent phenotype, investigation of velocardiofacial syndrome provides an exceptional opportunity for narrowing the search among possible neuroanatomical and genetic correlates of schizophrenia.
Links between velocardiofacial syndrome and schizophrenia have been suggested by clinical research on both entities. More than a decade after Shprintzen and colleagues (7) published the first description of velocardiofacial syndrome, evidence from clinical studies has demonstrated a higher prevalence of psychiatric disorders in the population of persons with the syndrome (8–10). One of the first investigations of velocardiofacial syndrome and risk for psychopathology noted an elevated incidence of schizophrenia and schizoaffective disorder among adults with the syndrome (8). A subsequent investigation (10) asserted an etiological link with bipolar disorder rather than schizophrenic disorders. More recently, scientific evidence has pointed again toward a predisposition for schizophrenia (11–13). Various estimates have been proposed for the frequency of velocardiofacial syndrome in the population of persons with schizophrenia. At least four studies have demonstrated an overrepresentation of the 22q11.2 deletion in samples of persons diagnosed with schizophrenia; 2%–6% of persons with schizophrenia in these samples had the 22q11.2 deletion (14–17). Although these clinical studies are credited with linking the two disorders, determining whether velocardiofacial syndrome is a subtype of schizophrenia and delineating any shared etiological pathways necessitate deeper investigation of the syndrome’s neuroanatomical features.
Magnetic resonance imaging (MRI) provides a tool for measuring the impact of the 22q11.2 deletion on brain structure and for identifying neuroanatomical features that are potentially shared by velocardiofacial syndrome and schizophrenia. The extensive body of MRI studies on schizophrenia and the relatively limited number of preliminary studies on velocardiofacial syndrome (9, 18–20) have separately yielded similar findings. For instance, investigations of morphological brain changes in persons diagnosed with schizophrenia have consistently shown decreased total cerebral volume (21–23). A parallel finding was obtained in the first quantitative MRI investigation of velocardiofacial syndrome (20), which showed smaller brain volumes, a finding congruent with the microcephaly observed qualitatively in clinical studies of children with the syndrome (6).
We could find no MRI studies that have targeted the temporal lobe, mesial temporal structures, and superior temporal gyrus in persons with velocardiofacial syndrome, although those structures have been extensively studied in the research on schizophrenia (21, 23). Results of MRI research on schizophrenia have demonstrated volumetric alterations in the temporal lobe and its component structures, including the superior temporal gyrus (21–23) and the hippocampus (23, 24). Although the 22q11.2 deletion has clear deleterious consequences for brain morphology, it is not known whether mesial temporal structures or the superior temporal gyrus, in particular, are affected. Consequently, whether the pathogenic mechanisms of velocardiofacial syndrome and schizophrenia are comparable in terms of their impact on the temporal lobe has yet to be elucidated.
The aims of the study reported here are twofold. First, we explore whether volumes of the temporal lobe and related structures are altered in a group of children and adolescents with velocardiofacial syndrome compared to typically developing children. Second, we investigate whether volumetric and age-related reductions in the regions of interest are congruent with the findings of MRI research on schizophrenia. If velocardiofacial syndrome is a subtype of schizophrenia or if the disorders have common neuropathogenic mechanisms, it is reasonable to hypothesize that the smaller structural and developmental volumes of the temporal lobe in children with velocardiofacial syndrome will be comparable to those observed in schizophrenia.
Method
Subjects
Children and adolescents with velocardiofacial syndrome were recruited through the VCF Support Group Network of Northern California and through advertisements on our web site (www. cap.stanford.edu/research/). Only persons with velocardiofacial syndrome who had a microdeletion on chromosome 22q11.2, as shown by fluorescent in situ hybridization, were included in the study. The study subjects were children and adolescents with confirmed velocardiofacial syndrome (N=23) and comparison subjects (N=23) who were matched individually for age and gender with the affected children. Eight female and 15 male subjects were included in each study group. The subjects ranged in age from 5.8 to 21.0 years (velocardiofacial syndrome group: mean=12.7 years, SD=3.9; comparison group: mean=12.9 years, SD=4.1). The comparison subjects were recruited through advertisements in local newspapers and newsletters for parent groups and among nonaffected siblings of children affected with identified genetic conditions (fragile X syndrome and Turner’s syndrome). A minimum IQ of 85 (one standard deviation below the population mean) and absence of previous neurological or psychiatric disorder were used as inclusion criteria for comparison subjects. Written informed consent was received from children and parents under protocols approved by the institutional review board of Stanford University.
MRI Protocol
Magnetic resonance images were obtained by using a GE Signa 1.5-T scanner (General Electric, Milwaukee). Coronal images were acquired with a three-dimensional volumetric radio frequency spoiled gradient echo with the following scan parameters: TR=35 msec, TE=6 msec, flip angle=45°, number of excitations=1, matrix size=256 × 192, field of view=24 cm2, slice thickness=1.5 mm, 124 slices.
Image Processing and Measurement
Spoiled gradient recall acquisition image data were imported into the software program BrainImage v4.x (25). This program permits semiautomated image analysis incorporating the following steps: 1) correction of nonuniformity in voxel intensity, 2) removal of images of nonbrain tissue, 3) segmentation of the brain into constituent tissue types by using a constrained fuzzy algorithm on the basis of voxel intensity and tissue boundaries, and 4) positional normalization and division of the data into anatomic subregions by using a stereotactic parcellation method (26). Details of these procedures have been published elsewhere (27–30). Derived data are presented in terms of left and right gray and white matter volumes for the whole brain and for the temporal lobe.
For both manual and automated procedures, raters were blinded to the identity and diagnosis of the subject. Circumscription of the mesial temporal structures (amygdala and hippocampus) was performed with a previously described protocol (31). Spoiled gradient recall acquisition datasets were expanded from a 256 × 256 to a 512 × 512 matrix by means of bicubic interpolation in order to increase the resolution at which a region of interest could be delineated.
The boundaries of the amygdala were drawn coronally, beginning on the slice where the anterior commissure first crosses the midline of the brain. The boundaries were drawn beginning inferolaterally, then moving medially at the border between the amygdala and the white matter tract inferior to it. The medial border was drawn at the CSF/gray matter border. The region of interest continued superomedially at the gray matter/white matter border and around the lateral amygdala to the starting point. In the posterior regions of the amygdala, the superior border was partially defined by the presence of the entorhinal sulcus. The boundary of the amygdala was drawn until it disappeared posteriorly.
The anterior-most slice of the hippocampus was determined by the presence of the following landmarks: the alveus, the point where the superior horn of the lateral ventricle first points medially, the intensification of gray matter signal and a greater separation between the gray and white matter, and the development of the laminar structure that distinguishes the hippocampus from the amygdala. The borders of the hippocampus were defined by the surrounding white matter and CSF (or the amygdala superiorly, when present), taking special care to include the alveus in the measurements of the hippocampus and to exclude the tail of the caudate nucleus (separated from the hippocampus by a white matter bridge) and the thalamus posteriorly (distinguished by its considerably lighter signal relative to the hippocampus). Circumscription continued until the hippocampus disappeared posteriorly, approximately at the point where the corpus callosum fuses with the fornix. Reliability analyses, based on 10 datasets, yielded intraclass correlation coefficients (ICCs) of 0.98 and 0.91 for the amygdala and hippocampus, respectively.
The superior temporal gyri were also defined manually, supplementing the semiautomated parcellation procedure. The superior temporal gyrus was measured in the rostrocaudal direction on a coronal stack oriented perpendicular to the anterior commissure and posterior commissure (voxel size=0.94 mm3). Boundaries of the superior temporal gyrus were defined laterally by the cortical surface and medially by a line connecting the deepest extension of the superior temporal sulcus to the furthest extent of the inferior ramus of the sylvian fissure. The most anterior slice of the superior temporal gyrus coincided with the halfway point between the head of the putamen and the anterior commissure. This designation ensured the operational exclusion of medial temporal gyral tissue, which merges with the superior temporal gyrus at the temporal pole. The most posterior slice of the superior temporal gyrus coincided with the first slice where the crus of the fornix was clearly identified laterally from the pulvinar. A reliability analysis of measurements by two independent raters resulted in an ICC of 0.96, indicating substantial interrater reliability for superior temporal gyrus measurement.
Statistical Analyses
The volumetric data met the criteria for employing parametric statistical analyses. Data analysis occurred in two stages. First, the mean brain volumes of subjects with velocardiofacial syndrome and the matched comparison subjects were statistically compared by using analyses of variance and covariance. Analysis of variance (ANOVA) models were used for comparisons of the absolute volumes of the whole brain, temporal lobe, superior temporal gyrus, and mesial temporal structures. For all subregional volumetric comparisons, analysis of covariance (ANCOVA) models were computed to statistically adjust for group differences in overall brain size and thus to measure whether any volumetric changes were diffuse or region-specific. Second, Pearson product-moment correlation was used to quantify the relationship between age and regional brain volumes within each group. Temporal lobe tissue, amygdalal, and hippocampal volumes were each residualized on the basis of simple linear regression with whole brain total tissue volume; temporal lobe gray matter and superior temporal gyrus gray matter were residualized on the basis of simple linear regression with whole brain gray matter volume. For both the ANOVA and the correlational analyses, an alpha of 0.05 (two-tailed) was used as the threshold for statistical significance. To determine whether the expected age-related volumetric reductions in the velocardiofacial syndrome group differed from the expected positive associations between age and brain volumes in the comparison subjects, correlation coefficients for each group were converted by using Fisher’s r-to-z transformation (32, 33). To test these directional hypotheses, a one-tailed alpha level of 0.05 was used.
Results
Group Comparisons of Mean Volumes
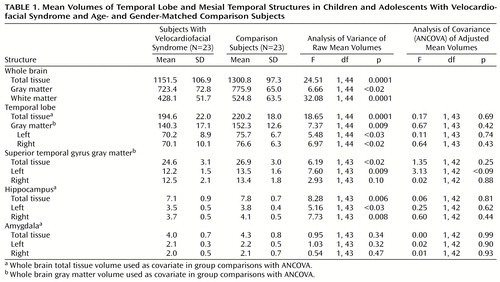
Results of ANOVA showed that subjects with velocardiofacial syndrome had a significantly lower whole brain total tissue volume (about 11% less) than the comparison subjects (Table 1). Absolute volumes of both gray and white matter were significantly smaller in the velocardiofacial syndrome group. Temporal lobe total tissue volume was significantly smaller among velocardiofacial syndrome subjects, and significantly smaller absolute volumes for temporal lobe gray matter in both hemispheres were detected in this group. Statistically significant differences also were observed for mean volumes of the superior temporal gyrus gray matter and the hippocampus. Average amygdala volumes did not statistically differ between the subjects with velocardiofacial syndrome and the comparison subjects.
Group comparisons of temporal and mesial temporal structures were reanalyzed with ANCOVA (Table 1). Whole brain total tissue volume was used as a covariate for comparisons of temporal lobe total tissue, hippocampus, and amygdala volumes, and whole brain gray matter volume was used as a covariate for comparisons of temporal lobe gray matter and superior temporal gyrus gray matter volumes. After statistically controlling for the 11% group difference in whole brain volume, adjusted mean volumes of the temporal lobe and hippocampus were not statistically different between the two groups. Similarly, when the whole brain gray matter volume was covaried statistically, the two groups did not differ in adjusted mean volumes of temporal lobe gray matter and superior temporal gyrus gray matter.
Changes in Brain Volumes With Age
Coefficients of correlation between age and regional brain volumes were computed by using residualized volumes of the temporal lobe, superior temporal gyrus, and mesial temporal structures. As shown in Table 2, Fisher r-to-z transformations were used to standardize the correlation coefficients and to compare observed group differences in the direction of the relationships between age and brain volumes. Differential directions were noted for the temporal lobe (total tissue and gray matter), as well as for the hippocampus. The correlation between temporal lobe total tissue volume and age was positive among comparison subjects and negative among subjects with velocardiofacial syndrome; this differential direction was statistically significant. A statistically significant group difference was also detected for the relationship between age and residualized temporal lobe gray matter volume. Comparison of the association between age and left hippocampus volume in comparison subjects approached significance, when compared to the negative association observed in the velocardiofacial syndrome group.
Discussion
The MRI data from the current study demonstrate that children and adolescents with velocardiofacial syndrome have marked neuroanatomical differences in the temporal lobe region, compared with age- and gender-matched normal children and adolescents, and that these aberrations occur within the context of a lower whole brain volume. Our observations for each region are discussed below, first in terms of localization (general versus region-specific) and second in terms of developmental trajectory (change with age).
Localization
The localization of the deletion’s impact is defined by whether the average volume of a structure is disproportionate relative to the overall lower whole brain volume in subjects with velocardiofacial syndrome (Table 1). It is important to discern whether volumetric aberrations in the temporal lobe and related structures are either due to a generalized neuroanatomical diminution that results in diffuse effects on brain structures or are region-specific. Both cases necessitate discussion, as any structure that has a lower volume, compared with either a relative or an absolute standard, may influence function.
Absolute volumes of the temporal lobe, superior temporal gyrus, and hippocampus were 9%–11% smaller in the children with velocardiofacial syndrome; however, these differences coincided with a lower whole brain volume of a similar magnitude. It is interesting to note that amygdala volumes in velocardiofacial syndrome children were not statistically different from those in their typically developing counterparts, despite the overall lower whole brain volume of the children with velocardiofacial syndrome. Further, correlational analyses indicated that amygdala volumes were preserved over time into adulthood. The amygdala is involved in emotion-related aspects of behavior, including learning and conditioning (34–36), and appears to be spared in the structures affected in velocardiofacial syndrome relative to other temporal and mesial temporal structures. These volumetric data raise an important methodological issue. Specifically, differential morphometric findings for the amygdala versus other temporal lobe structures in velocardiofacial syndrome indicate the need for separate measurement of the amygdala and hippocampus in imaging studies of velocardiofacial syndrome and schizophrenia, as previous studies have sometimes measured the two structures collectively as an amygdala-hippocampal complex (21).
Although the difference between groups in left superior temporal gyrus gray matter volume was not statistically significant (p=0.08), the possibility of a localized and early effect of the velocardiofacial syndrome deletion on this region (Table 1) deserves comment, given previous reports of an association between schizophrenic symptoms and left superior temporal gyrus morphology. Specifically, smaller left superior temporal gyrus volumes have been correlated with positive symptoms of psychosis in several studies (37–39). Frequently investigated in the schizophrenia literature, the superior temporal gyrus is the site of the primary and secondary auditory cortex (40), and abnormalities in this region have been associated with the presence of auditory hallucinations (22, 39, 41).
Developmental Trajectory
Most of our salient results regarding temporal lobe morphology in velocardiofacial syndrome arose from age-related analyses. These findings, although limited by a cross-sectional design (42), suggest that many of the distinguishable effects of the 22q11.2 deletion on brain morphology are manifested only in developmental changes of the central nervous system over time. Average volumes of some temporal lobe and related structures may not be selectively lower in samples of young children with velocardiofacial syndrome, compared with normal children, yet deviations from normal developmental trajectories (Table 2) may result in localized lower volumes that are observable later in life.
Correlational findings showed a significant difference between the velocardiofacial syndrome group and the comparison group in the relation between temporal lobe volumes and age. Among the comparison subjects, patterns of temporal lobe gray matter development were congruent with findings from a recent longitudinal MRI investigation of typically developing children and adolescents (43), showing an age-related increase in temporal lobe gray matter volume. Giedd and colleagues (43) suggested that temporal lobe gray matter continues to evolve throughout childhood and develops later than the occipital, frontal, and parietal lobes. This finding markedly contrasts with the age-related decline in temporal volumes shown in the children with velocardiofacial syndrome in our study. This decline in temporal lobe volumes with age was not accounted for by age-related differences in whole brain volume, suggesting that the 22q11.2 deletion has a selective developmental effect on the temporal lobe. Because the temporal lobe is linked to auditory and language processing (44, 45), early developmental aberrations in this structure may underlie the language and learning disabilities commonly observed in the velocardiofacial syndrome population.
Extrapolation from the observed pathways of temporal lobe development in velocardiofacial syndrome suggests that continued decrease in temporal lobe volumes with age may, by the time of adulthood, result in a localized diminution beyond an 11% overall difference in whole brain volume. This observation is of potential relevance to, and is consistent with, the more recent literature on velocardiofacial syndrome (19) and schizophrenia (21–23, 46). In particular, van Amelsvoort and associates (19) reported lower total and left temporal lobe volumes in seven adults with velocardiofacial syndrome and schizophrenia than in eight matched comparison subjects. Further, a longitudinal MRI investigation of childhood-onset schizophrenia (47) showed progressive developmental reduction in volumes of the temporal lobe, as well as of the superior temporal gyrus and the hippocampus.
In our study, the correlation between age and hippocampal volumes approached significance, suggesting that hippocampal volumes, specifically the left hemisphere volume, may decrease with age in children with velocardiofacial syndrome. Selective decreases in hippocampal volume with age are of potential importance to the developmental trajectory of cognitive problems in persons with velocardiofacial syndrome, given the role of this structure in working and long-term memory (48) and its connections to higher cortical centers (40, 49).
Potential age-related reductions of the hippocampus in velocardiofacial syndrome have interesting implications for the link with schizophrenia. Smaller hippocampal volumes have been consistently observed in individuals with schizophrenia (46, 50, 51). In a recent report, Razi and colleagues (24) suggested that differences in hippocampal volumes may not appear until later in life among schizophrenic patients, thus reflecting a consequence of the disorder rather than a causal predecessor. If neuropathological changes in velocardiofacial syndrome model those occurring in other etiologies for schizophrenia, our age-related findings would contrast with the assertion of Razi and colleagues and suggest that hippocampal alterations may actually precede the onset of significant psychiatric symptoms, supporting the idea that schizophrenia is a neurodevelopmental disorder (52, 53).
Findings of a selective neurodevelopmental impact on the hippocampus are congruent with recent genetic research on velocardiofacial syndrome, which points to the hippocampus as a region demonstrably affected by the 22q11.2 deletion. Yamagishi and associates (54) measured the expression of a newly identified gene, UFD1L, localized in the 2.0-megabase region commonly deleted in velocardiofacial syndrome. Of 182 subjects with velocardiofacial syndrome, all had a deletion involving that specific gene. Further, the mouse homolog gene, Ufd1, is known to play a key role in the embryonic development of the heart and the brain. Ufd1 is expressed specifically in palatal precursors, frontonasal regions, and the cells derived from the neural crest that form the conotruncal part of the heart. Most relevant is the fact that in the brain, Ufd1 is expressed with marked specificity in the medial telencephalon that forms the hippocampus. As Yamagishi and colleagues hypothesized, a smaller expression of UFD1L in subjects with a deletion in chromosome 22q11.2 might be responsible for abnormal development of the hippocampus.
Conclusions
The present study identifies several neurodevelopmental patterns common to both velocardiofacial syndrome and schizophrenia and suggests that the temporal lobe and mesial temporal structures may represent a shared substrate for the effects of the 22q11.2 deletion and for other etiological pathways that lead to schizophrenia. Longitudinal research will be needed to specify the immediate and unfolding influence of the 22q11.2 deletion on brain development in children affected with velocardiofacial syndrome and its relation to the onset of severe psychiatric disorders. In addition, MRI research involving adult subjects with velocardiofacial syndrome is clearly needed to confirm the developmental patterns identified in this cross-sectional group of children and adolescents. Given the greater risk for schizophrenia among individuals with velocardiofacial syndrome, it is especially important to track neuroanatomical changes into early adulthood, the typical age of onset for psychosis. Longitudinal tracking will require larger sample sizes and greater statistical power, given that only a portion of any sample of subjects with velocardiofacial syndrome will eventually develop schizophrenia. Morphological changes in the temporal lobe and mesial temporal structures during childhood may be more pronounced in those children with velocardiofacial syndrome who later experience a psychotic break. Ultimately, future research will help identify biological markers that predict the probability of severe psychiatric illness.
 |
 |
Received April 14, 2000; revisions received Aug. 22 and Sept. 22, 2000; accepted Oct. 3, 2000. From the Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine. Address reprint requests to Dr. Eliez, Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, 401 Quarry Rd., Stanford, CA 94305-5719; [email protected] (e-mail). Supported by a grant from the National Swiss Research Fund to Dr. Eliez, by grants MH-01142 from NIMH and HD-31715 from the National Institute of Child Health and Human Development to Dr. Reiss, and by a grant from the M.I.N.D. Institute to Dr. Reiss. The authors thank Wendy E. Brown, Carol A. Lin, and Anil Patwardhan for image processing.
1. Bassett AS, Chow EW:22q11 Deletion syndrome: a genetic subtype of schizophrenia. Biol Psychiatry 1999; 46:882–891Google Scholar
2. Dunham I, Shimizu N, Roe BA, Chissoe S, Hunt AR, Collins JE, Bruskiewich R, Beare DM, Clamp M, Smink LJ, Ainscough R, Almeida JP, Babbage A, Bagguley C, Bailey J, Barlow K, Bates KN, Beasley O, Bird CP, Blakey S, Bridgeman AM, Buck D, Burgess J, Burrill WD, O’Brien KP, et al.: The DNA sequence of human chromosome 22. Nature 1999; 402:489–495Crossref, Medline, Google Scholar
3. Driscoll DA, Salvin J, Sellinger B, Budarf ML, McDonald-McGinn DM, Zackai EH, Emanuel BS: Prevalence of 22q11 microdeletions in DiGeorge and velocardiofacial syndromes: implications for genetic counselling and prenatal diagnosis. J Med Genet 1993; 30:813–817Crossref, Medline, Google Scholar
4. Tezenas Du Montcel S, Mendizabai H, Ayme S, Levy A, Philip N: Prevalence of 22q11 microdeletion (letter). J Med Genet 1996; 33:719Crossref, Medline, Google Scholar
5. Goldberg R, Motzkin B, Marion R, Scambler PJ, Shprintzen RJ: Velo-cardio-facial syndrome: a review of 120 patients. Am J Med Genet 1993; 45:313–319Crossref, Medline, Google Scholar
6. Ryan AK, Goodship JA, Wilson DI, Philip N, Levy A, Seidel H, Schuffenhauer S, Oechsler H, Belohradsky B, Prieur M, Aurias A, Raymond FL, Clayton-Smith J, Hatchwell E, McKeown C, Beemer FA, Dallapiccola B, Novelli G, Hurst JA, Ignatius J, Green AJ, Winter RM, Brueton L, Brondum-Nielsen K, Stewart F, Van Essen T, Patton M, Paterson J, Scambler PJ: Spectrum of clinical features associated with interstitial chromosome 22q11 deletions: a European collaborative study. J Med Genet 1997; 34:798–804Crossref, Medline, Google Scholar
7. Shprintzen RJ, Goldberg RB, Lewin ML, Sidoti EJ, Berkman MD, Argamaso RV, Young D: A new syndrome involving cleft palate, cardiac anomalies, typical facies, and learning disabilities: velo-cardio-facial syndrome. Cleft Palate J 1978; 15:56–62Medline, Google Scholar
8. Shprintzen RJ, Goldberg R, Golding-Kushner KJ, Marion RW: Late-onset psychosis in the velo-cardio-facial syndrome. Am J Med Genet 1992; 42:141–142Crossref, Medline, Google Scholar
9. Chow EW, Mikulis DJ, Zipursky RB, Scutt LE, Weksberg R, Bassett AS: Qualitative MRI findings in adults with 22q11 deletion syndrome and schizophrenia. Biol Psychiatry 1999; 46:1436–1442Google Scholar
10. Papolos DF, Faedda GL, Veit S, Goldberg R, Morrow B, Kucherlapati R, Shprintzen RJ: Bipolar spectrum disorders in patients diagnosed with velo-cardio-facial syndrome: does a hemizygous deletion of chromosome 22q11 result in bipolar affective disorder? Am J Psychiatry 1996; 153:1541–1547Google Scholar
11. Gothelf D, Frisch A, Munitz H, Rockah R, Aviram A, Mozes T, Birger M, Weizman A, Frydman M: Velocardiofacial manifestations and microdeletions in schizophrenic inpatients. Am J Med Genet 1997; 72:455–461Crossref, Medline, Google Scholar
12. Bassett AS, Hodgkinson K, Chow EW, Correia S, Scutt LE, Weksberg R:22q11 Deletion syndrome in adults with schizophrenia. Am J Med Genet 1998; 81:328–337Google Scholar
13. Murphy KC, Jones LA, Owen MJ: High rates of schizophrenia in adults with velo-cardio-facial syndrome. Arch Gen Psychiatry 1999; 56:940–945Crossref, Medline, Google Scholar
14. Yan W, Jacobsen LK, Krasnewich DM, Guan XY, Lenane MC, Paul SP, Dalwadi HN, Zhang H, Long RT, Kumra S, Martin BM, Scrambler PJ, Trent JM, Sidrandky E, Ginns, EI, Rapoport JL: Chromosome 22q11.2 interstitial deletions among childhood-onset schizophrenics and “multidimensionally impaired.” Am J Med Genet 1998; 81:41–43Crossref, Medline, Google Scholar
15. Murphy KC, Jones RG, Griffiths E, Thompson PW, Owen MJ: Chromosome 22qII deletions: an under-recognised cause of idiopathic learning disability. Br J Psychiatry 1998; 172:180–183Crossref, Medline, Google Scholar
16. Nicolson R, Rapoport JL: Childhood-onset schizophrenia: rare but worth studying. Biol Psychiatry 1999; 46:1418–1428Google Scholar
17. Karayiorgou M, Morris MA, Morrow B, Shprintzen RJ, Goldberg R, Borrow J, Gos A, Nestadt G, Wolyniec PS, Lasseter VK, Eisen H, Childs B, Kazazian HH, Kucherlapati R, Antonarakis SE, Pulver AE, Housman DE: Schizophrenia susceptibility associated with interstitial deletions of chromosome 22q11. Proc Natl Acad Sci USA 1995; 92:7612–7616Google Scholar
18. Chow E, Zipursky RB, Mikulis D, Scutt L, Weksberg R, Bassett AS: MRI findings in adults with 22q11 deletion syndrome (22qDS) and schizophrenia (abstract). Schizophr Res 1999; 36:89Google Scholar
19. van Amelsvoort T, Daly E, Critchley H: A human genetic model for schizophrenia: brain, structure and function of people with velo-cardio-facial syndrome (abstract). Schizophr Res 1999; 36:213Google Scholar
20. Eliez S, Schmitt JE, White CD, Reiss AL: Children and adolescents with velocardiofacial syndrome: a volumetric MRI study. Am J Psychiatry 2000; 157:409–415Link, Google Scholar
21. Shenton ME, Wible CG, McCarley RW: A Review of Magnetic Resonance Imaging Studies of Brain Abnormalities in Schizophrenia. New York, Marcel Dekker, 1997Google Scholar
22. Lawrie SM, Abukmeil SS: Brain abnormality in schizophrenia: a systematic and quantitative review of volumetric magnetic resonance imaging studies. Br J Psychiatry 1998; 172:110–120Crossref, Medline, Google Scholar
23. Wright IC, Rabe-Hesketh S, Woodruff PWR, David AS, Murray RM, Bullmore ET: Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry 2000; 157:16–25Link, Google Scholar
24. Razi K, Greene KP, Sakuma M, Ge S, Kushner M, DeLisi LE: Reduction of the parahippocampal gyrus and the hippocampus in patients with chronic schizophrenia. Br J Psychiatry 1999; 174:512–519Crossref, Medline, Google Scholar
25. Reiss AL: BrainImage. Stanford, Calif, Stanford University, Stanford Psychiatry Neuroimaging Laboratory, 2000Google Scholar
26. Talairach J, Tournoux P: Co-Planar Stereotaxic Atlas of the Human Brain. New York, Thieme Medical, 1988Google Scholar
27. Kates WR, Warsofsky IS, Patwardhan A, Abrams MT, Liu AM, Naidu S, Kaufmann WE, Reiss AL: Automated Talairach atlas-based parcellation and measurement of cerebral lobes in children. Psychiatry Res 1999; 91:11–30Crossref, Medline, Google Scholar
28. Kaplan DM, Liu AM, Abrams MT, Warsofsky IS, Kates WR, White CD, Kaufmann WE, Reiss AL: Application of an automated parcellation method to the analysis of pediatric brain volumes. Psychiatry Res 1997; 76:15–27Crossref, Medline, Google Scholar
29. Andreasen NC, Rajarethinam R, Cizadlo T, Arndt S, Swayze VW II, Flashman LA, O’Leary DS, Ehrhardt JC, Yuh WTC: Automatic atlas-based volume estimation of human brain regions from MR images. J Comp Assist Tomogr 1996; 20:98–106Crossref, Medline, Google Scholar
30. Reiss AL, Hennessey JG, Rubin M, Beach L, Abrams MT, Warsofsky IS, Liu AM, Links JM: Reliability and validity of an algorithm for fuzzy tissue segmentation of MRI. J Comput Assist Tomogr 1998; 22:471–479Crossref, Medline, Google Scholar
31. Kates WR, Abrams MT, Kaufmann WE, Breiter SN, Reiss AL: Reliability and validity of MRI measurement of the amygdala and hippocampus in children with fragile X syndrome. Psychiatry Res 1997; 75:31–48Crossref, Medline, Google Scholar
32. Fisher RA: Statistical Methods for Research Workers. New York, Stechert, 1928Google Scholar
33. Viana MA: Statistical methods for summarizing independent correlational results. J Educational Statistics 1980; 5:83–104Crossref, Google Scholar
34. McGaugh JL: Memory—a century of consolidation. Science 2000; 287:248–251Crossref, Medline, Google Scholar
35. Maren S: Long-term potentiation in the amygdala: a mechanism for emotional learning and memory. Trends Neurosci 1999; 22:561–567Crossref, Medline, Google Scholar
36. Davidson RJ, Irwin W: The functional neuroanatomy of emotion and affective style. Trends Cogn Sci 1999; 3:11–21Crossref, Medline, Google Scholar
37. Marsh L, Harris D, Lim KO, Beal M, Hoff AL, Minn K, Csernansky JG, DeMent S, Faustman WO, Sullivan EV, Pfefferbaum A: Structural magnetic resonance imaging abnormalities in men with severe chronic schizophrenia and an early age at clinical onset. Arch Gen Psychiatry 1997; 54:1104–1112Google Scholar
38. Shenton ME, Kikinis R, Jolesz FA, Pollak SD, LeMay M, Wible CG, Hokama H, Martin J, Metcalf D, Coleman M: Abnormalities of the left temporal lobe and thought disorder in schizophrenia: a quantitative magnetic resonance imaging study. N Engl J Med 1992; 327:604–612Crossref, Medline, Google Scholar
39. Barta PE, Pearlson GD, Powers RE, Richards SS, Tune LE: Auditory hallucinations and smaller superior temporal gyrus volume in schizophrenia. Am J Psychiatry 1990; 147:1457–1462Google Scholar
40. Pandya DN: Anatomy of the auditory cortex. Rev Neurol (Paris) 1995; 151:486–494Medline, Google Scholar
41. Flaum M, Swayze VW II, O’Leary DS, Yuh WTC, Ehrhardt JC, Arndt SV, Andreasen NC: Effects of diagnosis, laterality, and gender on brain morphology in schizophrenia. Am J Psychiatry 1995; 152:704–714Link, Google Scholar
42. Kraemer HC, Yesavage JA, Taylor JL, Kupfer D: How can we learn about developmental processes from cross-sectional studies, or can we? Am J Psychiatry 2000; 157:163–171Google Scholar
43. Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL: Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci 1999; 2:861–863Crossref, Medline, Google Scholar
44. Chee MW, O’Craven KM, Bergida R, Rosen BR, Savoy RL: Auditory and visual word processing studied with fMRI. Hum Brain Mapp 1999; 7:15–28Crossref, Medline, Google Scholar
45. Muller RA, Rothermel RD, Behen ME, Muzik O, Mangner TJ, Chugani HT: Receptive and expressive language activations for sentences: a PET study. Neuroreport 1997; 8:3767–3770Google Scholar
46. McCarley RW, Wible CG, Frumin M, Hirayasu Y, Levitt JJ, Fischer IA, Shenton ME: MRI anatomy of schizophrenia. Biol Psychiatry 1999; 45:1099–1119Google Scholar
47. Jacobsen LK, Giedd JN, Castellanos FX, Vaituzis AC, Hamburger SD, Kumra S, Lenane MC, Rapoport JL: Progressive reduction of temporal lobe structures in childhood-onset schizophrenia. Am J Psychiatry 1998; 155:678–685Link, Google Scholar
48. Izquierdo I, Medina JH, Vianna MR, Izquierdo LA, Barros DM: Separate mechanisms for short- and long-term memory. Behav Brain Res 1999; 103:1–11Crossref, Medline, Google Scholar
49. Duvernoy HM, Bourgouin P: The Human Hippocampus: Functional Anatomy, Vascularization and Serial Sections With MRI, 2nd ed. New York, Springer Verlag, 1998Google Scholar
50. Giedd JN, Jeffries NO, Blumenthal J, Castellanos FX, Vaituzis AC, Fernandez T, Hamburger SD, Liu H, Nelson J, Bedwell J, Tran L, Lenane M, Nicolson R, Rapoport JL: Childhood-onset schizophrenia: progressive brain changes during adolescence. Biol Psychiatry 1999; 46:892–898Crossref, Medline, Google Scholar
51. Harrison PJ: The neuropathology of schizophrenia: a critical review of the data and their interpretation. Brain 1999; 122(part 4):593–624Google Scholar
52. Gur RE, Turetsky BI, Bilker WB, Gur RC: Reduced gray matter volume in schizophrenia. Arch Gen Psychiatry 1999; 56:905–911Crossref, Medline, Google Scholar
53. Raedler TJ, Knable MB, Weinberger DR: Schizophrenia as a developmental disorder of the cerebral cortex. Curr Opin Neurobiol 1998; 8:157–161Crossref, Medline, Google Scholar
54. Yamagishi H, Garg V, Matsuoka R, Thomas T, Srivastava D: A molecular pathway revealing a genetic basis for human cardiac and craniofacial defects. Science 1999; 283:1158–1161Google Scholar



