Cortical Responsiveness During Inner Speech in Schizophrenia: An Event-Related Potential Study
Abstract
OBJECTIVE: The study assessed the effects of inner speech on auditory cortical responsiveness in schizophrenia. METHOD: Comparison subjects (N=15) and patients with schizophrenia (N=15) were presented with acoustic and visual stimuli during three conditions: while subjects were silent, when spontaneous inner speech might occur; during directed inner speech, while subjects repeated a statement silently to themselves; and while subjects listened to recorded speech. N1 event-related potentials were recorded during the three conditions. RESULTS: N1 event-related potentials elicited by acoustic stimuli, but not by visual stimuli, were lower during directed inner speech than during the silent baseline condition in the comparison subjects but not in the patients. CONCLUSIONS: Abnormal auditory cortical responsiveness to inner speech in patients with schizophrenia may be a sign of corollary discharge dysfunction, which may potentially cause misattribution of inner speech to external voices.
One model of auditory hallucinations in schizophrenia proposes that ongoing inner speech is misidentified as external voices. Shergill et al. (1) used hemodynamic brain imaging to show that directed inner speech activates auditory speech processing areas. That inner speech uses auditory resources is suggested by ordinary experience: we complain of not being able to “think” when noises around us are too loud, and we fail to hear what others around us are saying when thinking intently.
The current study used the N1 component of the event-related potential elicited by unattended, irrelevant acoustic events to probe auditory cortical responsiveness. Peaking about 100 msec after stimulus onset, N1 is generated in the superior temporal gyrus of the auditory cortex by transient auditory stimuli of all kinds; the N1 amplitude is smaller when the auditory cortex is occupied with streams of other auditory stimuli (2).
We recently found that schizophrenic subjects have smaller than normal N1 amplitudes in response to auditory stimuli presented in silence, and we proposed that the smaller amplitudes were related to the subjects’ ongoing internal dialogue or thoughts (3). The inner speech or verbal thoughts of patients with a propensity to hear voices may be misattributed to external sources because of a failure of corollary discharge (4, 5) to alert the auditory cortex of impending input and dampen its responsiveness to that input.
In this study we compared responsiveness of the auditory cortex to irrelevant acoustic stimuli while subjects sat in silence (when spontaneous inner speech could occur), while they repeated statements silently in their own voice, and while they listened to recordings of statements in their own voice. Responsiveness to visual stimuli was also tested. We hypothesized that comparison subjects would show less engagement of the auditory cortex (indicated by larger N1 amplitudes in response to auditory stimuli) during spontaneous baseline inner speech than during directed inner speech, whereas patients would show similar engagement during baseline inner speech and during directed inner speech (indicated by small and equivalent N1 amplitudes).
Method
Fifteen healthy adults who had been screened for mental disorder with the Structured Clinical Interview for DSM-IV (13 men and two women; age 20–58 years) and 15 patients with DSM-IV schizophrenia who received medication (13 men and two women; age 22–53 years) gave written informed consent after procedures had been fully explained. The Brief Psychiatric Rating Scale (BPRS) (6) and Scale for the Assessment of Positive Symptoms (SAPS) (7) were administered, usually on the same day as the event-related potential testing.
Recordings were made of subjects saying seven typical hallucinatory statements (e.g., “That was really stupid.”) to be played back through headphones during the listening condition. Investigators matched the loudness of each sentence to that of a target sentence previously recorded from another speaker. Target sentence intensity was first set 25 dB above each subject’s speech comprehension threshold by using ascending and descending limits.
A series of three equally probable stimuli lasting 250 msec (speech sounds [/ba/], noises [broadband], and square checkerboards occupying 5×5 degrees of visual angle on a computer screen) were presented continuously throughout each condition in random order every 0.8–1.2 sec. During the silent baseline condition, the intensity of the speech sound and noise stimuli was set to 76 dB (SPL). During other conditions, the loudness was raised by an equal amount for all subjects to ensure that the stimuli were discriminable.
During the baseline condition, subjects were asked simply to focus on a fixation point on the screen while the stimuli were presented for 2 minutes and 42 seconds. During the listen and directed inner speech conditions, the first hallucinatory statement recorded by the subject earlier was played back repeatedly during a 30-second period, allowing about seven repetitions of the statement. Next, subjects repeated that same statement silently to themselves for 30 seconds. This sequence of listening and directed inner speech was repeated seven times, once for each of seven different statements, and lasted 7 minutes.
We report event-related potentials elicited by acoustic stimuli recorded at scalp sites Fz, Cza, and Cz and event-related potentials elicited by visual stimuli (checkerboards) at Pz. N1 was identified as the most negative peak between 75 and 200 msec. Methods for controlling eye activity and other artifacts are described elsewhere (3).
Results
N1 amplitude effects are illustrated in Figure 1. A four-way analysis of variance (ANOVA) of N1 amplitude assessed effects of group, condition, stimulus (/ba/, noise), and scalp site (Fz, Cza, Cz). N1 amplitudes in response to /ba/ were larger than those in response to noise (F=8.46, df=1, 28, p=0.007). The condition-by-group interaction indicated that the magnitude of the condition effect differed across groups (F=4.07, df=2, 56, p<0.03, with Greenhouse-Geisser correction), with condition being significant for both comparison subjects (F=33.44, df=2, 28, p<0.0001, with Greenhouse-Geisser correction) and patients (F=7.35, df=2, 28, p=0.004, with Greenhouse-Geisser correction). For comparison subjects, the N1 amplitude during the silent baseline condition was larger than during directed inner speech, which was larger than the amplitude during listening (Figure 1). In patients, the baseline N1 amplitude was not significantly larger than the amplitude during directed inner speech but was larger than the amplitude during listening (Figure 1). In addition, the baseline N1 amplitude was somewhat smaller in the patients than in the comparison subjects (F=3.62, df=1, 28, p=0.07), but the N1 amplitude for directed inner speech was equivalent in the two groups (F=0.10, df=1, 28, p=0.76), consistent with our hypothesis.
An ANOVA for N1 amplitude in response to the checkerboard did not approach significance for group, condition, or the interaction of group and condition.
None of the Spearman correlations between the N1 effect (i.e., N1 amplitude during the silent baseline condition minus the N1 amplitude during directed inner speech) and SAPS summary scores for hallucinations and delusions and BPRS scores for hallucinatory behavior and unusual thought content was significant.
Discussion
Using neurophysiological methods, we have demonstrated that directed inner speech engages the auditory cortex in comparison subjects, just as Shergill et al. (1) did by using functional magnetic resonance imaging methods. Further, we showed that the auditory cortex is similarly engaged in patients, even during a silent baseline condition, perhaps because of the nature of their spontaneous inner speech.
The reduction in the N1 amplitude in response to acoustic stimuli during directed inner speech compared to baseline seen in the comparison subjects was not seen in patients. The patients’ spontaneous verbal thoughts may differ in quantity or effect, through interactions of attention, acoustic interference, or corollary discharge. The effects of attention and interference on N1 amplitudes are probably too small to explain our results (3). However, if corollary discharge was not functioning properly in the patients with schizophrenia (4), auditory cortical responsiveness to inner speech might not have been dampened. Corollary discharge may also signal speech reception areas that speech-related activations are self-generated, preventing misperceptions that these thoughts have an external source.
The reduction in N1 amplitude during directed inner speech compared to listening was equivalent in the two groups. During those conditions, spontaneous thoughts may have been preempted by listening to the recording or by directed inner speech, rendering cortical resources equal in the two groups.
Shergill et al. (8) noted that areas engaged by directed inner speech are similar to those activated during auditory hallucinations (9). However, the lower N1 amplitude during baseline in patients was not related to the presence of auditory hallucinations or delusions during the week that the event-related potentials were recorded. Perhaps, this lower N1 amplitude reflects mechanisms underlying the potential to hallucinate and to have delusions rather than the current state of symptoms in medicated patients.
Received Feb. 7, 2001; revision received April 30, 2001; accepted May 3, 2001. From the Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine; the Psychiatry Service, Veterans Affairs Palo Alto Health Care System, Palo Alto, Calif.; the Department of Psychiatry, Yale University School of Medicine, New Haven, Conn.; and the Psychiatry Service, Veterans Affairs West Haven Health Care System, West Haven, Conn. Address reprint requests to Dr. Ford, Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, Stanford, CA 94305-5550; [email protected] (e-mail). Supported by NIMH grants MH-40052 and MH-58262 and by the Department of Veterans Affairs.
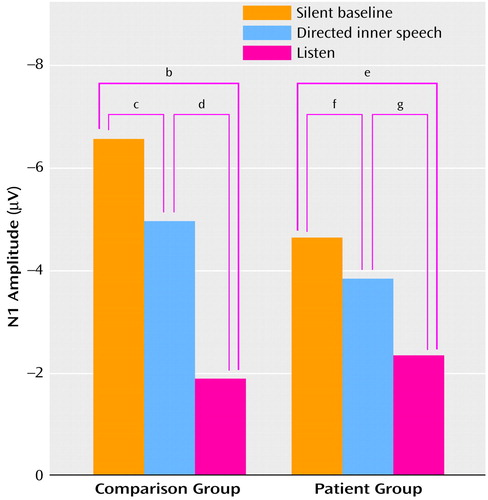
Figure 1. Mean Amplitudes of the N1 Component of the Event-Related Potential Elicited by Acoustic Stimuli in 15 Healthy Comparison Subjects and 15 Patients With Schizophrenia During Silent Baseline, Directed Inner Speech, and Listen Conditionsa
aN1 amplitudes were elicited by speech sounds and noise while the subjects sat in silence, while they repeated statements silently, and while they listed to recordings of statements in their own voice.
bF=47.84, df=1, 14, p<0.0001.
cF=9.64, df=1, 14, p=0.008.
dF=33.25, df=1, 14, p<0.0001.
eF=12.40, df=1, 14, p=0.003.
fF=2.51, df=1, 14, p=0.14.
gF=5.24, df=1, 14, p=0.04.
1. Shergill SS, Bullmore ET, Brammer MJ, Williams SC, Murray RM, McGuire PK: A functional study of auditory verbal imagery. Psychol Med 2001; 31:241-253Crossref, Medline, Google Scholar
2. Davis H, Zerlin S: Acoustic relations of the human vertex potential. J Acoust Soc Am 1966; 39:109-116Crossref, Medline, Google Scholar
3. Ford JM, Mathalon DH, Kalba S, Whitfield S, Faustman WO, Roth WT: Cortical responsiveness during talking and listening in schizophrenia: an event-related brain potential study. Biol Psychiatry (in press)Google Scholar
4. Feinberg I: Efference copy and corollary discharge: implications for thinking and its disorders. Schizophr Bull 1978; 4:636-640Crossref, Medline, Google Scholar
5. Frith CD: The positive and negative symptoms of schizophrenia reflect impairments in the perception and initiation of action. Psychol Med 1987; 17:631-648Crossref, Medline, Google Scholar
6. Overall JE, Gorham DR: The Brief Psychiatric Rating Scale. Psychol Rep 1962; 10:799-812Crossref, Google Scholar
7. Andreasen NC: Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, University of Iowa, 1984Google Scholar
8. Shergill SS, Bullmore E, Simmons A, Murray R, McGuire P: Functional anatomy of auditory verbal imagery in schizophrenic patients with auditory hallucinations. Am J Psychiatry 2000; 157:1691-1693Link, Google Scholar
9. Dierks T, Linden D, Jandl M, Formisano E, Goebel R, Lanfermann H, Singer W: Activation of Heschl’s gyrus during auditory hallucinations. Neuron 1999; 22:615-621Crossref, Medline, Google Scholar



