Fluoxetine for Bulimia Nervosa Following Poor Response to Psychotherapy
Abstract
OBJECTIVE: This was an investigation of whether treatment with fluoxetine is useful for individuals with bulimia nervosa who do not respond to psychotherapy or relapse afterward. METHOD: Twenty-two patients with bulimia nervosa who had not responded to, or had relapsed following, a course of cognitive behavior therapy or interpersonal psychotherapy were randomly assigned to receive placebo (N=9) or fluoxetine (60 mg/day, N=13) for 8 weeks. RESULTS: The median frequency of binge eating in the previous 28 days declined from 22 to four episodes in the fluoxetine group but increased from 15 to 18 episodes in the placebo group. Similarly, purging frequency in the previous 28 days declined from 30 to six episodes in the fluoxetine group but increased from 15 to 38 episodes in the placebo group. CONCLUSIONS: Fluoxetine may be a useful intervention for patients with bulimia nervosa who have not responded adequately to psychological treatment.
An extensive body of research in the last two decades has demonstrated that psychological treatment, particularly cognitive behavior therapy, is useful in the treatment of bulimia nervosa (1). The use of cognitive behavior therapy is associated with remission rates approaching 50% at the end of treatment, and this level of improvement is generally well maintained. One study (2) indicated that the response of bulimia nervosa to interpersonal psychotherapy may, over time, be comparable to that of cognitive behavior therapy. In that study, while interpersonal psychotherapy was inferior to cognitive behavior therapy at the conclusion of treatment, the outcomes of the two treatments at 1-year follow-up were similar. Despite the benefits of such interventions, a substantial number of patients remain symptomatic after psychological treatment, and others relapse after initial improvement. There is little systematic information on the response of such individuals to subsequent interventions.
The purpose of the current study was to determine the utility of a pharmacological intervention for patients whose response to psychological treatment was not satisfactory. Specifically, the current study compared fluoxetine to placebo for patients who had participated in a controlled comparison of cognitive behavior therapy and interpersonal psychotherapy for bulimia nervosa but who had not responded sufficiently or who had relapsed after the end of the psychological intervention.
Method
The patients in this study had previously participated in a controlled comparison of two forms of psychological treatment, cognitive behavior therapy and interpersonal psychotherapy, for bulimia nervosa (3). The comparison was conducted at two sites, Columbia University and Stanford University. At each site, 110 women who met the DSM-III-R criteria for bulimia nervosa and who used self-induced vomiting were randomly assigned to a course of either cognitive behavior therapy or interpersonal psychotherapy, each consisting of 19 individual sessions over 20 weeks. None of the patients received any other psychotherapy or pharmacotherapy during this time. Patients who participated in the comparison of cognitive behavior therapy and interpersonal psychotherapy but who continued to binge eat and to induce vomiting at least once weekly, on average, over 1 month were eligible to participate in the current study. Patients were permitted to enter the medication study at the conclusion of the first trial or during the succeeding 24 months; the average interval was 36 weeks.
The patients were randomly assigned to receive fluoxetine (60 mg/day) or placebo in double-blind fashion. They were seen weekly by a psychiatrist to assess response and side effects, but they received no formal psychological treatment.
The patients were assessed at the beginning and the conclusion of treatment by means of the Eating Disorder Examination, a structured interview of established reliability and validity (4). In addition, the patients completed the following self-report instruments at the beginning and conclusion of treatment: Beck Depression Inventory (5), Rosenberg Self-Esteem Scale (6), and Three-Factor Eating Questionnaire (7). The primary measures of outcome were the frequencies of binge eating and purging at the end of treatment, assessed with the Eating Disorder Examination, which evaluates the preceding 28-day period.
Data were analyzed by means of an analysis of covariance (ANCOVA). The dependent variable was the measure at the end of treatment, the covariate was the measure at baseline, and independent factors were drug (fluoxetine versus placebo) and site (Columbia versus Stanford). For the Beck Depression Inventory, an assumption required for using ANCOVA (parallel regression lines in the two groups between the dependent variable and the covariate) was not true; therefore, these data were analyzed by using a repeated measures analysis. Because the distributions of the frequencies of binge eating and purging were skewed, these data were square-root transformed before they were analyzed.
This study was approved by the appropriate institutional review boards of Columbia and Stanford Universities, and patients provided written informed consent before participating.
Results
Twenty-three patients began the study; 11 had received cognitive behavior therapy and 12 had received interpersonal psychotherapy. One patient at the Columbia site did not return after the baseline visit; her data were not included in the analyses. Of the remaining 13 patients at the Columbia site, seven were assigned to fluoxetine and six to placebo; at the Stanford site, six patients were assigned to fluoxetine and three to placebo. Twenty of the 22 patients completed the full 8-week trial.
The average ages of the patients in the fluoxetine and placebo groups were similar (32.0 years, SD=7.8, and 27.8 years, SD=5.2, respectively), as were the durations of binge eating (15.6 years, SD=8.9, and 13.9 years, SD=9.9). Two (15%) of the 13 patients in the fluoxetine group and two (22%) of the nine patients in the placebo group had past histories of anorexia nervosa. One patient in each group met criteria for both current major depressive disorder and current panic disorder. In addition, in the fluoxetine group, three patients met criteria for current major depressive disorder and one patient met criteria for current panic disorder.
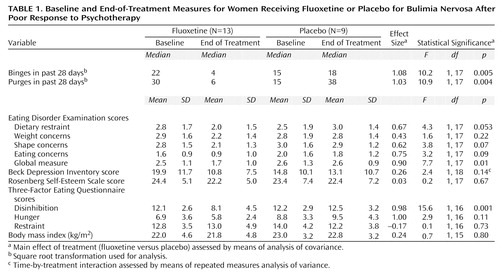
Table 1 summarizes the results. There were statistically significant drug-placebo differences at the end of treatment, favoring fluoxetine, in the frequency of objective binge-eating episodes, frequency of purging, global Eating Disorder Examination score, and Three-Factor Eating Questionnaire disinhibition score. Five (38%) of the 13 patients receiving fluoxetine reported no episodes of binge eating or purging during the last 28 days of the study, compared to none of the patients receiving placebo (Fisher’s exact test, p=0.054). There were significant site differences in the scores on the Eating Disorder Examination global measure and subscales for restraint, shape concerns, and eating concerns and on the Three-Factor Eating Questionnaire disinhibition measure (data not shown). On all of these measures, the mean scores of the patients at Columbia University were higher than those of the patients at Stanford. There were no site-by-treatment interactions.
Discussion
The results of this study extend previous data in demonstrating that fluoxetine is of benefit even to patients with bulimia nervosa who have not responded satisfactorily to state-of-the-art psychological treatment. In the 20 years since bulimia nervosa was recognized, relatively effective forms of treatment have been developed, but it is unclear how treatments should be sequenced and what strategies to pursue after an intervention has proved insufficient. This study suggests that pharmacotherapy may benefit some patients who do not respond satisfactorily to psychological treatment. In addition to its obvious clinical implications, this result has heuristic significance, in indicating that nonresponse to one mode of treatment does not necessarily imply nonresponse to another.
There are several limitations on our results. First, the study was of short duration (8 weeks), and it is unknown whether the benefits have persisted. Second, the number of patients was small, making it impossible for us to identify any potential predictors of pharmacological responsiveness. Finally, a number of eligible patients declined participation in this study because they found pharmacological treatment an unacceptable alternative, either because of concerns regarding side effects or because of a less specific reluctance to use medication for a behavioral problem. However, our results suggest that, for patients who remain symptomatic after a psychological treatment for bulimia nervosa and who are willing to consider a trial of medication, fluoxetine may be of benefit.
 |
Presented in part at the 152nd annual meeting of the American Psychiatric Association, Washington, D.C., May 15–20, 1999. Received Aug. 16, 1999; revision received Jan. 31, 2000; accepted March 2, 2000. From the Department of Psychiatry, College of Physicians and Surgeons, Columbia University, and the New York State Psychiatric Institute; and the Department of Psychiatry, Stanford University, Stanford, Calif. Address reprint requests to Dr. Walsh, Unit 98, New York State Psychiatric Institute, 1051 Riverside Dr., New York, NY 10032; [email protected] (e-mail).Supported in part by NIMH grant MH-49877 to Drs. Walsh and Agras, by Wellcome Principal Fellowship grant 046386 to Dr. Fairburn, and by Eli Lilly & Company.The authors thank Helena C. Kraemer, Ph.D., and Susan Bryson, M.A., M.S., for their assistance.
1. Wilson GT, Fairburn CG, Agras WS: Cognitive-behavioral therapy for bulimia nervosa, in Handbook of Treatment for Eating Disorders. Edited by Garner DM, Garfinkel PE. New York, Guilford, 1997, pp 67–93Google Scholar
2. Fairburn CG, Jones R, Peveler RC, Hope RA, O’Connor M: Psychotherapy and bulimia nervosa: longer-term effects of interpersonal psychotherapy, behavior therapy, and cognitive behavior therapy. Arch Gen Psychiatry 1993; 50:419–428Crossref, Medline, Google Scholar
3. Agras WS, Walsh BT, Fairburn CG, Wilson GT, Kraemer HC: A multicenter comparison of cognitive-behavioral therapy and interpersonal psychotherapy for bulimia nervosa. Arch Gen Psychiatry 2000; 57:459–466Crossref, Medline, Google Scholar
4. Cooper Z, Fairburn CG: The Eating Disorder Examination: a semi-structured interview for the assessment of the specific psychopathology of eating disorders. Int J Eating Disorders 1987; 6:1–8Crossref, Google Scholar
5. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J: An inventory for measuring depression. Arch Gen Psychiatry 1961; 4:561–571Crossref, Medline, Google Scholar
6. Rosenberg M. Conceiving the Self. New York, Basic Books, 1979Google Scholar
7. Stunkard AJ, Messick S: The three-factor eating questionnaire to measure dietary restraint, disinhibition, and hunger. J Psychosom Res 1985; 29:71–83Crossref, Medline, Google Scholar



