Novel Polymorphism in the Gene Region Encoding the Carboxyl-Terminal Intracellular Domain of the NMDA Receptor 2B Subunit: Analysis of Association With Schizophrenia
Abstract
OBJECTIVE: N-methyl-d-aspartate (NMDA) receptor antagonists are known to produce a syndrome resembling schizophrenia, probably due to their blockade of NMDA receptors. The NMDA receptor 2B (NR2B) subunit has been identified as one of the major proteins in the postsynaptic density at glutamatergic synapses, suggesting that the carboxyl-terminal domain of the NR2B subunit may play a significant role in intracellular signal transduction. METHOD: The authors screened for genetic variations in the region of the NR2B subunit gene encoding the carboxyl-terminal intracellular domain in patients with schizophrenia and studied the association between schizophrenia and a novel polymorphism of the NR2B subunit gene. RESULTS: One silent mutation (2664C/T) was identified. No significant differences in the frequencies of 2664C/T genotypes and alleles were found between patients with schizophrenia and healthy comparison subjects. CONCLUSIONS: The findings provided no evidence of an association between schizophrenia and the 2664C/T polymorphism of the NR2B subunit gene.
Twin, family, and adoption studies have consistently suggested a genetic contribution to the etiology of schizophrenia (1). Excitatory amino acid neurotransmission may be involved in the etiology of schizophrenia. The most compelling link between glutamatergic neurotransmission and schizophrenia is related to the mechanism of action of the psychotomimetic phencyclidine (PCP) (2). PCP and other N-methyl-d-aspartate (NMDA) receptor antagonists, such as ketamine and MK-801, produce a psychotic condition, probably due to their blockade of NMDA receptors (3). The ability of the NMDA receptor antagonists to induce a syndrome closely resembling schizophrenia suggests that dysfunction or dysregulation of NMDA receptor-mediated neurotransmission occurs in schizophrenia.
The NMDA receptor is thought to be formed from different combinations of the NMDA receptor 1 (NR1) subunit and one of the NMDA receptor 2 (NR2A-D) subunits. NMDA receptor heterogeneity may result from differential assembly of homologous NMDA receptor subunits. In the rat brain, the NR2B subunit was detected almost ubiquitously at birth, but during the first 3 postnatal weeks, the NR2B subunit became confined to anterior forebrain structures (4). Dysregulation in such transition of the distribution of the NR2B subunit in the brain may be involved in the etiology of schizophrenia, consistent with the neurodevelopmental hypothesis for the origins of the disorder (5).
The cloning of a cDNA encoding the human NR2B subunit has been described (6). The NR2B subunit is equipped with an unusually long calboxyl-terminal domain that is believed to extend into the cytoplasm. The NR2B subunit is the major tyrosine-phosphorylation protein in the postsynaptic density fraction (7), which contains enriched signal-transduction molecules regulating receptor localization and function in the brain. For example, a-actinin-2, a member of the actin-binding group of proteins, binds to the cytoplasmic tail of the NR2B subunit (8). A major phosphorylation site of the NR2B subunit by Ca2+/calmodulin-dependent protein kinase II has been located in the carboxyl-terminal domain (9). These lines of evidence suggest that the intracellular carboxyl-terminal domain of the NR2B subunit plays a crucial role in cellular signal transduction.
In the study reported here, we screened for genetic variations in the human NR2B subunit encoding the carboxyl-terminal intracellular domain, which might contribute to schizophrenia. To investigate a possible contribution of this polymorphism to the etiology of schizophrenia, we compared frequencies of genetic variations in unrelated patients with schizophrenia and a healthy comparison group.
Method
This study was approved by the ethics committee of Kobe University School of Medicine. Written informed consent was obtained from all subjects. The study group consisted of 164 unrelated Japanese patients who met DSM-IV criteria for schizophrenia (105 men and 59 women; mean age=51.5 years, SD=14.1). The comparison group consisted of 171 unrelated healthy volunteers (104 men and 67 women; mean age=47.8 years, SD=14.0).
The deduced amino acid sequence of the human NR2B subunit gene has an overall identity of 90% with the reported mouse sequence (10). Adopting the gene structure topology proposed for the region encoding the carboxyl-terminal domain of the mouse NMDA receptor e3 subunit (NR2C subunit in human) (11), which consists of a small-sized exon (26 amino acids) and a large-sized exon (378 amino acids), that region of the human NR2B subunit appears to consist mostly of a large-sized exon. Seven sets of polymerase chain reaction primers were prepared to produce approximately 300 base-pair overlapping fragments covering the complete deduced large-sized exon. Standard polymerase chain reaction was performed, and 22 patients with schizophrenia were screened by using single-strand conformation polymorphism analysis. The DNA fragments displaying a different pattern during single-strand conformation polymorphism analysis were subjected to direct sequencing. An endonuclease Aci I was used to confirm a mutation identified by polymerase chain reaction direct sequencing and to carry out an analysis of the presence of the mutation in the patients with schizophrenia and in the comparison group.
Differences in the allele and genotype frequencies between the patients with schizophrenia and the comparison group were tested for significance using the chi-square test.
Results
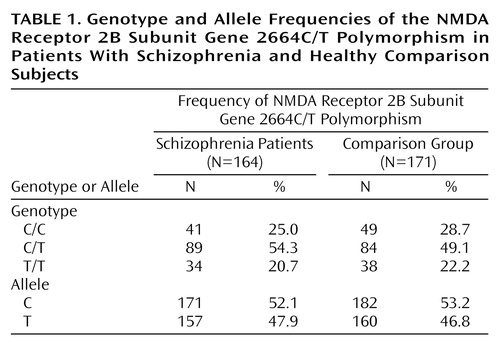
The fragment size of each polymerase chain reaction product obtained by the procedure mentioned above coincided with the expected size. We examined the mutations in the large-sized exon encoding the carboxyl-terminal intracellular domain of the human NR2B subunit. One point mutation was identified by single-strand conformation polymorphism and sequence analysis. This mutation was located at cDNA nucleotide position 2664, and was silent, changing codon 888 from ACC to ACT. We failed to detect other mutations that changed the amino acid sequence in the examined region. Table 1 shows the distribution of genotype and allele frequencies of the 2664C/T polymorphism for the patients with schizophrenia and the comparison group. The genotype and allele frequencies in the patients with schizophrenia did not differ from those in the comparison group (genotype, χ2=0.93, df=2, p>0.10; allele, χ2=0.08, df=1, p>0.10).
Discussion
We found one nucleotide sequence variant (2664C/T) in the region encoding the carboxyl-terminal intracellular domain of the NR2B subunit. This report is the first we are aware of to describe a polymorphism in the human NR2B subunit gene.
Based on our data indicating no association between schizophrenia and the 2664C/T polymorphism, it is unlikely that the human NR2B subunit gene contributes to the etiology of schizophrenia. However, NMDA receptors mediate neuronal signaling, affect neuronal gene expression, and are involved in neuronal plasticity, outgrowth, and survival. Especially, the human NR2B mRNA distribution is restricted to the frontoparietotemporal cortex and hippocampus (10), which are the sites that are probably affected in schizophrenia. Therefore, the NR2B gene is still a possible candidate for association with schizophrenia. There may be polymorphisms in the NR2B subunit gene that cause alternations in protein function, and such alternations in function could contribute to the etiology of schizophrenia. Further attempts are needed to find genetic markers and/or functional mutations at sites of the NR2B subunit gene other than the region encoding the carboxyl-terminal intracellular domain and to determine whether such polymorphisms, if they exist, are associated with schizophrenia.
 |
Received Oct. 7, 1999; revision received Dec. 27, 1999; accepted Dec. 30, 1999. From the Department of Psychiatry and Neurology, Kobe University School of Medicine. Address reprint requests to Dr. Shirakawa, Department of Psychiatry and Neurology, Kobe University School of Medicine, 7-5-1 Kusunoki-cho Chuo-ku, Kobe 650-0017, Japan; [email protected] (e-mail).Supported by a Grant-in Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture; a Research Grant for Nervous and Mental Disorders from the Ministry of Heath and Welfare; and the Medical Research Fund of Hyogo Medical Association.
1. Gottesman II: Schizophrenia epigenesis: past, present, and future. Acta Psychiatr Scand Suppl 1994; 90:26–33Crossref, Google Scholar
2. Halberstadt AL: The phencyclidine-glutamate model of schizophrenia. Clin Neuropharmacol 1995; 18:237–249Crossref, Medline, Google Scholar
3. Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremnen JD, Heninger GR, Bowers MB Jr, Charney DS: Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans: psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 1994; 51:199–214Crossref, Medline, Google Scholar
4. Wenzel A, Fritschy JM, Mohler H, Benkel D: NMDA receptor heterogeneity during postnatal development of the rat brain: differential expression of the NR2A, NR2B, and NR2C subunit proteins. J Neurochem 1997; 68:469–478Crossref, Medline, Google Scholar
5. Weinberger DR: Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry 1987; 44:660–669Crossref, Medline, Google Scholar
6. Hess SD, Daggett LP, Crona J, Deal C, Lu C-C, Urrutia A, Chavez-Noriega L, Ellis SB, Johnson EC, Veliçelebi G: Cloning and functional characterization of human heteromeric N-methyl-d-aspartate receptors. J Pharmacol Exp Ther 1996; 278:808–816Medline, Google Scholar
7. Moon IS, Apperson ML, Kennedy MB: The major tyrosin-phosphorylated protein in the postsynaptic density fraction in N-methyl-d-aspartate receptor subunit 2B. Proc Natl Acad Sci USA 1994; 91:3954–3958Google Scholar
8. Wyszynski M, Lin J, Rao A, Nigh E, Beggs AH, Craig AM, Sheng M: Competitive binding of a-actin and calmodulin to the NMDA receptor. Nature 1997; 385:439–442Crossref, Medline, Google Scholar
9. Omukumar RV, Kiely MJ, Rosenstein AJ, Min KT, Kennedy MB: Identification of a phosphorylation site for calcium/calmodulin-dependent protein kinase II in the NR2B subunit of the N-methyl-d-aspartate receptor. J Biol Chem 1996; 271:31670–31678Google Scholar
10. Schito AM, Pizzuti A, Di Maria E, Schenone A, Ratti A, Defferrari R, Bellone E, Mancardi GL, Ajmar F, Mandich P: mRNA distribution in adult human brain of GRIN2B, a N-methyl-d-aspartate (NMDA) receptor subunit. Neurosci Lett 1997; 239:49–53Crossref, Medline, Google Scholar
11. Nagasawa M, Sakimura K, Mori KJ, Bedell MA, Copeland NG, Jenkins NA, Mishina M: Gene structure and chromosomal localization of the mouse NMDA receptor channel subunits. Mol Brain Res 1996; 36:1–11Crossref, Medline, Google Scholar



