Low GSK-3β Immunoreactivity in Postmortem Frontal Cortex of Schizophrenic Patients
Abstract
OBJECTIVE: Glycogen synthase kinase-3 (GSK-3) is a protein kinase that is highly abundant in the brain. It is involved in signal transduction cascades of multiple cellular processes, particularly neurodevelopment. In an attempt to explore possible involvement of GSK-3β in psychiatric disorders, the authors examined its levels in postmortem brain tissue.METHOD: Western blot analysis was performed to measure GSK-3β in the frontal cortex of 14 schizophrenic patients, 15 patients with bipolar disorder, 15 patients with unipolar depression, and 14 normal comparison subjects.RESULTS: GSK-3β levels were 41% lower in the schizophrenic patients than in the comparison subjects. Other diagnostic groups did not differ from the comparison subjects.CONCLUSIONS: These results are consistent with the notion that schizophrenia involves neurodevelopmental pathology. It remains to be investigated whether the active fraction of GSK-3β, or its activity, is also low in frontal cortex of schizophrenic patients and if this is also reflected in other brain regions.
Glycogen synthase kinase-3 (GSK-3) is a highly conserved serine/threonine protein kinase that is involved in the signal transduction cascade of multiple cellular processes. GSK-3 is a juncture of at least three signal transduction cascades—the mitogen-activated protein kinase cascade (1), the phosphatidylinositol 3-kinase cascade (2), and the Wnt cascade, which plays a role in modulation of cell fate during development (3). Recently, GSK-3β has been identified as a potent kinase highly abundant in brain tissue (4) that apparently regulates diverse brain proteins, including microtubules (5), myelin basic protein (6), nerve growth factor (7), and neurofilaments (8). GSK-3β is also a potent tau kinase capable of phosphorylating tau on sites that are abnormally phosphorylated in Alzheimer’s disease brain and responsible for reducing microtubule binding involved in neuronal degradation (9). Lithium has been found to be an inhibitor of GSK-3β, with an inhibition constant within the therapeutic concentration range (10, 11). We were therefore interested in the possibility that GSK-3β might be elevated in patients with bipolar disorder. Because of theories that posit schizophrenia as a disorder of neurodevelopment (12), we were also intrigued by the possibility that GSK-3β levels might be abnormal in this disease.
Method
Frozen postmortem brain samples of frontal cortex (Brodmann’s area 10) were obtained from the Stanley Foundation Brain Bank. The 58 samples were taken from 14 schizophrenic patients, 15 patients with bipolar disorder, 15 with unipolar depression, and 14 normal comparison subjects. None of the comparison subjects had had a history of psychiatric disorder or had received antipsychotic medication, nor did any die as a result of suicide or a neurological disorder. The four groups were matched for age, sex, race, postmortem interval, and side of brain. The 14 schizophrenic patients (eight men and six women) were a mean age of 44.2 years (range=25–62), with a mean postmortem interval of 33.7 hours (range=12–61); 12 of these subjects were white, two were Asian. The 15 patients with bipolar disorder (nine men and six women) were a mean age of 42.3 years (range=25–61), with a mean postmortem interval of 32.5 hours (range=13–62); 14 of these subjects were white, one was black. The 15 patients with unipolar depression (nine men and six women) were a mean age of 46.5 years (range=30–65), with a mean postmortem interval of 27.5 hours (range=7–47); all were white. Finally, the 14 normal comparison subjects (eight men and six women) were a mean age of 48.1 years (range=29–68), with a mean postmortem interval of 23.7 hours (range=8–42); 13 of these subjects were white, one was black. The investigator who carried out the assays (N.K.) was blind to diagnosis and received specimens in a balanced way so that each run included matched samples, one from each of the four diagnostic groups. The study was approved by our hospital’s institutional review board.
We assessed postmortem stability of GSK-3β immunoreactivity by assessing GSK-3β in rat brain. Rats were sacrificed and left at room temperature for 0, 0.5, 2, 4, 8, 16, 24 and 48 hours (two rats per each time point, each assayed at least three times). Frontal cortex tissue was then frozen at –70˚C until assayed for GSK-3β immunoreactivity.
Sodium dodecyl sulfate/polyacrylamide gel electrophoretic separation and immunoblotting of GSK-3β were performed by means of a previously described procedure (13) with modifications. Briefly, samples (25–50 mg) of postmortem frontal cortex were sonicated for 10 seconds in 50% power capacity (Heat Systems Ultrasonic Inc.), and 0.1% (vol/vol) nonident P-40 was added. The cell lysate was diluted to 1 mg total protein/ml, and aliquots were stored at –20˚C until assayed. Aliquots of 9 and 18 ml (0.3 and 0.6 mg total protein) were separated, blotted, and probed for 16–18 hours at 4˚C with diluted (1:6000) anti-GSK-3β antibodies (Transduction Laboratories, Lexington, Ky.). Bands were detected with Chemiluminescence Western blot detection kit (Amersham, Oakville, Ont.). Densities of the immunoreactive bands were quantified using AIDA-2D image analysis system (Dinco and Rhenium Marketing, Jerusalem).
To minimize the effect of interblot variability, a calibration standard curve of known amounts of recombinant GSK-3β units (Upstate Biotechnology, Lake Placid, N.Y.) was run in each gel. The correlation coefficient of the standard curves was always higher than 0.9. Furthermore, each sample was analyzed at least three times and at two different protein concentrations, both within the linear range of detection. Thus, the absolute GSK-3β values (in units) of each band were derived from the standard curve run on the same gel. The mean value for each sample was calculated from all of the replicates. Statistical analyses of the data were preformed by the software STATISTICA for Windows.
Results
A single immunoreactive band of GSK-3β in the human brain frontal cortex (46 kDa) was confirmed. As seen in Figure 1, the schizophrenic patients had GSK-3β levels that were 41% lower than those of normal subjects (analysis of variance [ANOVA] F=3.09, df=3, 54, p=0.03; post hoc least significant difference, p=0.008).
There was no correlation between GSK-3β and age (r=0.11, df=56, n.s.) or postmortem interval (r=0.0002, df=56, n.s.). One-way ANOVA for the eight time points from 0 to 48 hours showed that postmortem interval did not have a significant effect on rat prefrontal cortex GSK-3β levels (F=1.2, df=7, 42, p=0.32). Values at 48 hours were 19% lower than those at time zero (post hoc least significant difference, p=0.44). There was no correlation between GSK-3β levels in the schizophrenic patients and their estimated lifetime antipsychotic consumption in chlorpromazine equivalents. Two-way ANOVAs revealed GSK-3β levels in the frontal cortex were lower in female than in male subjects (for diagnosis: F=3.37, df=3, 50, p=0.03; for sex: F=4.24, df=1, 50, p=0.04; for diagnosis-by-sex interaction: F=0.80, df=3, 50, p=0.50).
Discussion
The lower levels of GSK-3β found in our study seem specific for the patients with schizophrenia, since no changes were found in the levels of GSK-3β in patients with bipolar disorder or unipolar depression. In view of the fact that lithium inhibits GSK-3β (10, 11), it may seem surprising that GSK-3β is not altered in patients with bipolar disorder; however, the site of psychopharmacologic action of lithium is not necessarily the site of etiology for bipolar illness. The present study, supported by the previous lymphocyte findings of Yang et al. (14) and the recent report of Miyaoka et al. (15) of increased expression of Wnt-1 in the hippocampus of schizophrenic patients, suggest that alterations of neurodevelopment-linked proteins are markers of schizophrenia.
One of the multiple substrates of GSK-3, b-catenin, mediates signal transduction effects of this enzyme, particularly within the Wnt signaling cascade (3). By binding with cadherins b-catenin regulates cell adhesion and affects intracellular junctions such as synapses (16). Cotter et al. (17) recently found reduced immunohistochemically stained intraneuronal b- and g-catenin in the hippocampal subregions CA3 and CA4 of schizophrenic subjects. Indeed, schizophrenia has been shown to involve developmental brain changes reflected by abnormal neuronal lamination and synaptic organization (17, 18). Marker proteins that correlate with neurodevelopmental pathology have been reported in schizophrenia, e.g., lower mRNA levels of synaptophysin (19) and higher levels of growth-associated protein GAP-43 (20). Aberrant signal transduction as an underlying mechanism of schizophrenia has also been previously postulated (21).
The constitutively active form of GSK-3β is phosphorylated at the 216-tyrosine residue, while the inactivated form is phosphorylated at serine-9 residue (22). The anti-GSK-3β antibodies used in the present study do not discriminate between the active and the inactive forms; therefore, only total enzyme protein levels are quantitated.
The factors regulating total GSK-3 levels in the brain and other tissues are not known. Reduced total GSK-3β protein levels cannot be directly interpreted in terms of downstream effects, since the active fraction of the enzyme is the relevant one for such consideration. Therefore, it remains to be investigated whether the active fraction of GSK-3β, or its activity, is also low in frontal cortex of schizophrenic patients, and if this is also reflected in other brain regions.
Received July 22, 1999, revision received Nov. 29, 1999, accepted Dec. 1, 1999. From the Faculty of Health Sciences, Beersheva Mental Health Center, Ben-Gurion University. Reprints are not available. Address correspondence to Dr. Agam, Psychiatry Research Unit and Department of Clinical Biochemistry, Faculty of Health Sciences, Beersheva Mental Health Center, Ben-Gurion University of the Negev, P.O. Box 4600, Beersheva 84105, Israel; [email protected] (e-mail). Supported in part by a Leah Smith Postdoctoral Fellowship from the Israeli National Institute for Psychobiology (Dr. Kozlovsky). Postmortem brains were donated by the Stanley Foundation Brain Consortium courtesy of Drs. Llewellyn B. Bigelow, Juraj Cervenak, Mary M. Herman, Thomas M. Hyde, Joel E. Kleinman, Jose D. Paltan, Robert M. Post, E. Fuller Torrey, Maree J. Webster, and Robert H. Yolken.
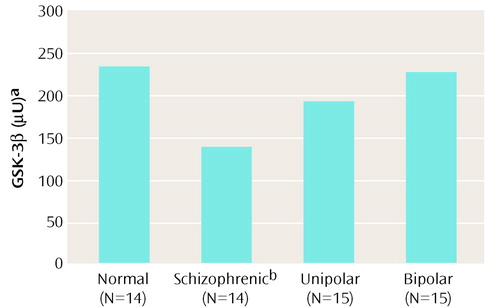
Figure 1. Mean Glycogen Synthase Kinase-3 (GSK-3β) Levels in Postmortem Frontal Cortex of Patients With Schizophrenia, Bipolar Disorder, or Unipolar Depression and of Normal Comparison Subjects
aTranslation of the densitometric immunoreactive levels into absolute units was carried out through derivation of values taken from the calibration standard curve of known amounts of recombinant GSK-3β units run in each gel, with the mean value of each group calculated from all the replicates.
bMean GSK-3β level significantly lower than that of normal comparison subjects.
1. Pelech SL, Charest DL: MAP kinase-dependent pathways in cell cycle control. Prog Cell Cycle Res 1995; 1:33–52Crossref, Medline, Google Scholar
2. Shaw M, Cohen P, Alessi DR: The activation of protein kinase B by H2O2 or heat shock is mediated by phosphoinositide 3-kinase and not by mitogen-activated protein kinase-activated protein kinase-2. Biochem J 1998; 336(part 1):241–246Google Scholar
3. Papkoff J, Aikawa M: WNT-1 and HGF regulate GSK3 beta activity and beta-catenin signaling in mammary epithelial cells. Biochem Biophys Res Commun 1998; 247:851–858Crossref, Medline, Google Scholar
4. Woodgett JR: Molecular cloning and expression of glycogen synthase kinase-3/factor A. Embo J 1990; 9:2431–2438Google Scholar
5. Singh TJ, Grundke-Iqbal I, Iqbal K: Differential phosphorylation of human tau isoforms containing three repeats by several protein kinases. Arch Biochem Biophys 1996; 328:43–50Crossref, Medline, Google Scholar
6. Yang SD: Identification of the ATP: Mg-dependent protein phosphatase activator (FA) as a myelin basic protein kinase in the brain. J Biol Chem 1986; 261:11786–11791Google Scholar
7. Taniuchi M, Johnson EM Jr, Roach PJ, Lawrence JC Jr: Phosphorylation of nerve growth factor receptor proteins in sympathetic neurons and PC12 cells: in vitro phosphorylation by the cAMP-independent protein kinase FA/GSK-3. J Biol Chem 1986; 261:13342–13349Google Scholar
8. Guan RJ, Khatra BS, Cohlberg JA: Phosphorylation of bovine neurofilament proteins by protein kinase FA (glycogen synthase kinase 3). J Biol Chem 1991; 266:8262–8267Google Scholar
9. Pei JJ, Tanaka T, Tung YC, Braak E, Iqbal K, Grundke-Iqbal I: Distribution, levels, and activity of glycogen synthase kinase-3 in the Alzheimer disease brain. J Neuropathol Exp Neurol 1997; 56:70–78Crossref, Medline, Google Scholar
10. Stambolic V, Ruel L, Woodgett JR: Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol 1996; 6:1664–1668; correction, 1997; 7:196Google Scholar
11. Klein PS, Melton DA: A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci USA 1996; 93:8455–8459Google Scholar
12. Weinberger DR: The biological basis of schizophrenia: new directions. J Clin Psychiatry 1997; 58(suppl 10):22–27Google Scholar
13. Mathews R, Li PP, Young LT, Kish SJ, Warsh JJ: Increased G alpha q/11 immunoreactivity in postmortem occipital cortex from patients with bipolar affective disorder. Biol Psychiatry 1997; 41:649–656Crossref, Medline, Google Scholar
14. Yang SD, Yu JS, Lee TT, Yang CC, Ni MH, Yang YY: Dysfunction of protein kinase FA/GSK-3 alpha in lymphocytes of patients with schizophrenic disorder. J Cell Biochem 1995; 59:108–116Crossref, Medline, Google Scholar
15. Miyaoka T, Seno H, Ishino H: Increased expression of Wnt-1 in schizophrenic brains. Schizophr Res 1999; 38:1–6Crossref, Medline, Google Scholar
16. Uchida N, Honjo Y, Johnson KR, Wheelock MJ, Takeichi M: The catenin/cadherin adhesion system is localized in synaptic junctions bordering transmitter release zones. J Cell Biol 1996; 135:767–779Crossref, Medline, Google Scholar
17. Cotter D, Kerwin R, al-Sarraji S, Brion JP, Chadwich A, Lovestone S, Anderton B, Everall I: Abnormalities of Wnt signalling in schizophrenia—evidence for neurodevelopmental abnormality. Neuroreport 1998; 9:1379–1383Google Scholar
18. Arnold SE, Trojanowski JQ: Recent advances in defining the neuropathology of schizophrenia. Acta Neuropathol (Berl) 1996; 92:217–231Crossref, Medline, Google Scholar
19. Eastwood SL, Burnet PW, Harrison PJ: Altered synaptophysin expression as a marker of synaptic pathology in schizophrenia. Neuroscience 1995; 66:309–319Crossref, Medline, Google Scholar
20. Perrone-Bizzozero NI, Sower AC, Bird ED, Benowitz LI, Ivins KJ, Neve RL: Levels of the growth-associated protein GAP-43 are selectively increased in association cortices in schizophrenia. Proc Natl Acad Sci USA 1996; 93:14182–14187Google Scholar
21. Hudson C, Young T, Li P, Warsh J: CNS signal transduction in the pathophysiology and pharmacotherapy of affective disorders and schizophrenia. Synapse 1993; 13:278–293Crossref, Medline, Google Scholar
22. Murai H, Okazaki M, Kikuchi A: Tyrosine dephosphorylation of glycogen synthase kinase-3 is involved in its extracellular signal-dependent inactivation. FEBS Lett 1996; 392:153–160Crossref, Medline, Google Scholar



