Response to Flumazenil in Women With Premenstrual Dysphoric Disorder
Abstract
OBJECTIVE: The authors sought to determine whether the administration of flumazenil would induce marked panic symptoms in women suffering from premenstrual dysphoric disorder.METHOD: Ten women with premenstrual dysphoric disorder and 11 comparison subjects were injected with flumazenil or placebo in a double-blind, randomized, balanced crossover design in a single session in the luteal phase of their menstrual cycles.RESULTS: Flumazenil induced a much greater panic response in the women with premenstrual dysphoric disorder than in the comparison subjects.CONCLUSIONS: These preliminary results are consistent with a dysregulation of the g-aminobutyric acid A/benzodiazepine receptor complex during the premenstruum of women suffering from premenstrual dysphoric disorder.
Methods for studying g-aminobutyric acid A (GABAA)/benzodiazepine receptor function in humans include intravenous injections with the benzodiazepine site antagonist flumazenil. The administration of flumazenil to normal comparison subjects has little or no effect. The induction of panic symptoms by the introduction of flumazenil (as described in patients with panic disorder) is suggestive of inverse agonist activity and has been interpreted as evidence for altered GABAA/benzodiazepine receptor sensitivity (1). Dysfunction of the central GABAA/benzodiazepine receptor has previously been suggested in women with premenstrual dysphoric disorder. Sundstrom et al. (2) found reduced sensitivity to the effects of diazepam, a benzodiazepine agonist, on saccadic eye movement and subjective sedation, particularly during the late luteal phase of the menstrual cycle in women with premenstrual dysphoric disorder. Abnormal plasma GABA levels have also been described in premenstrual women with dysphoric disorder, although their relevance to brain GABA is uncertain (3).
In the context of symptoms related to the menstrual cycle, it is worth noticing that GABA neurotransmission is influenced by progesterone metabolites. For example, allopregnanolone, a progesterone derivative, for which levels drop abruptly during the late luteal phase of the menstrual cycle, displays agonist and anxiolytic activity at the GABAA/benzodiazepine receptor level. GABAA/benzodiazepine receptor dysfunction in women with premenstrual dysphoric disorder would be more strongly implicated if there was also a change in response to flumazenil injection. We therefore hypothesized that the administration of flumazenil during the late luteal phase of the menstrual cycle in women with premenstrual dysphoric disorder would induce a greater panic response than in comparison subjects.
Method
Eleven healthy female volunteers (mean age=31 years, SD=10) and 10 women with premenstrual dysphoric disorder (mean age=34, SD=6) were recruited by means of advertisements. Written informed consent was obtained from the subjects after they had been given a full explanation of the proposed procedure. Comparison subjects had no lifetime axis I psychiatric disorders (4). The women with premenstrual dysphoric disorder were free of other current axis I psychiatric disorders. No subjects had a first-degree relative with panic disorder. Two women with premenstrual dysphoric disorder had a history of major depression, one of whom had also had posttraumatic stress disorder 13 years before this study. Because of the prevalence of depression in women with premenstrual dysphoric disorder, a history of depression was not an exclusion criterion, provided that the last episode had remitted at least 2 years previously.
The women with premenstrual dysphoric disorder were ascertained to have met the DSM-IV criteria for that disorder by prospective monitoring of three completed menstrual cycles by means of a modified Prospective Record of the Impact and the Severity of Menstrual Symptomatology (5) and a 100-mm visual analog scale. The subjects completed the calendar from the Prospective Record of the Impact and the Severity of Menstrual Symptomatology throughout each menstrual cycle. A 100-mm visual analog scale was completed 7–10 days after the onset of menses and 1–5 days before the onset of the next menses. The cyclicity and severity of menstrual symptoms were verified by comparing 100-mm visual analog scale ratings during the follicular and late luteal phases of the menstrual cycle. Affect cyclicity was ensured by a within-cycle (follicular to late luteal phase) increase of at least 50% in three of four menstrually related mood symptoms (tension, dysphoria, mood lability, or irritability) or a 100% increase in the severity of one of these symptoms. For the increase in severity to be considered clinically meaningful, the severity of the menstrual symptoms had to be rated as greater than 40 mm on the 100-mm visual analog scale during the late luteal phase and less than 20 mm on the 100-mm visual analog scale during the follicular phase. These measurements were required for at least two of the three menstrual cycles. All of the women with premenstrual dysphoric disorder also met the National Institute of Mental Health’s criteria for premenstrual syndrome (6). All subjects were physically healthy; none took psychotropic medication from 3 months before the study and until the end of the study.
All subjects received flumazenil and placebo injections 45 minutes apart, in random order, in a single session during the late luteal phase of each subject’s menstrual cycle (2–5 days before the menses). Five women with premenstrual dysphoric disorder and five comparison subjects received a flumazenil injection first; five women with premenstrual dysphoric disorder and six comparison subjects received a placebo injection first. The subjects sat in a reclining chair, and an intravenous catheter was placed in an antecubital vein, administering a slow normal saline drip. Flumazenil (2 mg) or normal saline placebo were administered as 20-ml intravenous infusions over 60 seconds.
Behavioral responses to flumazenil were assessed with the Panic Symptom Scale (7). Subjects rated the peak change in intensity of 18 DSM-IV panic symptoms on a scale of 0 (“not present”) to 4 (“extremely severe”). The Panic Symptom Scale provides a score for the increase from baseline in panic symptom intensity. To detect overreporting, we included four irrelevant symptoms in the Panic Symptom Scale (earache, itchy nose, back pain, and itchy feet), from which we derived an erroneous panic symptom score.
An analysis of variance with repeated measures was used to assess scores on the Panic Symptom Scale after placebo and flumazenil injections in women with premenstrual dysphoric disorder and comparison subjects. The variables were also compared cross-sectionally, by two-tailed t tests within “treatments” and by paired t tests within each “diagnosis.” Statistical inferences were based on a significance level of p<0.05.
Results
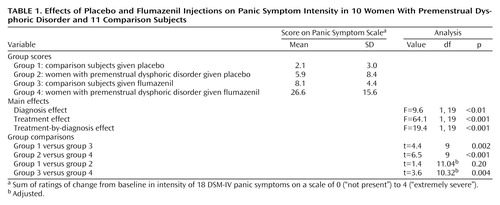
Mean values, multiple comparison results, and main effects for diagnosis, treatment, and diagnosis by treatment for scores on the Panic Symptom Scale are presented in Table 1. Effects from the order of injections are not presented here because statistical analysis failed to detect any influence. There were no statistically significant diagnosis (F=2.5, df=1, 19, p=0.13), treatment (F=2.5, df=1, 19, p=0.13), or diagnosis-by-treatment (F=2.5, df=1, 19, p=0.13) effects for erroneous panic symptom scores.
Discussion
This study shows that during the late luteal phase of the menstrual cycle, flumazenil induces marked panic symptoms in women with premenstrual dysphoric disorder but not in comparison subjects. One proposed explanation of such an altered response to flumazenil—that there might be a shift in benzodiazepine sensitivity toward inverse agonism (1)—is consistent with previous findings of a reduced sensitivity to diazepam in women with premenstrual dysphoric disorder (2). In this scenario, flumazenil, the benzodiazepine antagonist, becomes a partial inverse agonist, which would explain its panicogenic activity. It is interesting to note that our results contradict those of the proposed progesterone-withdrawal animal model for premenstrual syndrome (8). In this model, flumazenil displays an agonist activity at the GABAA/benzodiazepine receptor.
Taking into account that women with premenstrual dysphoric disorder are also more sensitive than comparison subjects to the panicogenic activity of various other neurochemical agents (9), a cognitive factor that may promote symptom amplification must be considered. Such a phenomenon is unlikely to explain our results since flumazenil induces very few symptoms in healthy volunteers. The primary biological interpretation of our data is further supported by the fact that patients with premenstrual dysphoric disorder did not overreport the irrelevant symptoms included in our Panic Symptom Scale.
In summary, this study suggests the possibility of a dysregulation of GABAA/benzodiazepine receptor function in women with premenstrual dysphoric disorder. This conclusion could be lessened in importance because of the spectrum of neurochemical agents that provokes panic symptoms in women with premenstrual dysphoric disorder (9). To assess the clinical relevance of our findings, it will be critical to investigate whether the observed panicogenic activity of flumazenil in women with premenstrual dysphoric disorder is greater during the symptomatic late luteal phase than during the asymptomatic mid-follicular phase of the menstrual cycle.
 |
Presented in part at the 36th annual meeting of the American College of Neuropsychopharmacology, Waikoloa, Hawaii, December 8–12, 1997. Received Dec. 15, 1997; revisions received Sept. 21 and Nov. 1, 1999; accepted Dec. 10, 1999. From the Clinical Investigation Unit, Department of Psychiatry, University of Alberta. Address reprint requests to Dr. Le Mellédo, Department of Psychiatry, University of Alberta, 1E7.26 Mackenzie Centre, Edmonton, Alberta T6G 2B7, Canada; [email protected] (e-mail). Supported by grant MA 49 893 from the Medical Research Council of Canada to Dr. Le Mellédo; and the research fund of the University of Alberta’s Psychopharmacological Research Unit. Studentships for Ms. Van Driel and Ms. Lott supported by the Alberta Heritage Foundation for Medical Research. The authors thank registered nurses Linda Broenink, Sue Miller, Tina Tolvay, and Lori Zuk of the Clinical Investigation Unit and Linda Hein of the Department of Psychiatry for their help.
1. Nutt DJ, Glue P, Lawson CW, Wilson S: Flumazenil provocation of panic attacks: evidence for altered benzodiazepine sensitivity in panic disorder. Arch Gen Psychiatry 1990; 47:917–925Crossref, Medline, Google Scholar
2. Sundstrom I, Ashbrook D, Backstrom T: Reduced benzodiazepine sensitivity in patients with premenstrual syndrome: a pilot study. Psychoneuroendocrinology 1997; 22:25–38Crossref, Medline, Google Scholar
3. Halbreich U, Petty F, Yonkers K, Kramer K, Rush AJ, Bibi KW: Low plasma g-aminobutyric acid levels during the late luteal phase of women with premenstrual dysphoric disorder. Am J Psychiatry 1996; 153:718–720Link, Google Scholar
4. Spitzer RL, Williams JBW, Gibbon M: Structured Clinical Interview for DSM-IV (SCID). New York, New York State Psychiatric Institute, Biometrics Research, 1995Google Scholar
5. Reid RL, Robinson GE: Premenstrual syndrome. Curr Obstet Gynecol Fertil 1985; 8:1–57Google Scholar
6. Anderson M, Severino SK, Hurt SW, Williams NA: Premenstrual syndrome research: using the NIHM guidelines. J Clin Psychiatry 1988; 49:484–486Medline, Google Scholar
7. Bradwejn J, Koszycki D, Shriqui C: Enhanced sensitivity to cholecystokinin tetrapeptide in panic disorder: clinical and behavioral findings. Arch Gen Psychiatry 1991; 48:603–610Crossref, Medline, Google Scholar
8. Smith SS, Gong QH, Hsu F-C, Markowitz RS, ffrench-Mullen JMH, Li X: GABAA receptor a4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature 1998; 392:926–930Crossref, Medline, Google Scholar
9. Yonkers KA: Anxiety symptoms and anxiety disorders: how are they related to premenstrual disorders? J Clin Psychiatry 1997; 58(suppl):62–69Google Scholar



