Divalproex Treatment for Youth With Explosive Temper and Mood Lability: A Double-Blind, Placebo-Controlled Crossover Design
Abstract
OBJECTIVE: The authors sought to replicate open-label findings showing that specific criteria for explosive temper and mood lability identify disruptive youth who improve while receiving the anticonvulsant divalproex sodium.METHOD: Twenty outpatient children and adolescents (ages 10–18) with a disruptive behavior disorder (oppositional defiant disorder or conduct disorder) met the specific criteria for explosive temper and mood lability. They received 6 weeks of divalproex treatment and 6 weeks of placebo by random assignment. Independent evaluators blind to group assignment assessed response at the end of each phase.RESULTS: At the end of phase 1, eight of 10 subjects had responded to divalproex; zero of 10 had responded to placebo. Of the 15 subjects who completed both phases, 12 has superior response taking divalproex.CONCLUSIONS: This preliminary study replicates open-label findings showing that divalproex is an efficacious treatment for explosive temper and mood lability in disruptive children and adolescents.
The disruptive behavior disorders listed in DSM-IV (oppositional defiant disorder and conduct disorder) have no standard psychopharmacological treatment. Since children and adolescents with these disorders are at high risk for delinquency and addiction, identifying medication-responsive subtypes could have public health implications. We previously reported that 10 adolescents with a disruptive behavior disorder who met operationalized criteria for explosive temper and mood lability showed marked improvement under open-label treatment with the anticonvulsant/mood stabilizer divalproex sodium (1). Here we test this finding under double-blind, placebo-controlled conditions.
Method
The patients were referred by pediatric clinics, school programs for emotionally disturbed youth, and substance abuse counselors in the New York City area and were treated as outpatients. The subjects met the DSM-IV criteria for conduct disorder or oppositional defiant disorder plus these criteria:
A. An explosive temper, defined as four or more outbursts of rage, property destruction, or fighting per month on minimal provocation.
B. Mood lability, defined as multiple daily distinct shifts from normal to irritable mood with withdrawn or boisterous behavior, occurring without a clear precipitant.
C. Chronic symptoms, defined as at least of 1 year’s duration.
D. Impairment from these symptoms in two or more areas, including school, the law, family, substance use, peers, and work.
E. Symptoms not limited to phases of substance toxicity or withdrawal.
F. Symptoms not limited to a particular place or to particular intimate relationships.
Patients with significant medical problems, mental retardation (IQ less than 70), major depression, PTSD, head trauma, and a history of bipolar I or bipolar II disorder were excluded. The New York State Psychiatric Institute’s institutional review board approved the study. The parents gave written informed consent, and the children gave written assent. The study had a two-phase, double-blind crossover design, with patients randomly assigned to phase 1 with divalproex treatment for 6 weeks, immediately followed by phase 2 with placebo for 6 weeks (or placebo then divalproex treatment).
There were three main measures—all integrating information from the child, parent, and school and based on the clinician’s best estimates. For the diagnosis, before group assignment, a child and adolescent research psychiatrist (S.J.D.) used the Structured Clinical Interview for DSM-IV (2) with supplemental questions on attention deficit hyperactivity disorder (ADHD) and oppositional defiant disorder from the Diagnostic Inventory Scale for Children (3). For outcome, at the end of each phase, an independent evaluator (an experienced research psychiatrist who was blind to group assignment [J.W.S. or F.M.Q.]) administered the Modified Overt Aggression Scale (4) and six items from the anger-hostility subscale of the SCL-90 (5).
The Modified Overt Aggression Scale weighted explosive temper outbursts from the previous week in severity, from verbal aggression (cursing and screaming) to aggression against objects (smashing things) to self-aggression (cutting) to hitting people. Its score combines the frequency and severity of explosive outbursts. A similar scale was sensitive to medication effects in outpatient adults (6) and children (4). The anger-hostility subscale of the SCL-90 (5) measures irritability per se—urges and overt acts too mild to register on the Modified Overt Aggression Scale but indicative of an angry, dysphoric, and unpredictable mood. We used these items on a clinician-administered rating form.
We examined joint test-retest reliability in 10 patients, comparing scores of the independent evaluator and the treating psychiatrist on the end-of-phase Modified Overt Aggression Scale and the SCL-90 anger-hostility items. Both were blind to group assignment. This yielded an intraclass correlation coefficient of 0.87 for the Modified Overt Aggression Scale and 0.92 for the SCL-90 anger-hostility items, suggesting excellent reliability in our study group.
The independent evaluator’s assessments were dichotomized into “response” and “no response” categories on the basis of whether there was a substantial (≥70%) reduction from baseline in scores on both the Modified Overt Aggression Scale and the SCL-90 anger-hostility items. This was intended to operationalize a clinically meaningful improvement.
Patients received nonspecific support to maximize retention. Dosing was not tapered between phases, and abrupt withdrawal produced no untoward effects. The divalproex dose was increased over 2 weeks to 10 mg/lb/day. A nonblind psychiatrist (E.V.N.) reviewed the drug’s blood level and allowed a single increase of one pill (250 mg/day) if at the end of week 2 the blood level was less than 90 mg/ml. The final dose range was 750–1500 mg/day. To preserve the blind, dose increases were allowed on an equal number of patients in the placebo phase.
Results
Twenty children and adolescents, ages 10–18 (mean age=13.8, SD=2.4) entered the study. Sixteen (80%) were boys; 15% (N=3) were Caucasian, 60% (N=12) were Hispanic, and 25% (N=5) were African American. Eighteen (90%) were truant or in special education classes (classified as “emotionally disturbed”). Self- and parental reports, as well as the results of urine toxicology tests, indicated that marijuana and (prescribed) stimulants were the only other psychotropic medications used by the group. The DSM-IV diagnoses found in these children were ADHD (four subjects), marijuana abuse (six subjects), and disruptive behavior disorder (oppositional defiant disorder or conduct disorder) (one subject); diagnoses were a priori in all cases. Seventeen (85%) of 20 subjects completed at least one phase of the study; 15 (75%) of 20 subjects completed both phases.
Divalproex was well tolerated. Increased appetite was the only significant side effect, which occurred in four subjects (20%) and did not require any patient to discontinue treatment. The mean blood drug level (excluding one noncompliant patient) during the active phase was 82.2 mg/ml (SD=19.1).
All patients who entered phase 2 completed it. One phase-1 responder (taking divalproex) and one phase-1 nonresponder (taking placebo) refused to enter the crossover phase of the study. Three subjects dropped out in the first 2 weeks of phase 1. Two cited lack of efficacy (one taking medication and one taking placebo); one subject randomly assigned to divalproex treatment was jailed for parole violation.
We analyzed the data in two ways. We first considered phase-1 results only and adopted the intent-to-treat principle. For the second analysis, we examined the full crossover design and did not consider the five subjects who dropped out.
The first 6 weeks of the trial was a parallel-groups design. During this first phase, 10 subjects each were randomly assigned to receive divalproex and placebo. Eight patients randomly assigned to received medication responded at the end of this first phase, whereas none of the patients randomly assigned to receive placebo were judged to have responded. These results indicate that divalproex was effective, compared to placebo, after 6 weeks (p<0.001, Fisher’s exact test, two-tailed).
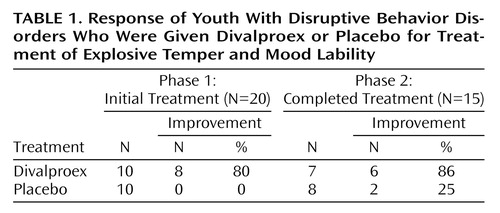
Six of the seven phase-1 nonresponders (taking placebo) who entered phase 2 responded to divalproex. Additionally, 1 to 2 weeks into phase 2, six of the eight phase-1 medication responders began relapsing, ending phase 2 with average scores of 33% (Modified Overt Aggression Scale) and 27% (SCL-90 anger-hostility items)—better than at the beginning of phase 1. One subject did not enter phase 2, and 1 did not relapse in phase 2. Table 1 summarizes the study results.
All 15 patients who began phase 2 completed it. Twelve met the response criteria only during the medication phase, one only during the placebo phase, one in both phases, and one in neither phase. These results indicate that patients are much more likely to obtain a superior response taking divalproex than placebo (p=0.003, sign test).
Discussion
When we replicated an open-label study (1), children and adolescents with a disruptive behavior disorder plus explosive temper and mood lability preferentially responded to divalproex under double-blind, placebo-controlled conditions. Clinically meaningful placebo response did not occur in the first 6 weeks. In the second 6 weeks, however, one patient did not relapse on placebo, and one changed his status from nonresponder (taking medication) to responder (taking placebo). The phase-2 placebo response of 25% suggests that some patients who remain in a study for 12 weeks may receive nonspecific, clinically meaningful benefit.
Mood stabilizers have shown antiaggressive effects in other populations (see review, reference 7). Many youngsters with inattention impulsivity and hyperactivity (ADHD) have a stimulant-responsive form of disruptive behavior. The present data suggest that those with explosive temper and mood lability may have another pharmacologically treatable form of disruptive behavior.
Replication in larger parallel-groups, placebo-controlled trials is indicated to estimate medication and placebo effects in this population. Studies combining medication with psychosocial interventions targeting explosive temper and mood lability should be considered. Long-term follow-up is needed to determine whether treatment may help prevent substance abuse, criminality, and other negative outcomes associated with the disruptive behavior disorders.
 |
Received July 1, 1999; revision received Nov. 24, 1999; accepted Dec. 1, 1999. From the Department of Therapeutics, Division of Epidemiology of Brain Disorders, New York State Psychiatric Institute; and the Mailman School of Public Health, Columbia University, New York. Address reprint requests to Dr. Donovan, Department of Therapeutics, Division of Epidemiology of Brain Disorders, New York State Psychiatric Institute, 1051 Riverside Dr., Suite 51, New York, NY 10032; [email protected] (e-mail). Supported by grant DA-00246 to Dr. Donovan from the National Institute on Drug Abuse. Abbott Laboratories Inc. supplied the divalproex and the matching placebo.
1. Donovan SJ, Susser ES, Nunes EV, Stewart JW, Quitkin FM, Klein DF: Divalproex treatment of disruptive adolescents: a report of 10 cases. J Clin Psychiatry 1997; 58:12–15Crossref, Google Scholar
2. Spitzer RL, Williams JBW, Gibbon M: Structured Clinical Interview for DSM-IV (SCID). New York, New York State Psychiatric Institute, Biometrics Research, 1995Google Scholar
3. Shaffer D, Fisher P, Dulcan MK, Davies M, Piacentini J, Schwab-Stone ME, Lahey BB, Bourdon K, Jensen PS, Bird HR, Canino G, Regier DA: The NIMH Diagnostic Interview Schedule for Children Version 2.3 (DISC-2.3): description, acceptability, prevalence rates and performance in the MECA Study. Methods for the Epidemiology of Child and Adolescent Mental Disorders Study. J Am Acad Child Adolesc Psychiatry 1996; 35:865–877Crossref, Medline, Google Scholar
4. Malone RP, Luebbert RP, Pena-Ariet M, Biesecker K, Delaney MA: The Overt Aggression Scale in a study of lithium in aggressive conduct disorder. Psychopharmacol Bull 1994; 30:215–218Medline, Google Scholar
5. Derogatis LR, Lipman RS, Covi L: SCL-90: an outpatient psychiatric rating scale—preliminary report. Psychopharmacol Bull 1973; 9:13–28Medline, Google Scholar
6. Kavoussi RJ, Cocarro EF: Divalproex sodium for impulsive aggressive behavior in patients with personality disorder. J Clin Psychiatry 1998; 59:676–680Crossref, Medline, Google Scholar
7. Donovan SJ, Nunes EV: Treatment of comorbid affective and substance use disorders: therapeutic potential of anticonvulsants. Am J Addict 1998; 7:210–220Medline, Google Scholar



